The Effect of Carbohydrate Intake on Strength and Resistance Training Performance: A Systematic Review
Abstract
1. Introduction
2. Methods
2.1. Search Strategy
2.2. Inclusion Criteria
2.3. Study Selection and Data Extraction
2.4. Quality Assessment
3. Results
3.1. The Effect of Acute Carbohydrate Manipulation on Strength Training Performance
3.1.1. Main Findings
3.1.2. Carbohydrate Dosage and Training Volume
3.1.3. Results from Published Abstracts
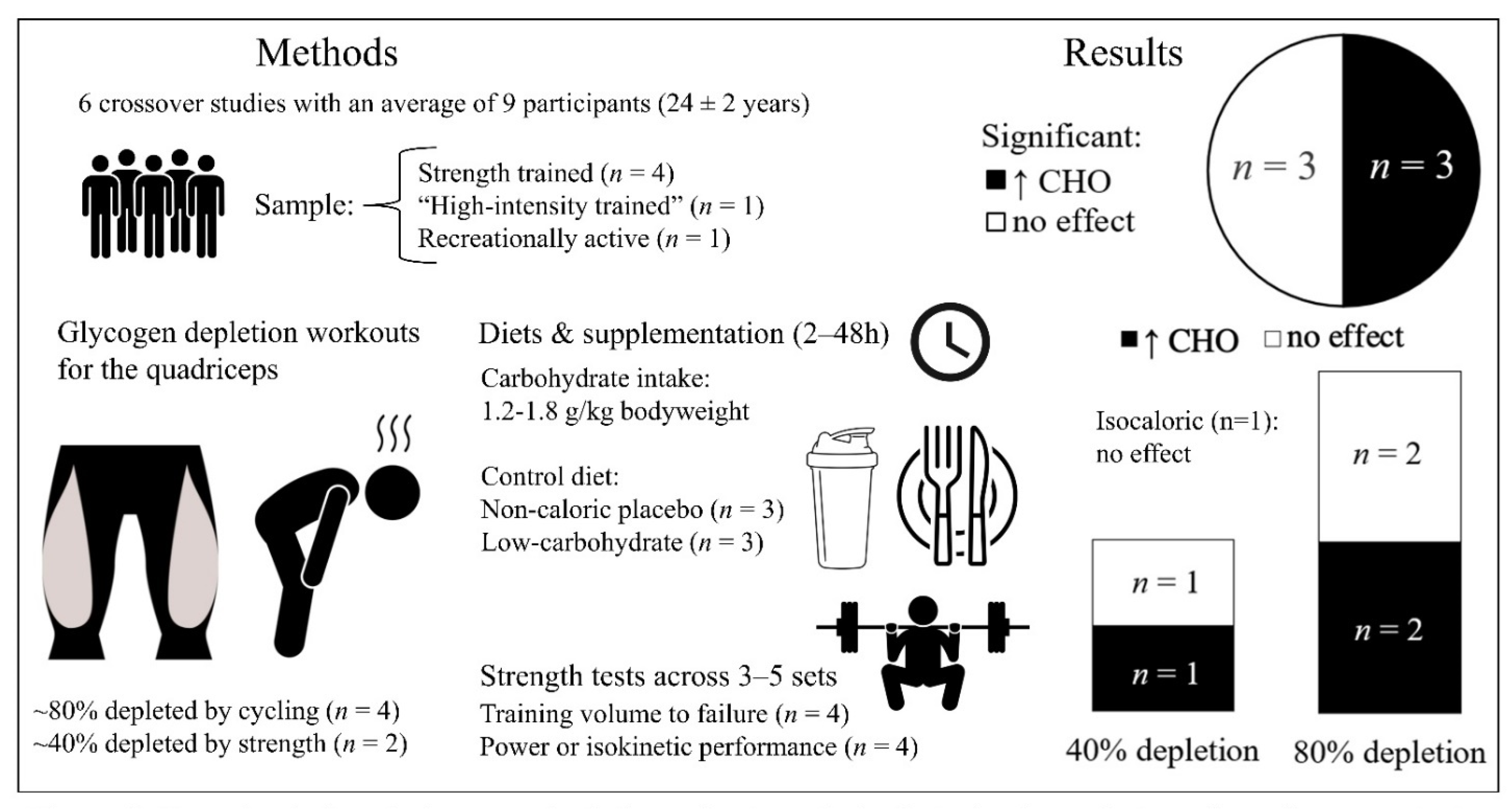
3.2. The Effect of Exercise-Induced Glycogen Depletion and Carbohydrate Manipulation on Acute Strength Training Performance
3.2.1. Main Findings
3.2.2. Carbohydrate Dosage and Training Volume
3.3. The Effect of Short-Term Carbohydrate Manipulation on Acute Strength Training Performance
3.3.1. Main Findings
3.3.2. Results from Published Abstracts
3.4. The Effect of Longer-Term Carbohydrate Diets and Strength-Training on Changes in Strength Performance
Main Findings
4. Discussion
4.1. The Effect of Acute Carbohydrate Manipulation on Strength Training Performance
4.2. The Effect of Exercise-Induced Glycogen Depletion and Carbohydrate Manipulation on Acute Strength Performance
4.3. The Effect of Short-Term Carbohydrate Manipulation on Acute Strength Training Performance
4.4. The Effect of Longer-Term Carbohydrate Diets and Strength Training on Changes in Strength Performance
4.5. Conclusions and Practical Applications
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Study | 1 | 2 | 3 | 4 | 5 | 6a | 6b | 8a | 8b | 9 | 12 | Total (Max 11) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acute studies | ||||||||||||
| Baty et al. [32] | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 10 |
| Dalton et al. [33] | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 8 |
| Fairchild et al. [27] | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 10 |
| Fayh et al. [34] | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 8 |
| Haff et al. [28] | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 8 |
| Krings et al. [29] | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 9 |
| Kulik et al. [35] | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 9 |
| Lambert et al. [26] | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 8 |
| Laurenson-Dubè [30] | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 7 |
| Lynch et al. [36] | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 8 |
| Maroufi et al. [44] | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 8 |
| Naharudin et al. [39] | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 8 |
| Naharudin et al. [41] | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 10 |
| Raposo [37] | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 8 |
| Rountree et al. [38] | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 9 |
| Santos et al. [42] | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 8 |
| Smith et al. [31] | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 9 |
| Welikonich [40] | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 7 |
| Wilburn et al. [43] | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 9 |
| Glycogen-depletion studies | ||||||||||||
| Haff et al. [49] | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 8 |
| Haff et al. [52] | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 8 |
| Leveritt-Abernethy [15] | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 6 |
| Mitchell et al. [48] | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 7 |
| Oliver et al. [50] | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 10 |
| Symons-Jacobs [51] | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 7 |
| Short-term studies | ||||||||||||
| Dipla et al. [54] | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 8 |
| Escobar et al. [53] | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 7 |
| Hatfield et al. [55] | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 7 |
| Kreider et al. [56] | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 6 |
| Meirelles et al. [57] | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 8 |
| Moura et al. [59] | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 8 |
| Sawyer et al. [58] | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 8 |
| Study | 1 | 2 | 3 | 4 | 5 | 6a | 6b | 6c | 7 | 8a | 8b | 9 | 11 | 12 | Total (Max 14) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Agee [68] | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 9 |
| Greene et al. [61] | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 * | 0 | 9 |
| Gregory et al. [62] | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 * | 0 | 11 |
| Kephart et al. [69] | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 * | 0 | 8 |
| Kreider et al. [66] | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 * | 1 | 1 | 1 | 1 | 0 | 11 |
| LaFountain et al. [72] | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 10 |
| Meirelles-Gomes [63] | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 1 * | 1 | 1 | 1 | 1 | 1 | 11 |
| Michalski et al. [75] | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 * | 0 | 8 |
| De Oliveira et al. [71] | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 1 * | 1 | 1 | 1 | 1 | 0 | 8 |
| Paoli et al. [76] | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 * | 1 | 1 | 1 | 1 * | 0 | 11 |
| Paoli et al. [70] | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 1 * | 1 | 1 | 1 | 1 * | 0 | 9 |
| Rhyu and Cho [67] | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 1 * | 1 | 1 | 1 | 1 * | 0 | 9 |
| Rozenek et al. [74] | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 10 |
| Van Zant et al. [64] | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 1 * | 1 | 1 | 1 | 1 * | 0 | 9 |
| Vargas-Molina et al. [73] | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | 8 |
| Vidić et al. [77] | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 8 |
| Wilson et al. [65] | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 8 |
References
- Burke, L.M.; Hawley, J.A.; Wong, S.H.S.; Jeukendrup, A.E. Carbohydrates for training and competition. J. Sports Sci. 2011, 29 (Suppl. 1), S17–S27. [Google Scholar] [CrossRef]
- Hughes, D.C.; Ellefsen, S.; Baar, K. Adaptations to Endurance and Strength Training. Cold Spring Harb. Perspect. Med. 2017, 8, a029769. [Google Scholar] [CrossRef]
- Knuiman, P.; Hopman, M.T.E.; Mensink, M. Glycogen availability and skeletal muscle adaptations with endurance and resistance exercise. Nutr. Metab. 2015, 12, 59. [Google Scholar] [CrossRef]
- Tesch, P.A.; Ploutz-Snyder, L.L.; Yström, L.; Castro, M.J.; Dudley, G.A. Skeletal Muscle Glycogen Loss Evoked by Resistance Exercise. J. Strength Cond. Res. 1998, 12, 67–73. [Google Scholar] [CrossRef]
- Robergs, R.A.; Pearson, D.R.; Costill, D.L.; Fink, W.J.; Pascoe, D.D.; Benedict, M.A.; Lambert, C.P.; Zachweija, J.J. Muscle glycogenolysis during differing intensities of weight-resistance exercise. J. Appl. Physiol. 1991, 70, 1700–1706. [Google Scholar] [CrossRef]
- Lambert, C.P.; Flynn, M.G. Fatigue during High-Intensity Intermittent Exercise. Sports Med. 2002, 32, 511–522. [Google Scholar] [CrossRef]
- Ørtenblad, N.; Westerblad, H.; Nielsen, J. Muscle glycogen stores and fatigue. J. Physiol. 2013, 591, 4405–4413. [Google Scholar] [CrossRef]
- Hokken, R.; Laugesen, S.; Aagaard, P.; Suetta, C.; Frandsen, U.; Ørtenblad, N.; Nielsen, J. Subcellular localization- and fibre type-dependent utilization of muscle glycogen during heavy resistance exercise in elite power and Olympic weightlifters. Acta Physiol. 2020, 231, e13561. [Google Scholar] [CrossRef]
- Duhamel, T.A.; Perco, J.G.; Green, H.J. Manipulation of dietary carbohydrates after prolonged effort modifies muscle sarcoplasmic reticulum responses in exercising males. Am. J. Physiol. Integr. Comp. Physiol. 2006, 291, R1100–R1110. [Google Scholar] [CrossRef]
