Oral Supplementation with L-Carnosine Attenuates Social Recognition Deficits in CD157KO Mice via Oxytocin Release
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Three-Chamber Social Approach Test
2.3. Real-Time Reverse Transcriptase Quantitative PCR
2.4. Tissue Preparation
2.5. Immunocytochemistry
2.6. Image Quantification
2.7. Sampling of Cerebrospinal Fluid (CSF)
2.8. Enzyme Immunoassay for OT
2.9. Statistical Analysis
3. Results
3.1. Effects of L-Carnosine Supplementation on Social Behavior
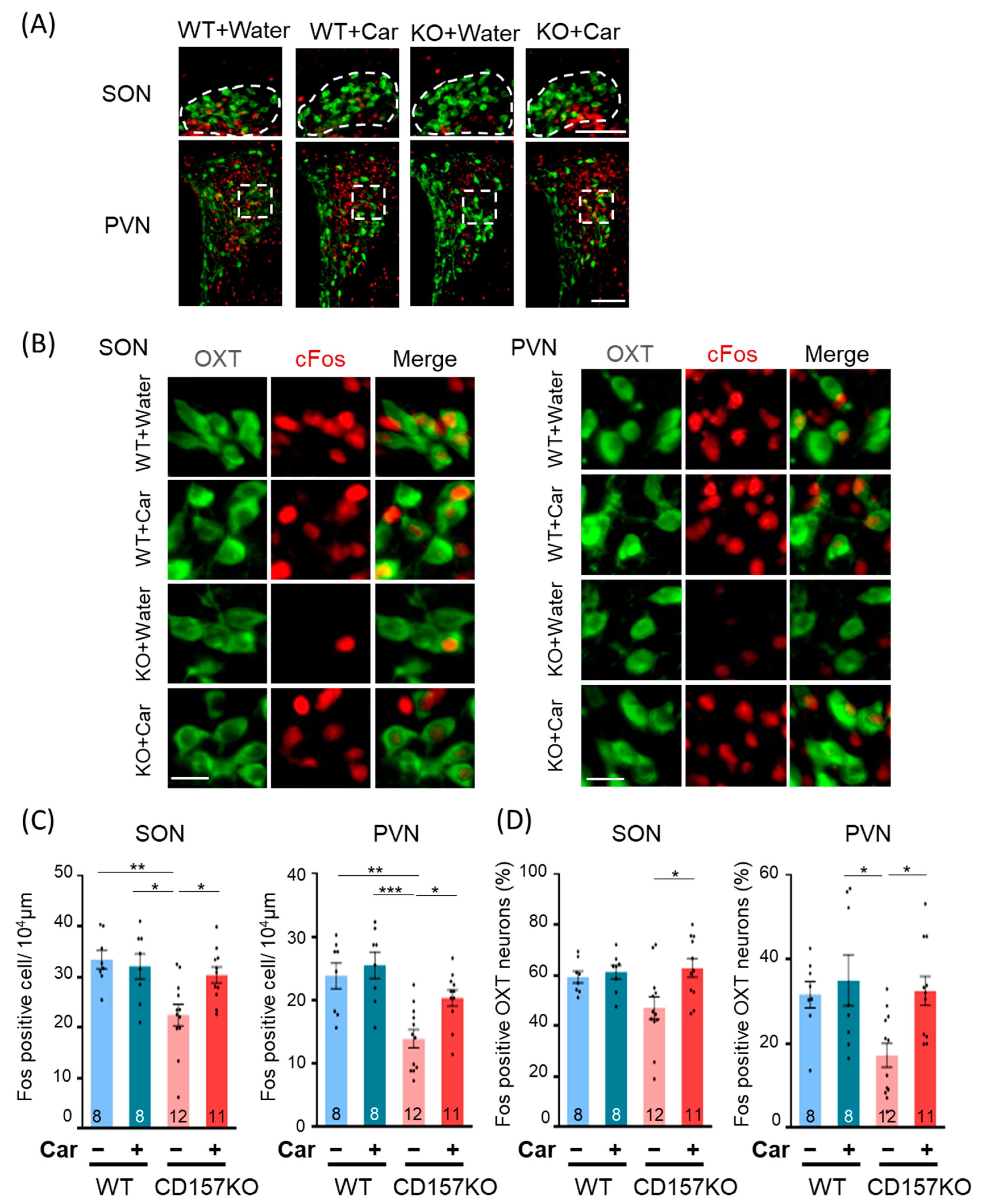
3.2. c-Fos Activation in the SON and PVN
3.3. Effects of L-Carnosine Supplementation on c-Fos Expression in the BLA and MePV in CD157KO Mice
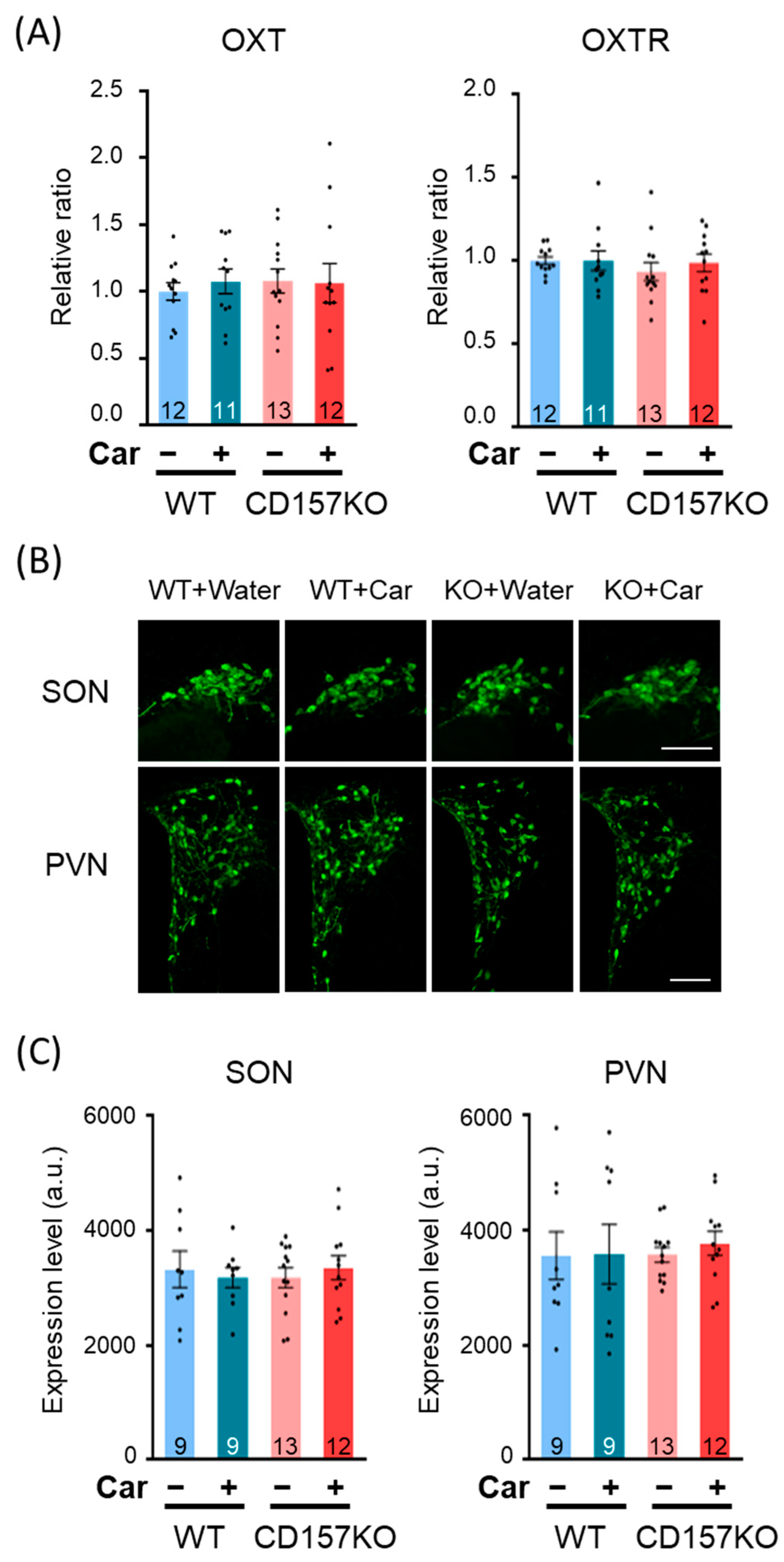
3.4. L-Carnosine Treatment Increased Oxytocin Concentration in CSF
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chiarotti, F.; Venerosi, A. Epidemiology of Autism Spectrum Disorders: A Review of Worldwide Prevalence Estimates Since 2014. Brain Sci. 2020, 10, 274. [Google Scholar] [CrossRef] [PubMed]
- Jurek, B.; Neumann, I.D. The Oxytocin Receptor: From Intracellular Signaling to Behavior. Physiol. Rev. 2018, 98, 1805–1908. [Google Scholar] [CrossRef] [PubMed]
- Modi, M.E.; Young, L.J. The oxytocin system in drug discovery for autism: Animal models and novel therapeutic strategies. Horm. Behav. 2012, 61, 340–350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Domes, G.; Heinrichs, M.; Michel, A.; Berger, C.; Herpertz, S.C. Oxytocin improves "mind-reading" in humans. Biol. Psychiatry 2007, 61, 731–733. [Google Scholar] [CrossRef]
- Savaskan, E.; Ehrhardt, R.; Schulz, A.; Walter, M.; Schachinger, H. Post-learning intranasal oxytocin modulates human memory for facial identity. Psychoneuroendocrinology 2008, 33, 368–374. [Google Scholar] [CrossRef]
- Yamasue, H.; Domes, G. Oxytocin and Autism Spectrum Disorders. Curr. Top. Behav. Neurosci. 2018, 35, 449–465. [Google Scholar] [CrossRef]
- Kosfeld, M.; Heinrichs, M.; Zak, P.J.; Fischbacher, U.; Fehr, E. Oxytocin increases trust in humans. Nature 2005, 435, 673–676. [Google Scholar] [CrossRef] [Green Version]
- Zak, P.J.; Stanton, A.A.; Ahmadi, S. Oxytocin increases generosity in humans. PLoS ONE 2007, 2, e1128. [Google Scholar] [CrossRef]
- Young, L.J.; Barrett, C.E. Neuroscience. Can oxytocin treat autism? Science 2015, 347, 825–826. [Google Scholar] [CrossRef] [Green Version]
- Boldyrev, A.A.; Aldini, G.; Derave, W. Physiology and pathophysiology of carnosine. Physiol. Rev. 2013, 93, 1803–1845. [Google Scholar] [CrossRef]
- Rajanikant, G.K.; Zemke, D.; Senut, M.C.; Frenkel, M.B.; Chen, A.F.; Gupta, R.; Majid, A. Carnosine is neuroprotective against permanent focal cerebral ischemia in mice. Stroke 2007, 38, 3023–3031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fleisher-Berkovich, S.; Abramovitch-Dahan, C.; Ben-Shabat, S.; Apte, R.; Beit-Yannai, E. Inhibitory effect of carnosine and N-acetyl carnosine on LPS-induced microglial oxidative stress and inflammation. Peptides 2009, 30, 1306–1312. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, J.C.; Fisher, H.; Flancbaum, L. Mobilization of renal carnosine and histidine to histamine during compound-48/80-induced shock. Nephron 1991, 59, 299–303. [Google Scholar] [CrossRef] [PubMed]
- Kasziba, E.; Flancbaum, L.; Fitzpatrick, J.C.; Schneiderman, J.; Fisher, H. Simultaneous determination of histidine-containing dipeptides, histamine, methylhistamine and histidine by high-performance liquid chromatography. J. Chromatogr. 1988, 432, 315–320. [Google Scholar] [CrossRef]
- Shen, Y.; Hu, W.W.; Fan, Y.Y.; Dai, H.B.; Fu, Q.L.; Wei, E.Q.; Luo, J.H.; Chen, Z. Carnosine protects against NMDA-induced neurotoxicity in differentiated rat PC12 cells through carnosine-histidine-histamine pathway and H(1)/H(3) receptors. Biochem. Pharmacol. 2007, 73, 709–717. [Google Scholar] [CrossRef]
- Chesnoy-Marchais, D. Persistent GABAA/C responses to gabazine, taurine and beta-alanine in rat hypoglossal motoneurons. Neuroscience 2016, 330, 191–204. [Google Scholar] [CrossRef]
- Ouyang, L.; Tian, Y.; Bao, Y.; Xu, H.; Cheng, J.; Wang, B.; Shen, Y.; Chen, Z.; Lyu, J. Carnosine decreased neuronal cell death through targeting glutamate system and astrocyte mitochondrial bioenergetics in cultured neuron/astrocyte exposed to OGD/recovery. Brain Res. Bull. 2016, 124, 76–84. [Google Scholar] [CrossRef]
- Tomonaga, S.; Hayakawa, T.; Yamane, H.; Maemura, H.; Sato, M.; Takahata, Y.; Morimatsu, F.; Furuse, M. Oral administration of chicken breast extract increases brain carnosine and anserine concentrations in rats. Nutr. Neurosci. 2007, 10, 181–186. [Google Scholar] [CrossRef]