- Ørtenblad, N.; Nielsen, J.; Saltin, B.; Holmberg, H.-C. Role of glycogen availability in sarcoplasmic reticulum Ca2+kinetics in human skeletal muscle. J. Physiol. 2011, 589, 711–725. [Google Scholar] [CrossRef]
- Vigh-Larsen, J.F.; Ørtenblad, N.; Spriet, L.L.; Overgaard, K.; Mohr, M. Muscle Glycogen Metabolism and High-Intensity Exercise Performance: A Narrative Review. Sports Med. 2021, 51, 1855–1874. [Google Scholar] [CrossRef]
- Lemon, P.W.; Mullin, J.P. Effect of initial muscle glycogen levels on protein catabolism during exercise. J. Appl. Physiol. 1980, 48, 624–629. [Google Scholar] [CrossRef]
- Howarth, K.R.; Phillips, S.M.; MacDonald, M.J.; Richards, D.; Moreau, N.A.; Gibala, M.J. Effect of glycogen availability on human skeletal muscle protein turnover during exercise and recovery. J. Appl. Physiol. 2010, 109, 431–438. [Google Scholar] [CrossRef]
- Jacobs, I.; Kaiser, P.; Tesch, P. Muscle strength and fatigue after selective glycogen depletion in human skeletal muscle fibers. Eur. J. Appl. Physiol. Occup. Physiol. 1981, 46, 47–53. [Google Scholar] [CrossRef]
- Leveritt, M.; Abernethy, P.J. Effects of Carbohydrate Restriction on Strength Performance. J. Strength Cond. Res. 1999, 13, 52–57. [Google Scholar] [CrossRef]
- Pendergast, D.R.; Meksawan, K.; Limprasertkul, A.; Fisher, N.M. Influence of exercise on nutritional requirements. Eur. J. Appl. Physiol. 2011, 111, 379–390. [Google Scholar] [CrossRef]
- Haff, G.G.; Lehmkuhl, M.J.; McCoy, L.B.; Stone, M.H. Carbohydrate supplementation and resistance training. J. Strength Cond. Res. Natl. Strength Cond. Assoc. 2003, 17, 187–196. [Google Scholar] [CrossRef]
- Bell, D.G.; Jacobs, I. Muscle fiber-specific glycogen utilization in strength-trained males and females. Med. Sci. Sports Exerc. 1989, 21, 649–654. [Google Scholar] [CrossRef]
- Lambert, C.P.; Frank, L.L.; Evans, W.J. Macronutrient Considerations for the Sport of Bodybuilding. Sports Med. 2004, 34, 317–327. [Google Scholar] [CrossRef]
- Slater, G.; Phillips, S.M. Nutrition guidelines for strength sports: Sprinting, weightlifting, throwing events, and bodybuilding. J. Sports Sci. 2011, 29 (Suppl. 1), S67–S77. [Google Scholar] [CrossRef]
- Burke, L.M.; Cox, G.R.; Cummings, N.K.; Desbrow, B. Guidelines for Daily Carbohydrate Intake. Sports Med. 2001, 31, 267–299. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Pieper, D.; Rombey, T. Where to prospectively register a systematic review. Syst. Rev. 2022, 11, 8. [Google Scholar] [CrossRef]
- Smart, N.A.; Waldron, M.; Ismail, H.; Giallauria, F.; Vigorito, C.; Cornelissen, V.; Dieberg, G. Validation of a new tool for the assessment of study quality and reporting in exercise training studies. Int. J. Evid.-Based Health 2015, 13, 9–18. [Google Scholar] [CrossRef]
- Nunes, J.P.; Grgic, J.; Cunha, P.M.; Ribeiro, A.S.; Schoenfeld, B.J.; de Salles, B.F.; Cyrino, E.S. What influence does resistance exercise order have on muscular strength gains and muscle hypertrophy? A systematic review and meta-analysis. Eur. J. Sport Sci. 2020, 21, 149–157. [Google Scholar] [CrossRef]
- Lambert, C.P.; Flynn, M.G.; Boone, J.B.; Michaud, T.J.; Rodriguez-Zayas, J. Effects of Carbohydrate Feeding on Multiple-bout Resistance Exercise. J. Strength Cond. Res. 1991, 5, 192–197. [Google Scholar] [CrossRef]
- Fairchild, T.J.; Dillon, P.; Curtis, C.; Dempsey, A.R. Glucose Ingestion Does Not Improve Maximal Isokinetic Force. J. Strength Cond. Res. 2016, 30, 194–199. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Haff, G.G.; Schroeder, C.A.; Koch, A.J.; Kuphal, K.E.; Comeau, M.J.; Potteiger, J.A. The effects of supplemental carbohydrate ingestion on intermittent isokinetic leg exercise. J. Sports Med. Phys. Fit. 2001, 41, 216–222. [Google Scholar]
- Krings, B.M.; Rountree, J.A.; McAllister, M.J.; Cummings, P.M.; Peterson, T.J.; Fountain, B.J.; Smith, J.W. Effects of acute carbohydrate ingestion on anaerobic exercise performance. J. Int. Soc. Sports Nutr. 2016, 13, 40. [Google Scholar] [CrossRef]
- Laurenson, D.M.; Dubé, D.J. Effects of carbohydrate and protein supplementation during resistance exercise on respiratory exchange ratio, blood glucose, and performance. J. Clin. Transl. Endocrinol. 2015, 2, 1–5. [Google Scholar] [CrossRef]
- Smith, J.W.; Krings, B.M.; Shepherd, B.D.; Waldman, H.S.; Basham, S.A.; McAllister, M.J. Effects of carbohydrate and branched-chain amino acid beverage ingestion during acute upper body resistance exercise on performance and postexercise hormone response. Appl. Physiol. Nutr. Metab. 2018, 43, 504–509. [Google Scholar] [CrossRef] [PubMed]
- Baty, J.J.; Hwang, H.; Ding, Z.; Bernard, J.R.; Wang, B.; Kwon, B.; Ivy, J.L. The Effect of a Carbohydrate and Protein Supplement on Resistance Exercise Performance, Hormonal Response, and Muscle Damage. J. Strength Cond. Res. 2007, 21, 321–329. [Google Scholar] [CrossRef]
- Dalton, R.A.; Rankin, J.W.; Sebolt, D.; Gwazdauskas, F. Acute Carbohydrate Consumption Does Not Influence Resistance Exercise Performance during Energy Restriction. Int. J. Sport Nutr. 1999, 9, 319–332. [Google Scholar] [CrossRef] [PubMed]
- Fayh, A.P.T.; Umpierre, D.; Sapata, K.B.; Neto, F.M.D.; De Oliveira, A.R.; Ii, I. Efeitos da ingestão prévia de carboidrato de alto índice glicêmico sobre a resposta glicêmica e desempenho durante um treino de força. Rev. Bras. Med. Do Esporte 2007, 13, 416–420. [Google Scholar] [CrossRef][Green Version]
- Kulik, J.R.; Touchberry, C.D.; Kawamori, N.; ABlumert, P.; Crum, A.J.; Haff, G.G. Supplemental Carbohydrate Ingestion Does Not Improve Performance of High-Intensity Resistance Exercise. J. Strength Cond. Res. 2008, 22, 1101–1107. [Google Scholar] [CrossRef] [PubMed]
- Lynch, S. The differential effects of a complex protein drink versus isocaloric carbohydrate drink on performance indices following high-intensity resistance training: A two arm crossover design. J. Int. Soc. Sports Nutr. 2013, 10, 31. [Google Scholar] [CrossRef]
- Raposo, K. The Effects of Pre-Exercise Carbohydrate Supplementation on Resistance Training Performance during an Acute Resistance Training Session; University of South Florida: Tampa, FL, USA, 2011. [Google Scholar]
- Rountree, J.A.; Krings, B.M.; Peterson, T.J.; Thigpen, A.G.; McAllister, M.J.; Holmes, M.E.; Smith, J.W. Efficacy of Carbohydrate Ingestion on CrossFit Exercise Performance. Sports 2017, 5, 61. [Google Scholar] [CrossRef]
- Bin Naharudin, M.N.; Yusof, A.; Shaw, H.; Stockton, M.; Clayton, D.J.; James, L.J. Breakfast Omission Reduces Subsequent Resistance Exercise Performance. J. Strength Cond. Res. 2019, 33, 1766–1772. [Google Scholar] [CrossRef]
- Welikonich, M.J.; Nagle, E.F.; Goss, F.L.; Robertson, R.J.; Crawford, K. Effect Of Carbohydrate-Protein Supplementation On Resistance Exercise Performance, Perceived Exertion, And Salivary Cortisol. Med. Sci. Sports Exerc. 2011, 43, 586–587. [Google Scholar] [CrossRef]
- Naharudin, M.N.; Adams, J.; Richardson, H.; Thomson, T.; Oxinou, C.; Marshall, C.; Clayton, D.J.; Mears, S.A.; Yusof, A.; Hulston, C.J.; et al. Viscous placebo and carbohydrate breakfasts similarly decrease appetite and increase resistance exercise performance compared with a control breakfast in trained males. Br. J. Nutr. 2020, 124, 232–240. [Google Scholar] [CrossRef]
- Dos Santos, M.P.P.; Spineli, H.; Silva, H.V.R.S.; Learsi, S.K.; De Araujo, G.G. Ingestion of a drink containing carbohydrate increases the number of bench press repetitions. Rev. Nutr. 2019, 32, e190056. [Google Scholar] [CrossRef]
- Wilburn, D.T.; Machek, S.B.; Cardaci, T.D.; Hwang, P.S.; Willoughby, D.S. Acute Maltodextrin Supplementation During Resistance Exercise. J. Sports Sci. Med. 2020, 19, 282–288. [Google Scholar] [PubMed]