- Leng, G.; Onaka, T.; Caquineau, C.; Sabatier, N.; Tobin, V.A.; Takayanagi, Y. Oxytocin and appetite. Prog. Brain Res. 2008, 170, 137–151. [Google Scholar] [CrossRef]
- Chez, M.G.; Buchanan, C.P.; Aimonovitch, M.C.; Becker, M.; Schaefer, K.; Black, C.; Komen, J. Double-blind, placebo-controlled study of L-carnosine supplementation in children with autistic spectrum disorders. J. Child Neurol. 2002, 17, 833–837. [Google Scholar] [CrossRef]
- Ann Abraham, D.; Narasimhan, U.; Christy, S.; Muhasaparur Ganesan, R. Effect of L-Carnosine as adjunctive therapy in the management of children with autism spectrum disorder: A randomized controlled study. Amino Acids 2020, 52, 1521–1528. [Google Scholar] [CrossRef] [PubMed]
- Abraham, D.A.; Undela, K.; Narasimhan, U.; Rajanandh, M.G. Effect of L-Carnosine in children with autism spectrum disorders: A systematic review and meta-analysis of randomised controlled trials. Amino Acids 2021, 53, 575–585. [Google Scholar] [CrossRef] [PubMed]
- Kaisho, T.; Ishikawa, J.; Oritani, K.; Inazawa, J.; Tomizawa, H.; Muraoka, O.; Ochi, T.; Hirano, T. BST-1, a surface molecule of bone marrow stromal cell lines that facilitates pre-B-cell growth. Proc. Natl. Acad. Sci. USA 1994, 91, 5325–5329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Funaro, A.; Ortolan, E.; Ferranti, B.; Gargiulo, L.; Notaro, R.; Luzzatto, L.; Malavasi, F. CD157 is an important mediator of neutrophil adhesion and migration. Blood 2004, 104, 4269–4278. [Google Scholar] [CrossRef]
- Podesta, M.; Benvenuto, F.; Pitto, A.; Figari, O.; Bacigalupo, A.; Bruzzone, S.; Guida, L.; Franco, L.; Paleari, L.; Bodrato, N.; et al. Concentrative uptake of cyclic ADP-ribose generated by BST-1+ stroma stimulates proliferation of human hematopoietic progenitors. J. Biol. Chem. 2005, 280, 5343–5349. [Google Scholar] [CrossRef] [Green Version]
- Malavasi, F.; Deaglio, S.; Ferrero, E.; Funaro, A.; Sancho, J.; Ausiello, C.M.; Ortolan, E.; Vaisitti, T.; Zubiaur, M.; Fedele, G.; et al. CD38 and CD157 as receptors of the immune system: A bridge between innate and adaptive immunity. Mol. Med. 2006, 12, 334–341. [Google Scholar] [CrossRef] [Green Version]
- Satake, W.; Nakabayashi, Y.; Mizuta, I.; Hirota, Y.; Ito, C.; Kubo, M.; Kawaguchi, T.; Tsunoda, T.; Watanabe, M.; Takeda, A.; et al. Genome-wide association study identifies common variants at four loci as genetic risk factors for Parkinson's disease. Nat. Genet. 2009, 41, 1303–1307. [Google Scholar] [CrossRef]