- Maroufi, K.; Razavi, R.; Gaeini, A.A.; Nourshahi, M. The effects of acute consumption of carbohydrate-protein supplement in varied ratios on CrossFit athletes’ performance in two CrossFit exercises: A randomized cross-over trial. J. Sports Med. Phys. Fit. 2021, 61, 1362–1368. [Google Scholar] [CrossRef] [PubMed]
- Conley, M.; Stone, M.; Marsit, J.L.; O’Bryant, H.S.; Nieman, D.C.; Johnson, J.L.; ButTerworth, D.; Keith, R. Effects of Carbohydrate Ingestion on Resistance exercise. J. Strength Cond. Res. 1995, 9, 192–206. [Google Scholar]
- Vincent, K.R.; Clarkson, P.M.; Freedson, P.S.; DeCheke, M. 1092 effect of a pre-exercise liquid, high carbohydrate feeding on resistance exercise performance. Med. Sci. Sports Exerc. 1993, 25, S194. [Google Scholar] [CrossRef]
- Lepeley, A. The Effects Of Protein Versus Carbohydrate Consumption on Resistance Exercise Performance And Ratings of Perceived Exertion In Women. Ph.D. Dissertation, TUI University, Cypress, CA, USA, 2012. [Google Scholar]
- Mitchell, J.B.; DiLauro, P.C.; Pizza, F.X.; Cavender, D.L. The effect of preexercise carbohydrate status on resistance exercise performance. Int. J. Sport Nutr. 1997, 7, 185–196. [Google Scholar] [CrossRef]
- Haff, G.G.; Stone, M.H.; Warren, B.J.; Keith, R.; Johnson, R.L.; Nieman, D.C.; Williams, F.; Kirksey, K.B. The Effect of Carbohydrate Supplementation on Multiple Sessions and Bouts of Resistance Exercise. J. Strength Cond. Res. 1999, 13, 111–117. [Google Scholar] [CrossRef]
- Oliver, J.M.; Almada, A.L.; Van Eck, L.E.; Shah, M.; Mitchell, J.B.; Jones, M.T.; Jagim, A.R.; Rowlands, D.S. Ingestion of high molecular weight carbohydrate enhances subsequent repeated maximal power: A randomized controlled trial. PLoS ONE 2016, 11, e0163009. [Google Scholar] [CrossRef]
- Symons, J.D.; Jacobs, I. High-intensity exercise performance is not impaired by low intramuscular glycogen. Med. Sci. Sports Exerc. 1989, 21, 550–557. [Google Scholar] [CrossRef]
- Haff, G.G.; Koch, A.J.; Potteiger, J.A.; Kuphal, K.E.; Magee, L.M.; Green, S.B.; Jakicic, J.J. Carbohydrate supplementation attenuates muscle glycogen loss during acute bouts of resistance exercise. Int. J. Sport Nutr. Exerc. Metab. 2000, 10, 326–339. [Google Scholar] [CrossRef]
- Escobar, K.A.; Morales, J.; Vandusseldorp, T.A. The Effect of a Moderately Low and High Carbohydrate Intake on Crossfit Performance. Int. J. Exerc. Sci. 2016, 9, 460–470. [Google Scholar] [PubMed]
- Dipla, K.; Makri, M.; Zafeiridis, A.; Soulas, D.; Tsalouhidou, S.; Mougios, V.; Kellis, S. An isoenergetic high-protein, moderate-fat diet does not compromise strength and fatigue during resistance exercise in women. Br. J. Nutr. 2008, 100, 283–286. [Google Scholar] [CrossRef] [PubMed]
- Hatfield, D.L.; Kraemer, W.J.; Volek, J.S.; Rubin, M.R.; Grebien, B.; Gomez, A.L.; French, D.N.; Scheett, T.P.; Ratamess, N.A.; Sharman, M.J.; et al. The Effects of Carbohydrate Loading on Repetitive Jump Squat Power Performance. J. Strength Cond. Res. 2006, 20, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Kreider, R.B.; Hill, D.; Horton, G.; Downes, M.; Smith, S.; Anders, B. Effects of Carbohydrate Supplementation during Intense training on Dietary Patterns, Psychological Status, and Performance. Int. J. Sport Nutr. 1995, 5, 125–135. [Google Scholar] [CrossRef]
- Meirelles, C.; Candido, T.; Gomes, P.S. Effects of short-term very low-carbohydrate or conventional diet on strength performance. J. Sports Med. Phys. Fit. 2010, 50, 189–195. [Google Scholar]
- Sawyer, J.C.; Wood, R.J.; Davidson, P.W.; Collins, S.M.; Matthews, T.D.; Gregory, S.M.; Paolone, V.J. Effects of a Short-Term Carbohydrate-Restricted Diet on Strength and Power Performance. J. Strength Cond. Res. 2013, 27, 2255–2262. [Google Scholar] [CrossRef]
- Moura, R.F.; De Moraes, W.M.A.M.; De Castro, B.M.; Nogueira, A.L.P.; Trindade, T.B.; Schoenfeld, B.J.; Prestes, J. Carbohydrate refeed does not modify GVT-performance following energy restriction in bodybuilders. Clin. Nutr. ESPEN 2021, 43, 308–316. [Google Scholar] [CrossRef]
- Mirdha, P.; Nalgirkar, V.; Patil, A.; Potaliya, P.; Gupta, V.K. Effect of Carbohydrate Loading on Resistance Exercise and Muscle Mass: A Prospective Study. Mymensingh Med. J. MMJ 2021, 30, 826–829. [Google Scholar]
- Greene, D.A.; Varley, B.J.; Hartwig, T.; Chapman, P.; Rigney, M. A Low-Carbohydrate Ketogenic Diet Reduces Body Mass Without Compromising Performance in Powerlifting and Olympic Weightlifting Athletes. J. Strength Cond. Res. 2018, 32, 3373–3382. [Google Scholar] [CrossRef]
- Gregory, R.M.; Hamdan, H.; Torisky, D.M.; Akers, J.D. A Low-Carbohydrate Ketogenic Diet Combined with 6-Weeks of Crossfit Training Improves Body Composition and Performance. Master’s Thesis, James Madison University, Harrisonburg, VA, USA, 2016. [Google Scholar]
- Meirelles, C.M.; Gomes, P.S.C. Effects of Short-Term Carbohydrate Restrictive and Conventional Hypoenergetic Diets and Resistance Training on Strength Gains and Muscle Thickness. J. Sports Sci. Med. 2016, 15, 578–584. [Google Scholar]
- Van Zant, R.S.; Conway, J.M.; Seale, J.L. A moderate carbohydrate and fat diet does not impair strength performance in moderately trained males. J. Sports Med. Phys. Fit. 2002, 42, 31–37. [Google Scholar]
- Wilson, J.M.; Lowery, R.P.; Roberts, M.D.; Sharp, M.H.; Joy, J.M.; Shields, K.A.; Partl, J.; Volek, J.S.; D’Agostino, D. The Effects of Ketogenic Dieting on Body Composition, Strength, Power, and Hormonal Profiles in Resistance Training Males. J. Strength Cond. Res. 2021, 34, 3463–3474. [Google Scholar] [CrossRef] [PubMed]
- Kreider, R.B.; Rasmussen, C.; Kerksick, C.M.; Wilborn, C.; Taylor, L.; Campbell, B.; Magrans-Courtney, T.; Fogt, D.; Ferreira, M.; Li, R.; et al. A Carbohydrate-Restricted Diet during Resistance Training Promotes More Favorable Changes in Body Composition and Markers of Health in Obese Women with and without Insulin Resistance. Physician Sportsmed. 2011, 39, 27–40. [Google Scholar] [CrossRef]
- Rhyu, H.-S.; Cho, S.-Y. The effect of weight loss by ketogenic diet on the body composition, performance-related physical fitness factors and cytokines of Taekwondo athletes. J. Exerc. Rehabil. 2014, 10, 326–331. [Google Scholar] [CrossRef] [PubMed]
- Agee, J.L. Effects of a Low-Carbohydrate Ketogenic Diet on Power Lifting Performance and Body Composition. Master’s Thesis, James Madison University, Harrisonburg, VA, USA, 2015. [Google Scholar]
- Kephart, W.C.; Pledge, C.D.; Roberson, P.A.; Mumford, P.W.; Romero, M.A.; Mobley, C.B.; Martin, J.S.; Young, K.C.; Lowery, R.P.; Wilson, J.M.; et al. The three month effects of a ketogenic diet on body composition, blood parameters, and performance metrics in crossfit trainees: A pilot study. Sports 2018, 6, 1. [Google Scholar] [CrossRef]
- Paoli, A.; Grimaldi, K.; D’Agostino, D.; Cenci, L.; Moro, T.; Bianco, A.; Palma, A. Ketogenic diet does not affect strength performance in elite artistic gymnasts. J. Int. Soc. Sports Nutr. 2012, 9, 34. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, P.V.; Baptista, L.; Moreira, F.; Junior, A.H.L. Correlação entre a suplementação de proteína e carboidrato e variáveis antropométricas e de força em indivíduos submetidos a um programa de treinamento com pesos. Rev. Bras. Med. Do Esporte 2006, 12, 51–55. [Google Scholar] [CrossRef]
- LaFountain, R.A.; Miller, V.J.; Barnhart, E.C.; Hyde, P.N.; Crabtree, C.D.; McSwiney, F.; Beeler, M.K.; Buga, A.; Sapper, T.N.; A Short, J.; et al. Extended Ketogenic Diet and Physical Training Intervention in Military Personnel. Mil. Med. 2019, 184, e538–e547. [Google Scholar] [CrossRef]
- Vargas-Molina, S.; Petro, J.L.; Romance, R.; Kreider, R.B.; Schoenfeld, B.J.; Bonilla, D.A.; Benítez-Porres, J. Effects of a ketogenic diet on body composition and strength in trained women. J. Int. Soc. Sports Nutr. 2020, 17, 19. [Google Scholar] [CrossRef]
- Rozenek, R.; Ward, P.; Long, S.; Garhammer, J. Effects of high-calorie supplements on body composition and muscular strength following resistance training. J. Sports Med. Phys. Fit. 2002, 42, 340–347. [Google Scholar]
- Durkalec-Michalski, K.; Nowaczyk, P.M.; Główka, N.; Ziobrowska, A.; Podgórski, T. Is a Four-Week Ketogenic Diet an Effective Nutritional Strategy in CrossFit-Trained Female and Male Athletes? Nutrients 2021, 13, 864. [Google Scholar] [CrossRef]
- Paoli, A.; Cenci, L.; Pompei, P.; Sahin, N.; Bianco, A.; Neri, M.; Caprio, M.; Moro, T. Effects of Two Months of Very Low Carbohydrate Ketogenic Diet on Body Composition, Muscle Strength, Muscle Area, and Blood Parameters in Competitive Natural Body Builders. Nutrients 2021, 13, 374. [Google Scholar] [CrossRef] [PubMed]
- Vidić, V.; Ilić, V.; Toskić, L.; Janković, N.; Ugarković, D. Effects of calorie restricted low carbohydrate high fat ketogenic vs. non-ketogenic diet on strength, body-composition, hormonal and lipid profile in trained middle-aged men. Clin. Nutr. 2021, 40, 1495–1502. [Google Scholar] [CrossRef] [PubMed]
- Børsheim, E.; Cree, M.G.; Tipton, K.D.; Elliott, T.A.; Aarsland, A.; Wolfe, R.R. Effect of carbohydrate intake on net muscle protein synthesis during recovery from resistance exercise. J. Appl. Physiol. 2004, 96, 674–678. [Google Scholar] [CrossRef] [PubMed]
- MacDougall, J.D.; Ray, S.; Sale, D.G.; McCartney, N.; Lee, P.; Garner, S. Muscle Substrate Utilization and Lactate Production During Weightlifting. Can. J. Appl. Physiol. 1999, 24, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Murray, B.; Rosenbloom, C. Fundamentals of glycogen metabolism for coaches and athletes. Nutr. Rev. 2018, 76, 243–259. [Google Scholar] [CrossRef]
- Brewer, C.B.; Booher, B.M.; Lawton, N. Comparison of Acute Energy Expenditure and Rating of Perceived Exertion in Equivalent Bouts of Circuit Training and Treadmill Running. J. Strength Cond. Res. 2021, 35, 680–687. [Google Scholar] [CrossRef]
- Herzog, W. Why are muscles strong, and why do they require little energy in eccentric action? J. Sport Health Sci. 2018, 7, 255–264. [Google Scholar] [CrossRef]
- Grgic, J.; Schoenfeld, B.J.; Skrepnik, M.; Davies, T.B.; Mikulic, P. Effects of Rest Interval Duration in Resistance Training on Measures of Muscular Strength: A Systematic Review. Sports Med. 2017, 48, 137–151. [Google Scholar] [CrossRef]
- Vianna, J.M.; Lima, J.P.; Saavedra, F.J.; Reis, V.M. Aerobic and Anaerobic Energy During Resistance Exercise at 80% 1RM. J. Hum. Kinet. 2011, 29A, 69–74. [Google Scholar] [CrossRef]
- Brunelli, D.T.; Finardi, E.A.R.; Bonfante, I.L.P.; Gáspari, A.F.; Sardeli, A.V.; Souza, T.M.F.; Chacon-Mikahil, M.P.T.; Cavaglieri, C. Acute low- compared to high-load resistance training to failure results in greater energy expenditure during exercise in healthy young men. PLoS ONE 2019, 14, e0224801. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.S.; McCormick, M.C.; Robergs, R.A. Interaction among Skeletal Muscle Metabolic Energy Systems during Intense Exercise. J. Nutr. Metab. 2010, 2010, 905612. [Google Scholar] [CrossRef]
- Beneke, R.; Pollmann, C.H.; Bleif, I.; Leithäuser, R.; Hütler, M. How anaerobic is the Wingate Anaerobic Test for humans? Eur. J. Appl. Physiol. 2002, 87, 388–392. [Google Scholar] [CrossRef] [PubMed]
- Sherman, W.M.; Costill, D.L.; Fink, W.J.; Miller, J.M. Effect of Exercise-Diet Manipulation on Muscle Glycogen and Its Subsequent Utilization During Performance. Int. J. Sports Med. 1981, 2, 114–118. [Google Scholar] [CrossRef] [PubMed]
- Churchley, E.G.; Coffey, V.G.; Pedersen, D.J.; Shield, A.; Carey, K.A.; Cameron-Smith, D.; Hawley, J.A. Influence of preexercise muscle glycogen content on transcriptional activity of metabolic and myogenic genes in well-trained humans. J. Appl. Physiol. 2007, 102, 1604–1611. [Google Scholar] [CrossRef]
- Tesch, P.A. Glycogen and triglyceride utilization in relation to muscle metabolic characteristics in men performing heavy-resistance exercise. Eur. J. Appl. Physiol. Occup. Physiol. 1990, 61, 5–10. [Google Scholar] [CrossRef]
- Koopman, R.; Manders, R.J.F.; Jonkers, R.A.M.; Hul, G.B.J.; Kuipers, H.; van Loon, L.J.C. Intramyocellular lipid and glycogen content are reduced following resistance exercise in untrained healthy males. Eur. J. Appl. Physiol. 2005, 96, 525–534. [Google Scholar] [CrossRef]
- Pascoe, D.D.; Costill, D.L.; Fink, W.J.; ARobergs, R.; Zachwieja, J.J. Glycogen resynthesis in skeletal muscle following resistive exercise. Med. Sci. Sports Exerc. 1993, 25, 349–354. [Google Scholar] [CrossRef]
- Roy, B.D.; Tarnopolsky, M.A. Influence of differing macronutrient intakes on muscle glycogen resynthesis after resistance exercise. J. Appl. Physiol. 1998, 84, 890–896. [Google Scholar] [CrossRef]
- Tesch, P.A.; Colliander, E.B.; Kaiser, P. Muscle metabolism during intense, heavy-resistance exercise. Eur. J. Appl. Physiol. Occup. Physiol. 1986, 55, 362–366. [Google Scholar] [CrossRef]
- Taylor, R.; Magnusson, I.; Rothman, D.L.; Cline, G.W.; Caumo, A.; Cobelli, C.; Shulman, G.I. Direct assessment of liver glycogen storage by 13C nuclear magnetic resonance spectroscopy and regulation of glucose homeostasis after a mixed meal in normal subjects. J. Clin. Investig. 1996, 97, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Ejensen, J.; Rustad, P.I.; Kolnes, A.J.; Lai, Y.-C. The Role of Skeletal Muscle Glycogen Breakdown for Regulation of Insulin Sensitivity by Exercise. Front. Physiol. 2011, 2, 112. [Google Scholar] [CrossRef]
- Mears, S.A.; Dickinson, K.; Bergin-Taylor, K.; Dee, R.; Kay, J.; James, L.J. Perception of Breakfast Ingestion Enhances High-Intensity Cycling Performance. Int. J. Sports Physiol. Perform. 2018, 13, 504–509. [Google Scholar] [CrossRef] [PubMed]
- Waterworth, S.P.; Spencer, C.C.; Porter, A.L.; Morton, J.P. Perception of Carbohydrate Availability Augments High-Intensity Intermittent Exercise Capacity Under Sleep-Low, Train-Low Conditions. Int. J. Sport Nutr. Exerc. Metab. 2020, 30, 105–111. [Google Scholar] [CrossRef]
- Naharudin, M.N.; Yusof, A.; Clayton, D.J.; James, L.J. Starving Your Performance? Reduced Preexercise Hunger Increases Resistance Exercise Performance. Int. J. Sports Physiol. Perform. 2021, 1, 1–7. [Google Scholar] [CrossRef]
- Decimoni, L.S.; Curty, V.M.; Almeida, L.; Koch, A.J.; Willardson, J.M.; Machado, M. Carbohydrate mouth rinsing improves resistance training session performance. Int. J. Sports Sci. Coach. 2018, 13, 804–809. [Google Scholar] [CrossRef]
- Green, M.S.; Kimmel, C.S.; Martin, T.D.; Mouser, J.G.; Brune, M.P. Effect of Carbohydrate Mouth Rinse on Resistance Exercise Performance. J. Strength Cond. Res. 2020; publish ahead of print. [Google Scholar] [CrossRef]
- Costill, D.L.; Pearson, D.R.; Fink, W.J. Impaired muscle glycogen storage after muscle biopsy. J. Appl. Physiol. 1988, 64, 2245–2248. [Google Scholar] [CrossRef]
- Gollnick, P.D.; Piehl, K.; Saltin, B. Selective glycogen depletion pattern in human muscle fibres after exercise of varying intensity and at varying pedalling rates. J. Physiol. 1974, 241, 45–57. [Google Scholar] [CrossRef]
- Ivy, J.L. Muscle Glycogen Synthesis Before and After Exercise. Sports Med. 1991, 11, 6–19. [Google Scholar] [CrossRef]
- Pascoe, D.D.; Gladden, L.B. Muscle Glycogen Resynthesis after Short Term, High Intensity Exercise and Resistance Exercise. Sports Med. 1996, 21, 98–118. [Google Scholar] [CrossRef]
- Bortz, W.M.; Paul, P.; Haff, A.C.; Holmes, W.L. Glycerol turnover and oxidation in man. J. Clin. Investig. 1972, 51, 1537–1546. [Google Scholar] [CrossRef] [PubMed]
- Ruderman, N.B. Muscle Amino Acid Metabolism and Gluconeogenesis. Annu. Rev. Med. 1975, 26, 245–258. [Google Scholar] [CrossRef]