- Tan, E.K.; Kwok, H.H.; Tan, L.C.; Zhao, W.T.; Prakash, K.M.; Au, W.L.; Pavanni, R.; Ng, Y.Y.; Satake, W.; Zhao, Y.; et al. Analysis of GWAS-linked loci in Parkinson disease reaffirms PARK16 as a susceptibility locus. Neurology 2010, 75, 508–512. [Google Scholar] [CrossRef]
- Liu, X.; Cheng, R.; Verbitsky, M.; Kisselev, S.; Browne, A.; Mejia-Sanatana, H.; Louis, E.D.; Cote, L.J.; Andrews, H.; Waters, C.; et al. Genome-wide association study identifies candidate genes for Parkinson’s disease in an Ashkenazi Jewish population. BMC Med Genet. 2011, 12, 104. [Google Scholar] [CrossRef] [Green Version]
- Saad, M.; Lesage, S.; Saint-Pierre, A.; Corvol, J.C.; Zelenika, D.; Lambert, J.C.; Vidailhet, M.; Mellick, G.D.; Lohmann, E.; Durif, F.; et al. Genome-wide association study confirms BST1 and suggests a locus on 12q24 as the risk loci for Parkinson's disease in the European population. Hum. Mol. Genet. 2011, 20, 615–627. [Google Scholar] [CrossRef]
- Yokoyama, S.; Al Mahmuda, N.; Munesue, T.; Hayashi, K.; Yagi, K.; Yamagishi, M.; Higashida, H. Association Study between the CD157/BST1 Gene and Autism Spectrum Disorders in a Japanese Population. Brain Sci. 2015, 5, 188–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ceroni, F.; Sagar, A.; Simpson, N.H.; Gawthrope, A.J.; Newbury, D.F.; Pinto, D.; Francis, S.M.; Tessman, D.C.; Cook, E.H.; Monaco, A.P.; et al. A deletion involving CD38 and BST1 results in a fusion transcript in a patient with autism and asthma. Autism Res. 2014, 7, 254–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopatina, O.; Yoshihara, T.; Nishimura, T.; Zhong, J.; Akther, S.; Fakhrul, A.A.; Liang, M.; Higashida, C.; Sumi, K.; Furuhara, K.; et al. Anxiety- and depression-like behavior in mice lacking the CD157/BST1 gene, a risk factor for Parkinson's disease. Front. Behav. Neurosci. 2014, 8, 133. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, A.; Cherepanov, S.M.; Kikuchi, Y.; Fakhrul, A.A.; Akther, S.; Deguchi, K.; Yoshihara, T.; Ishihara, K.; Shuto, S.; Higashida, H. Lipo-oxytocin-1, a Novel Oxytocin Analog Conjugated with Two Palmitoyl Groups, Has Long-Lasting Effects on Anxiety-Related Behavior and Social Avoidance in CD157 Knockout Mice. Brain Sci. 2015, 5, 3–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Higashida, H.; Liang, M.; Yoshihara, T.; Akther, S.; Fakhrul, A.; Stanislav, C.; Nam, T.S.; Kim, U.H.; Kasai, S.; Nishimura, T.; et al. An immunohistochemical, enzymatic, and behavioral study of CD157/BST-1 as a neuroregulator. BMC Neurosci. 2017, 18, 35. [Google Scholar] [CrossRef] [Green Version]