- Phielix, E.; Begovatz, P.; Gancheva, S.; Bierwagen, A.; Kornips, E.; Schaart, G.; Hesselink, M.K.C.; Schrauwen, P.; Roden, M. Athletes feature greater rates of muscle glucose transport and glycogen synthesis during lipid infusion. JCI Insight 2019, 4, e127928. [Google Scholar] [CrossRef]
- Thomas, K.; Brownstein, C.G.; Dent, J.; Parker, P.; Goodall, S.; Howatson, G. Neuromuscular Fatigue and Recovery after Heavy Resistance, Jump, and Sprint Training. Med. Sci. Sports Exerc. 2018, 50, 2526–2535. [Google Scholar] [CrossRef] [PubMed]
- Creer, A.; Gallagher, P.; Slivka, D.; Jemiolo, B.; Fink, W.; Trappe, S. Influence of muscle glycogen availability on ERK1/2 and Akt signaling after resistance exercise in human skeletal muscle. J. Appl. Physiol. 2005, 99, 950–956. [Google Scholar] [CrossRef] [PubMed]
- Knuiman, P.; Hopman, M.T.E.; Wouters, J.A.; Mensink, M. Select Skeletal Muscle mRNAs Related to Exercise Adaptation Are Minimally Affected by Different Pre-exercise Meals that Differ in Macronutrient Profile. Front. Physiol. 2018, 9, 28. [Google Scholar] [CrossRef]
- Camera, D.M.; West, D.W.D.; Burd, N.A.; Phillips, S.M.; Garnham, A.P.; Hawley, J.A.; Coffey, V.G. Low muscle glycogen concentration does not suppress the anabolic response to resistance exercise. J. Appl. Physiol. 2012, 113, 206–214. [Google Scholar] [CrossRef]
- Murphy, C.; Koehler, K. Energy deficiency impairs resistance training gains in lean mass but not strength: A meta-analysis and meta-regression. Scand. J. Med. Sci. Sports 2021, 32, 125–137. [Google Scholar] [CrossRef]
- Volek, J.S.; Freidenreich, D.J.; Saenz, C.; Kunces, L.J.; Creighton, B.C.; Bartley, J.M.; Davitt, P.M.; Munoz, C.X.; Anderson, J.M.; Maresh, C.M.; et al. Metabolic characteristics of keto-adapted ultra-endurance runners. Metabolism 2016, 65, 100–110. [Google Scholar] [CrossRef]
- Phinney, S.D.; Bistrian, B.; Evans, W.; Gervino, E.; Blackburn, G.L. The human metabolic response to chronic ketosis without caloric restriction: Preservation of submaximal exercise capability with reduced carbohydrate oxidation. Metabolism 1983, 32, 769–776. [Google Scholar] [CrossRef]
- Helge, J.W.; Watt, P.W.; Richter, E.A.; Rennie, M.J.; Kiens, B. Fat utilization during exercise: Adaptation to a fat-rich diet increases utilization of plasma fatty acids and very low density lipoprotein-triacylglycerol in humans. J. Physiol. 2001, 537, 1009–1020. [Google Scholar] [CrossRef] [PubMed]
- Zderic, T.W.; Davidson, C.J.; Schenk, S.; Byerley, L.O.; Coyle, E.F. High-fat diet elevates resting intramuscular triglyceride concentration and whole body lipolysis during exercise. Am. J. Physiol. Metab. 2004, 286, E217–E225. [Google Scholar] [CrossRef] [PubMed]
- Phinney, S.D.; Horton, E.S.; Sims, E.A.H.; Hanson, J.S.; Danforth, E.; Lagrange, B.M. Capacity for Moderate Exercise in Obese Subjects after Adaptation to a Hypocaloric, Ketogenic Diet. J. Clin. Investig. 1980, 66, 1152–1161. [Google Scholar] [CrossRef]
- Taber, C.B.; Vigotsky, A.; Nuckols, G.; Haun, C.T. Exercise-Induced Myofibrillar Hypertrophy is a Contributory Cause of Gains in Muscle Strength. Sports Med. 2019, 49, 993–997. [Google Scholar] [CrossRef] [PubMed]
- Folland, J.P.; Williams, A.G. The Adaptations to Strength Training. Sports Med. 2007, 37, 145–168. [Google Scholar] [CrossRef] [PubMed]
- Toomey, C.M.; McCormack, W.G.; Jakeman, P. The effect of hydration status on the measurement of lean tissue mass by dual-energy X-ray absorptiometry. Eur. J. Appl. Physiol. 2017, 117, 567–574. [Google Scholar] [CrossRef]
- Deemer, S.E.; Plaisance, E.P.; Martins, C. Impact of ketosis on appetite regulation—A review. Nutr. Res. 2020, 77, 1–11. [Google Scholar] [CrossRef]
- Tzur, A.; Roberts, B.M. The Ketogenic Diet for Bodybuilders and Physique Athletes. Strength Cond. J. 2020, 42, 108–115. [Google Scholar] [CrossRef]
- Valenzuela, P.L.; Castillo-García, A.; Lucia, A.; Naclerio, F. Effects of Combining a Ketogenic Diet with Resistance Training on Body Composition, Strength, and Mechanical Power in Trained Individuals: A Narrative Review. Nutrients 2021, 13, 3083. [Google Scholar] [CrossRef]




| Criterion | n | % | |
|---|---|---|---|
| Study quality | 1. Eligibility criteria specified | 21 | 66 |
| 2. Randomization specified | 5 | 16 | |
| 3. Allocation concealment | 30 | 94 | |
| 4. Groups similar at baseline | 32 | 100 | |
| 5. Blinding of assessor (for at least one key outcome) | 16 | 50 | |
| Study reporting | 6a. Outcome measures assesses in 85% of participants | 30 | 94 |
| 6b. Adverse events reported | 1 | 3 | |
| 8a. Between-group statistics reported—primary | 32 | 100 | |
| 8b. Between-group statistics reported—secondary | 32 | 100 | |
| 9. Points measures and measures of variability reported | 31 | 97 | |
| 12. Exercise volume and energy expenditure | 29 | 91 |
| Criterion | n | % | |
|---|---|---|---|
| Study quality | 1. Eligibility criteria specified | 16 | 94 |
| 2. Randomization specified | 0 | 0 | |
| 3. Allocation concealment | 12 | 71 | |
| 4. Groups similar at baseline | 15 | 88 | |
| 5. Blinding of assessor (for at least one key outcome) | 1 | 6 | |
| Study reporting | 6a. Outcome measures assesses in 85% of participants | 14 | 82 |
| 6b. Adverse events reported | 7 | 41 | |
| 6c. Exercise attendance reported | 13 | 76 | |
| 7. Intention-to-treat analysis | 7 | 41 | |
| 8a. Between-group statistics reported—primary | 17 | 100 | |
| 8b. Between-group statistics reported—secondary | 17 | 100 | |
| 9. Points measures and measures of variability reported | 17 | 100 | |
| 11. Relative exercise intensity remained constant | 16 | 94 | |
| 12. Exercise volume and energy expenditure | 5 | 29 |
| Study | Design and Population | Training Protocol and Performance Outcomes | Carbohydrate (CHO) Intakes | Fasted or Fed | Results |
|---|---|---|---|---|---|
| Baty et al. [32] | RCT: Healthy untrained men (n = 32), carbohydrate-protein group vs. placebo group. | Training: 7 exercises (high pull, lat pull-down, standing overhead press, knee extension, leg curl, leg press and bench press) with the two first sets as 8 RM, and the third set with the same load as set 2 but until voluntary failure. Outcome: total weight lifted (kg) during the last set per exercise and weight lifted scaled per lean body mass multiplied by the number of repetitions completed during the last set per exercise. | CHO: 0.59 g/kg (44 g [6.2%] and 1.5% protein). Placebo: non-caloric. Timing: 355 mL 30 min prior to exercise, 177 mL immediately before and after the fourth exercise. | Fasted (12 h overnight). | No significant differences between conditions in weight lifted the last set or total training volume (total load CHO-PRO: 534 ± 80 kg vs. placebo: 556 ± 82 kg; weight scaled per lean body mass × repetitions CHO-PRO: 93 ± 17 vs. placebo: 92 ± 21). |
| Dalton et al. [33] | RCT: Strength-trained subjects (n = 22), carbohydrates (n = 8) vs. placebo (n = 8) vs. control (n = 6) in caloric deficit | Training: lower-body exercises (squat, leg press and knee extension) and bench press at 60–80% of 10 RM, 5-sets per exercise. Outcome: last set of knee extension and bench press 80% of 1 RM to failure. | CHO: 1 g/kg beverage supplement. Placebo: non-caloric supplement. Control: no supplement. Timing: 30 min before testing. | Overnight fasted. | No significant differences in repetitions to failure between conditions (knee extension CHO: 17 ± 1, placebo: 17 ± 2, control: 17 ± 2; bench press CHO: 17 ± 2, placebo: 17 ± 2, control: 16 ± 3). |
| Fairchild et al. [27] | Counterbalanced crossover: Strength-trained men (n = 11) and women (n = 6), carbohydrate vs. placebo. | Training: one set of 3 RM knee extensions in an isokinetic dynamometer, and again after 5, 15, 30, 45, 60, 75 and 90 min. Outcome: peak and average isokinetic torque. | CHO: 1.1 g/kg (75 g). Placebo: non-caloric supplement. Timing: after the first baseline 3 RM. | Fasted (>12 h overnight). | There was no interaction effect but when adjusting for baseline values a significant main effect between conditions were observed where the CHO condition resulted in a decline (~2%-points) and maintenance in average and peak torque, respectively, compared to an increase (~4–5%-points) in both for placebo. |
| Fayh et al. [34] | Crossover: Strength-trained subjects (n = 8), carbohydrate vs. placebo. | Training: seven exercises (bench press, lat pulldown, rear deltoid, barbell curl, hammer curl, leg press and squat) with three sets with an intensity of 70% 1 RM to failure. Outcome: total training volume (repetitions × sets × load). | CHO: 1 g/kg (84 g) of maltodextrin beverage supplement. Placebo: non-caloric supplement. Timing: 15 min before training. | Fed (2 h pre). | No significant differences in total training volume between conditions (CHO: 12,944 ± 2548 kg vs. placebo: 12,876 ± 2025 kg). |
| Haff et al. [28] | Crossover: Strength-trained men (n = 8), carbohydrate vs. placebo. | Training: 16 sets of 10 repetitions with isokinetic knee extension and flexion Outcome: total and average work (J) across all sets, peak and average isokinetic torque (Nm) across all sets. | CHO: 1.0 g/kg prior to exercise and 0.51 g/kg during exercise (143 g in total). Placebo: non-caloric. Timing: before exercise and after set 1, 6 and 11. | Fed (3 h pre). | Significant greater total work (CHO: 24 ± 2 J, placebo: 22 ± 2 J), average work (CHO: 1.5 ± 0.1 J, placebo: 1.4 ± 0.5 J), and average torque per set (CHO: 105 ± 8 Nm, placebo: 98 ± 8 Nm) in knee extension in the CHO condition. No differences were observed between conditions in peak torque in the knee extension or any of the measurements for the knee flexors. |
| Krings et al. [29] | Crossover: Strength-trained men (n = 7), carbohydrates, amino acids and electrolytes vs. amino acids and electrolytes (placebo). | Training: explosive high-intensity training and resistance training: hang clean at 50–70% 1 RM, front squat at 45–90% 1 RM, box jumps, dumbbell bench press and barbell bent-over row at 60–73% 1 RM, barbell reverse lunge at 55–70% 1 RM, single-arm shoulder press at 65–70% 1 RM, dumbbell biceps curl and dumbbell overhead triceps extension at 60% 1 RM. Three to seven sets for all exercises. Outcome: last set to failure in dumbbell bench press, barbell bent-over row, dumbbell biceps curl and dumbbell overhead triceps extension. Sprints, jump distance, overhead medicine ball throws and agility tests. | CHOs: 15, 30 or 60 g/h corresponding to a 3, 6 and 12% solution. In addition to 5.5 g amino acids (AA) and electrolytes. Placebo: 5.5 g AA and electrolytes. Timing: before exercise and every 15 min during exercise, total 5 dosages. | Fasted (at least 10 h overnight). | No significant differences in total repetitions between CHOs and placebo, but 15 g/h > 60 g/h. For the bench press, all CHO groups outperformed placebo without dose-response. No significant differences for the other three exercises, two jumps or four run times, except 60 g/h > placebo for the 27-m sprint. |
| Kulik et al. [35] | Counterbalanced crossover: Strength-trained men (n = 8), carbohydrate vs. placebo. | Training: sets of five repetitions at 85% 1 RM until subjects could no longer squat to parallel, failed to do a repetition every 8 s, or reached voluntary failure, with 3-min rest between sets. Outcome: repetitions and sets to failure, in addition to volume load (load × sets × repetitions) and total work (kJ). | CHO: 0.3 g/kg (28 g). Placebo: non-caloric. Timing: before and after every other set of 5 repetitions. | Fed (3 h pre). | No significant differences between conditions in repetitions and sets to failure or volume load and total load (repetitions CHO: 20 ± 15 vs. placebo: 20 ± 13, sets CHO: 4 ± 3 vs. placebo: 4 ± 3, volume load CHO: 2929 ± 2220 kg vs. placebo: 2773 ± 1951 kg, work CHO: 30 ± 22 kJ vs. placebo: 29 ± 20 kJ). |
| Lambert et al. [26] | Crossover: Strength-trained men (n = 7), carbohydrate vs. placebo. | Training: knee extensions at 80% of 10 RM, first set was performed with 10 repetitions, then subsequent sets were performed until one failed to perform 7 repetitions in a single set. Outcome: repetitions and sets to failure. | CHO: 1 g/kg before exercise, and 0.17 g/kg dosages during exercise (97 or 125 g in total). Placebo: non-caloric. Timing: before exercise, an after set 5, 10 and 15. | Relatively fed (4-h pre). | No significant difference in repetitions and sets to failure between the conditions. However, there was a tendency for more repetitions (149 ± 16 vs. 129 ± 12, p = 0.067) and sets (17 ± 2 vs. 14 ± 2, p = 0.056) in the CHO condition. |
| Laurenson and Dubé [30] | Crossover: Strength-trained men (n = 10), carbohydrates vs. placebo. | Training: Seven sets of squat and bench press (60% 1 RM), first 6 with a predetermined number of repetitions. Outcome: last set was performed to repetition failure where the total volume (kg load × repetitions) and peak power output was measured. | CHO: 0.43 g/kg (36 g and 12 g of protein). Placebo: non-caloric. Timing: two dosages, 12 and 26 min into exercise. | Fasted (8–10 h). | Significantly more total bench press volume in the CHO condition (921 ± 365 vs. 783 ± 332). However, no differences was observed in total squat volume (CHO: 1009 ± 433 vs. 909 ± 472, p = 0.1) or peak power for either bench press or squat. |
| Lynch [36] | Crossover: Strength-trained men (n = 15), carbohydrates vs. high-protein (including carbohydrates, protein and fat). | Training: high-intensity resistance training for 2 min (overhead push-press, dumbbell push-press, squats and dumbbell push-ups) for as many rounds as possible. Outcome: performance tests 2 h after the workout; agility T-test, push-up (repetitions to failure), and 40-yard sprint. | CHO: A total of 0.84 g/kg (68 g). High-protein: 40 g protein, 11 g of carbohydrate and 6 g fat (isocaloric to CHO). Timing: within 5 min of completing the first workout. | Not specified. | No significant difference between conditions in agility T-test, push-ups to failure or sprint. However, analyzing all three performance variables simultaneously yielded a significant greater effect of the high-protein condition compared to the carbohydrate condition. |
| Maroufi et al. [44] | Crossover: Male CrossFit athletes (n = 8), carbohydrate-protein supplement in two ratios (2:2 or 3:1) vs. placebo. | Training: two 15–17 min CrossFit workouts. Outcome: repetitions to failure. | CHO-protein (ratio 3:1): 67.5 g CHO and 22.5 g protein. CHO-protein (ratio 2:2): 45 g CHO and 45 g protein. Placebo: non-caloric. Timing: 1 h and immediately before testing. | Fasted (overnight) | No significant difference between conditions in repetitions to failure (3:1 ratio 341 ± 56, 2:2 ratio 366 ± 61, placebo 346 ± 65). |
| Naharudin et al. [39] | Counterbalanced crossover: Strength-trained men (n = 16), breakfast vs. a water-only breakfast. | Training: Four sets to failure with squat and bench press at 90% of 10 RM. Outcome: repetitions to failure. | CHO: A total of 1.5 g/kg (116 g), standardized breakfast meal, ~20% of estimated energy needs. Control: water only. Timing: 2 h before testing. | Fasted (~10 h overnight). | Significantly more repetitions to failure in the CHO condition for squat (68 ± 14 vs. placebo: 58 ± 11, effect size [ES] = 0.98) and bench press (40 ± 5 vs. placebo: 38 ± 5, ES = 1.06). |
| Naharudin et al. [41] | Counterbalanced crossover: Strength-trained men (n = 22), breakfast vs. placebo-breakfast vs. water-only. | Training: Four sets to failure with squat and bench press at 90% of 10 RM. Outcome: repetitions to failure. | CHO: A total of 1.5 g/kg (117 g), standardized breakfast meal, 496 kcal. Placebo: semi-solid, 29 kcal with low-energy flavored squash and water. Control: water only. Timing: ~2 h before testing. | Fasted (10–13 h overnight). | Significantly more repetitions to failure in the CHO and placebo breakfast conditions in the squat exercise (CHO: 44 ± 10, placebo: 43 ± 10, water-only: 38 ± 10), but not during bench press (CHO: 39 ± 7, placebo: 38 ± 7, water-only: 37 ± 7). While there was no significant difference in repetitions completed in the CHO- vs. the placebo condition. |