- Ishihara, K.; Hirano, T. BST-1/CD157 regulates the humoral immune responses in vivo. Chem. Immunol. 2000, 75, 235–255. [Google Scholar] [CrossRef]
- Gerasimenko, M.; Cherepanov, S.M.; Furuhara, K.; Lopatina, O.; Salmina, A.B.; Shabalova, A.A.; Tsuji, C.; Yokoyama, S.; Ishihara, K.; Brenner, C.; et al. Nicotinamide riboside supplementation corrects deficits in oxytocin, sociability and anxiety of CD157 mutants in a mouse model of autism spectrum disorder. Sci. Rep. 2020, 10, 10035. [Google Scholar] [CrossRef]
- Lim, N.K.; Moestrup, V.; Zhang, X.; Wang, W.A.; Moller, A.; Huang, F.D. An Improved Method for Collection of Cerebrospinal Fluid from Anesthetized Mice. J. Vis. Exp. 2018, 133, e56774. [Google Scholar] [CrossRef]
- Tanimizu, T.; Kenney, J.W.; Okano, E.; Kadoma, K.; Frankland, P.W.; Kida, S. Functional Connectivity of Multiple Brain Regions Required for the Consolidation of Social Recognition Memory. J. Neurosci. 2017, 37, 4103–4116. [Google Scholar] [CrossRef]
- Shelley, D.N.; Meisel, R.L. The effects of mating stimulation on c-Fos immunoreactivity in the female hamster medial amygdala are region and context dependent. Horm. Behav. 2005, 47, 212–222. [Google Scholar] [CrossRef]
- Szymanski, L.A.; Keller, M. Activation of the olfactory system in response to male odors in female prepubertal mice. Behav. Brain Res. 2014, 271, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Higashida, H.; Salmina, A.B.; Olovyannikova, R.Y.; Hashii, M.; Yokoyama, S.; Koizumi, K.; Jin, D.; Liu, H.X.; Lopatina, O.; Amina, S.; et al. Cyclic ADP-ribose as a universal calcium signal molecule in the nervous system. Neurochem. Int. 2007, 51, 192–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Higashida, H.; Hashii, M.; Tanaka, Y.; Matsukawa, S.; Higuchi, Y.; Gabata, R.; Tsubomoto, M.; Seishima, N.; Teramachi, M.; Kamijima, T.; et al. CD38, CD157, and RAGE as Molecular Determinants for Social Behavior. Cells 2019, 9, 62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomonaga, S.; Tachibana, T.; Takagi, T.; Saito, E.S.; Zhang, R.; Denbow, D.M.; Furuse, M. Effect