| Raposo et al. [37] | Counterbalanced crossover: Strength-trained women (n = 13), carbohydrates vs. placebo. | Training: Five sets with 75% of 1 RM of bench press and 85% of 1 RM for leg press. Outcome: repetitions to failure and total volume (sets × repetitions × load) for each exercise and all exercises together. | CHO: A total of 1 g/kg (81 g). Placebo: non-caloric. Timing: A total of 1 h before exercise. | Fasted (overnight). | No significant differences between conditions in repetitions to failure and training volume (repetitions bench press, CHO: 45 ± 11 vs. 45 ± 10; leg press, CHO: 112 ± 59 vs. 98 ± 38. Training volume bench press, CHO: 1451 ± 414 vs. 1430 ± 387; leg press, CHO: 19,960 ± 13,477 vs. 17,103 ± 8927). |
| Rountree et al. [38] | Crossover: Strength-trained men (n = 8), carbohydrates vs. placebo. | Training: Five rounds of wall throws with a 9 kg medicine ball, box jumps, sumo deadlift high pulls with 34 kg, push presses with 34 kg for as many repetitions as possible within 1 min, and rowing ergometer at maximum effort for 1 min. Outcome: repetitions to failure and caloric expenditure during rowing. | CHO: A total of 0.2 g/kg (16 g). Placebo: non-caloric. Timing: before exercise and during the training session (6 total dosages of 2.7 g each). | Fasted (10–12 h overnight). | No significant differences between conditions in repetitions to failure (total repetitions CHO: 279 vs. placebo: 272) and caloric expenditure during 1 min all out rowing (kilocalories CHO: 42 vs. placebo: 45). |
| Santos et al. [42] | Crossover: Strength-trained men (n = 8), carbohydrates vs. placebo. | Training: one set of bench-press, 70% of 1 RM to failure. Outcome: repetitions to failure. | CHO: A total of 0.27 g/kg (20 g). Placebo: non-caloric. Timing: 1 h before training. | Not specified. | Significantly more repetitions in the CHO condition (13 ± 2 vs. 11 ± 2). |
| Smith et al. [31] | Crossover: Strength-trained men (n = 13), carbohydrates vs. carbohydrates + BCAA vs. BCAA vs. placebo. | Training: barbell bench press, landmine bent-over row, barbell incline press, and landmine close-grip row. All exercises were performed with 5 sets to failure at 65% of 1 RM. Outcome: repetitions to failure. | CHO: A total of 0.44 g/kg (36 g). CHO + BCAA: A total of 36 g and 7.5 g BCAA. BCAA: A total of 7.5 g Placebo: non-caloric. Timing: the total dosage was distributed to be ingested before and after warm-up, and after the last set of each exercise. | Fasted (10 h overnight). | No significant time × treatment interactions for any exercise for repetition performance. However, there was a treatment effect for CHO + BCAA compared to the other treatments, but it was confounded by an order effect. Additionally, close-grip row repetitions to failure were greater in the CHO-BCAA condition compared to the other conditions. |
| Welikonich [40] | RCT: Recreational strength-trained men (n = 27), carbohydrates vs. CHO-protein vs. placebo. | Training: multiple sets with leg press of 8–10 repetitions at 70% of 1 RM until fatigue (unable to reach 8 repetitions) Outcome: total number of repetitions in addition to sets to failure, measured as total training volume (load × repetitions × sets). | CHO: A total of 0.81 g/kg (~60 g), 0 g PRO CHO-PRO: A total of 0.65 g/kg (~50 g) CHO, ~14 g PRO Placebo: non-caloric (15 kcal) Timing: A total of 15 min before training (~30 g) and between every other set (in total ~30 g). | Fed (standardized liquid meal 5 h pre). | Significantly more repetitions in the CHO and CHO-PRO condition (CHO: 136 ± 55/36, respectively) vs. placebo (90 ± 15). However, no difference was observed between groups in total volume of work (CHO: 28,052 ± 19,198 kg vs. CHO-PRO: 24,836 ± 9737 vs. placebo: 15,934 ± 3276 kg (p = 0.13). |
| Wilburn et al. [43] | Crossover: Recreational strength-trained men (n = 10), carbohydrates vs. placebo. | Training: Four sets of leg press at 70% of 1 RM to failure. Outcome: repetitions to failure. | CHO: A total of 2 g/kg (180 g). Placebo: non-caloric. Timing: 30 min before training. | Fed (3 h pre, instructed not to change dietary habits). | No significant differences between conditions (total repetitions CHO: 52 ± 7, placebo: 54 ± 8, p = 0.80). |
| Study | Design and Population | Training Protocol and Performance Outcomes | Carbohydrate- (CHO) Intakes | Fasted or Fed | Results |
|---|---|---|---|---|---|
| Haff et al. [49] | Counterbalanced crossover: Strength-trained men (n = 6), carbohydrate vs. placebo. | Glycogen depleting workout: Five sets of 10 repetitions in squats (65% 1 RM), speed squats (45% 1 RM) and 1-legged squat (10% 1 RM). Training: A total of 4 h after the first workout, session two was performed; as many sets of 10 squats with 55% of 1 RM as possible (to failure) with a 3-min rest interval. Outcome: completion of as many sets with 10 repetitions as possible. | Both conditions received a standardized high-carbohydrate (~1.2 g/kg) lunch 2.5 h prior to strength tests (~825 kcal). CHO: 1.2 g/kg/h during the morning session, 0.38 g/kg/h during the 4 h recovery period between workouts, while a non-specified dosage was provided every second set (total carbohydrate intake not specified). Placebo: non-caloric. Timing: morning, recovery period and during exercise. | Fed (2.5 h pre). | Significantly more repetitions and sets to failure in the CHO condition (total repetitions CHO: 199 ± 115 vs. placebo: 131 ± 67, total sets CHO: 19 ± 12 vs. placebo: 11 ± 7). There was no significant difference in the total work performed between conditions, but a tendency for a difference in favor of the CHO condition (336 ± 217 vs. placebo: 224 ± 114, p = 0.066). |
| Haff et al. [52] | Crossover: Strength-trained men (n = 8), carbohydrate vs. placebo. | Training: Three sets of 10 repetitions of knee extension and flexion in an isokinetic dynamometer, pre and post depletion workout: 3 sets of 10 repetitions of squats (65% 1 RM), speed squats (45% 1 RM) and 1-legged squat (10% 1 RM). Outcome: total and average work (J) across sets, peak and average isokinetic torque (Nm) before and after the training bout. | CHO: A total of 1 g/kg pre and 0.3 g/kg 3 × during the depletion workout (163 g in total) Placebo: non-caloric. Timing: before exercise and 3 drinks during. | Fed (3 h pre). | No significant differences in the isokinetic measurements between conditions. (Glycogen levels were reduced by ~41% in the placebo condition and ~27% in the carbohydrate condition.) |
| Leveritt and Abernethy [15] | Crossover: Recreationally active men (n = 5) and women (n = 1); first tested strength, then performed a glycogen depletion workout 5 days later and 2 days of a low carbohydrate diet (~100 g per day) prior to strength tests again. | Glycogen depletion workout: cycling at 75% of VO2 max for 1 h, 3 min rest, followed by four 1 min bouts at 100% of VO2 max with 3-min rest intervals. Outcome: Three sets of isoinertial squat at 80% of 1 RM performed until failure with 3-min rest-intervals, in addition to 5 repetitions with isokinetic knee extension torque at five different contraction speeds. | Lower carbohydrate: ~1884 kcal 1.21 g/kg (90 g carbohydrates). Control diet: Not reported. | Not specified. | Significantly more repetitions at set 1 and 2 during squats in the control diet group compared to the low-carbohydrate diet (set 1: CHO: 18 ± 8, control: 12 ± 5, set 2: CHO: 14 ± 6, control: 10 ± 4. No significant difference was observed in set 3 (CHO: 10 ± 7, control: 11 ± 4) or in torque during knee extensions. |