of central administration of carnosine and its constituents on behaviors in chicks. Brain Res. Bull. 2004, 63, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Rubtsov, A.M. Molecular mechanisms of regulation of the activity of sarcoplasmic reticulum Ca-release channels (ryanodine receptors), muscle fatigue, and Severin's phenomenon. Biochemistry 2001, 66, 1132–1143. [Google Scholar] [CrossRef] [PubMed]
- Jin, D.; Liu, H.X.; Hirai, H.; Torashima, T.; Nagai, T.; Lopatina, O.; Shnayder, N.A.; Yamada, K.; Noda, M.; Seike, T.; et al. CD38 is critical for social behaviour by regulating oxytocin secretion. Nature 2007, 446, 41–45. [Google Scholar] [CrossRef]
- Gangopadhyay, P.; Chawla, M.; Dal Monte, O.; Chang, S.W.C. Prefrontal-amygdala circuits in social decision-making. Nat. Neurosci. 2021, 24, 5–18. [Google Scholar] [CrossRef]
- Huang, W.C.; Zucca, A.; Levy, J.; Page, D.T. Social Behavior Is Modulated by Valence-Encoding mPFC-Amygdala Sub-circuitry. Cell Rep. 2020, 32, 107899. [Google Scholar] [CrossRef]
- Newman, S.W. The medial extended amygdala in male reproductive behavior. A node in the mammalian social behavior network. Ann. N. Y. Acad. Sci. 1999, 877, 242–257. [Google Scholar] [CrossRef]
- Masuoka, N.; Yoshimine, C.; Hori, M.; Tanaka, M.; Asada, T.; Abe, K.; Hisatsune, T. Effects of Anserine/Carnosine Supplementation on Mild Cognitive Impairment with APOE4. Nutrients 2019, 11, 1626. [Google Scholar] [CrossRef] [Green Version]
- Arabzadeh, S.; Shahhossenie, M.; Mesgarpour, B.; Rezaei, F.; Shalbafan, M.R.; Ghiasi, Z.; Akhondzadeh, S. L-carnosine as an adjuvant to fluvoxamine in treatment of obsessive compulsive disorder: A randomized double-blind study. Hum. Psychopharmacol. 2017, 32, e2584. [Google Scholar] [CrossRef] [PubMed]
- Corona, C.; Frazzini, V.; Silvestri, E.; Lattanzio, R.; La Sorda, R.; Piantelli, M.; Canzoniero, L.M.; Ciavardelli, D.; Rizzarelli, E.; Sensi, S.L. Effects of dietary supplementation of carnosine on mitochondrial dysfunction, amyloid pathology, and cognitive deficits in 3xTg-AD mice. PLoS ONE 2011, 6, e17971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghajar, A.; Khoaie-Ardakani, M.R.; Shahmoradi, Z.; Alavi, A.R.; Afarideh, M.; Shalbafan, M.R.; Ghazizadeh-Hashemi, M.; Akhondzadeh, S. L-carnosine as an add-on to risperidone for treatment of negative symptoms in patients with stable schizophrenia: A double-blind, randomized placebo-controlled trial. Psychiatry Res. 2018, 262, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Jackson, M.C.; Kucera, C.M.; Lenney, J.F. Purification and properties of human serum carnosinase. Clin. Chim. Acta 1991, 196, 193–205. [Google Scholar] [CrossRef]
- Yeum, K.J.; Orioli, M.; Regazzoni, L.; Carini, M.; Rasmussen, H.; Russell, R.M.; Aldini, G. Profiling histidine dipeptides in plasma and urine after ingesting beef, chicken or chicken broth in humans. Amino Acids 2010, 38, 847–858. [Google Scholar] [CrossRef]
- Rokicki, J.; Li, L.; Imabayashi, E.; Kaneko, J.; Hisatsune, T.; Matsuda, H. Daily Carnosine and Anserine Supplementation Alters Verbal Episodic Memory and Resting State Network Connectivity in Healthy Elderly Adults. Front. Aging Neurosci. 2015, 7, 219. [Google Scholar] [CrossRef] [Green Version]
- Pegova, A.; Abe, H.; Boldyrev, A. Hydrolysis of carnosine and related compounds by mammalian carnosinases. Comp. Biochem. Physiol. B-Biochem. Mol. Biol. 2000, 127, 443–446. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsuji, T.; Furuhara, K.; Gerasimenko, M.; Shabalova, A.; Cherepanov, S.M.; Minami, K.; Higashida, H.; Tsuji, C. Oral Supplementation with L-Carnosine Attenuates Social Recognition Deficits in CD157KO Mice via Oxytocin Release. Nutrients 2022, 14, 803. https://doi.org/10.3390/nu14040803
Tsuji T, Furuhara K, Gerasimenko M, Shabalova A, Cherepanov SM, Minami K, Higashida H, Tsuji C. Oral Supplementation with L-Carnosine Attenuates Social Recognition Deficits in CD157KO Mice via Oxytocin Release. Nutrients. 2022; 14(4):803. https://doi.org/10.3390/nu14040803
Chicago/Turabian StyleTsuji, Takahiro, Kazumi Furuhara, Maria Gerasimenko, Anna Shabalova, Stanislav M Cherepanov, Kana Minami, Haruhiro Higashida, and Chiharu Tsuji. 2022. "Oral Supplementation with L-Carnosine Attenuates Social Recognition Deficits in CD157KO Mice via Oxytocin Release" Nutrients 14, no. 4: 803. https://doi.org/10.3390/nu14040803
APA StyleTsuji, T., Furuhara, K., Gerasimenko, M., Shabalova, A., Cherepanov, S. M., Minami, K., Higashida, H., & Tsuji, C. (2022). Oral Supplementation with L-Carnosine Attenuates Social Recognition Deficits in CD157KO Mice via Oxytocin Release. Nutrients, 14(4), 803. https://doi.org/10.3390/nu14040803