| Mitchell et al. [48] | Counterbalanced crossover: Strength-trained men (n = 11); high-carbohydrate and a low-carbohydrate condition for 48 h after glycogen depletion. | Glycogen depletion workout: cycling at 70% of VO2 max for 1 h, followed by 1-min sprints at 115% of VO2 max with 1-min rest-intervals. Outcome: after the 48 h diet period; five sets at 15 RM of squats, leg presses and knee extensions to failure. Performance was quantified as total volume lifted. | Lower carbohydrate: 3094 kcal 0.4 g/kg CHO/protein/fat 32/226/230 g Higher carbohydrate: 3206 kcal 7.7 g/kg CHO/protein/fat 643/84/33 g | Not specified. | No significant differences in total training volume between groups (high-carbohydrate: ~15,800 kg, low-carbohydrate: ~15,500 kg). |
| Oliver et al. [50] | Crossover: Strength-trained men (n = 16), two carbohydrate conditions with different molecular weight and osmolarity vs. placebo. | Glycogen depletion workout: cycling for 60 min at 70% VO2 max, followed by six 1-min sprints at 120% of maximal aerobic power. Training: A total of 2 h after cessation of the first workout, session two was performed; squats at 75% of 1 RM, five sets of 10 as explosive as possible. Outcome: average power output, force and velocity across all squat sets. | CHO: 1.2 g/kg (106 g), high molecular weight and low osmolarity (HMW), and low molecular weight and high osmolarity (LMW). Placebo: non-caloric. Timing: after the glycogen depletion bout (2 h before strength training). | Fasted (overnight 12 h). | The carbohydrate conditions achieved significantly greater average power outputs and movement velocities than placebo, but the differences between groups in total training volume or average force output were insignificant or of ‘trivial’ magnitude. |
| Symons and Jacobs [51] | Counterbalanced crossover: Men (n = 8) with experience with high-intensity training; glycogen depleted the knee extensors with cycling, then subjects followed two diets the next two days; low carbohydrate diet or a mixed diet. | Outcome: knee extension electrically evoked isometric muscle force, voluntary isometric strength and isokinetic total work across all repetitions (J), peak and average torque from 50 maximal unilateral knee extensions, in addition to muscle fatigue (average torque of the last three contractions divided by the peak torque). | Lower carbohydrate: A total of 3000 kcal 140 g, 1.8 g/kg/day (19%) carbohydrates. Higher carbohydrate: Not reported. | No significant differences between groups in any of the performance measurements. |
| Study | Design and Population | Training Protocol and Performance Outcomes | Diet | Results |
|---|---|---|---|---|
| Isocaloric studies | ||||
| Dipla et al. [54] | Counterbalanced crossover: Recreationally active women (n = 10); control diet or a high-protein lower-carbohydrate diet for 1 week each. | Outcome: handgrip strength and four sets of 16 maximal repetitions (120° per seconds) with isokinetic knee extensors and flexors contractions. Isokinetic peak torque determined by three maximal efforts, and muscle fatigue as the percentage reduction in work produced in the last set relative to the first set. | Lower carbohydrate: A total of 1305 kcal CHO/protein/fat 99/131/43 g Higher carbohydrate: A total of 1315 kcal CHO/protein/fat 179/53/43 g | No significant differences in peak torque or muscle fatigue between groups. |
| Non-isocaloric, protein non-equated studies | ||||
| Escobar et al. [59] | RCT: Male (n = 7) and female (n = 11) CrossFit athletes (n = 18); high-carbohydrate (n = 9) or a control group (n = 9). Subjects consumed their regular diet for 5 days, then the carbohydrate group increased carbohydrate intake to 6–8 g/kg/day. | Training: CrossFit workouts on day 6 and 7. Outcome: number of repetitions performed in a 12-min CrossFit workout on day 1, 5 and 9. | Lower carbohydrate: 1846 kcal CHO/protein/fat 213/105/64 g Higher carbohydrate: 2938 kcal CHO/protein/fat 428/129/79 g | No significant differences between groups in number of repetitions during CrossFit training. |
| Hatfield et al. [55] | Counterbalanced crossover: Strength-trained men (n = 8); diet with 50% or 80% of calories from carbohydrates for 4 days. | Outcome: Four sets of 12 squat jumps at 30% 1 RM. Power output and total work (J) was measured. | Lower carbohydrate: A total of 50% carbohydrates Higher carbohydrate: A total of 80% carbohydrates | No significant differences between groups in any of the performance measurements. |
| Kreider et al. [56] | RCT: Male athletes (n = 14); carbohydrate supplement group (4 g/kg/day) or a placebo group for 7 days. | Training: One to two intensive hockey training sessions per day. Outcome: vertical jump, 1 RM bench press and leg press. | Lower carbohydrate: A total of 2398 kcal 346 g (58%) carbohydrates Higher carbohydrate: A total of 3685 kcal 628 g (68%) carbohydrates | No significant differences between groups in any of the performance measurements. |
| Meirelles et al. [57] | RCT: Sedentary women (n = 24); 500–800 kcal deficit conventional diet (n = 12) or ad libitum very low carbohydrate diet (VLCD, n = 12) for 1 week. | Outcome: Three sets of 15 maximal effort knee extensions in the concentric phase at a velocity of 60°/s. Peak torque, average power, set total work (J), and total work across all sets were measured. | Lower carbohydrate: A total of <40 g carbohydrates per day Higher carbohydrate: CHO/protein/fat 48/22/30% 165 g carbohydrates | No significant differences between groups in any of the isokinetic measurements. |
| Moura et al. [59] | RCT: Enhanced male bodybuilders (n = 11); moderate energy deficit (n = 6) or severe energy deficit (n = 5) with acute strength tests in the fourth diet week after two days of low-calorie lower-carbohydrate intake and then after 2 days of refeed with higher-carbohydrate intake. | Training: followed their usual resistance training with five sessions per week. Outcome: total repetitions to failure, 10 sets of leg press at 70% 1 RM with 10 RM and 30 s rest-intervals. | Lower carbohydrate: Moderate energy deficit: 2968 kcal CHO/protein/fat 227/295/98 g Severe energy deficit: 2507 kcal CHO/protein/fat 235/271/54 Higher carbohydrate: Refeed after moderate energy deficit: 4039 kcal CHO/protein/fat 687/151/76 g Refeed after severe energy deficit: 3715 kcal CHO/protein/fat 655/116/70 g Combined moderate and energy deficit groups: Lower carbohydrate: A total of 2758 kcal CHO/protein/fat 231/284/78 g Higher carbohydrate refeed: A total of 3892 kcal CHO/protein/fat 672/135/73 g | No significant differences between groups in number of repetitions. |
| Sawyer et al. [58] | Crossover: Strength-trained men (n = 16) and women (n = 15); habitual diet for 7 days, and then a carbohydrate restricted diet for 7 days. | Training: required to complete a 1-week resistance trained log, so likely continued their usual training. Outcome: handgrip strength, bench press and back squat 1 RM, bench press peak power, followed by repetitions to failure, in addition to countermovement vertical jump height and peak power output from a 30 s Wingate. | Lower carbohydrate: A total of 2157 kcal CHO/protein/fat 31/201/137 g Higher carbohydrate: A total of 2537 kcal CHO/protein/fat 265/145/100 g | Significantly greater handgrip strength, squat 1 RM, and vertical jump height in the carbohydrate restricted condition compared to the control condition, with no difference in the other measurements. |
| Study | Design and Population | Strength Training and Performance Outcomes | Diet | Results |
|---|---|---|---|---|
| Isocaloric, isonitrogenous studies | ||||
| Greene et al. [61] | Crossover: Intermediate to elite male (n = 9) and female (n = 5) powerlifters and Olympic weightlifters; low-carbohydrate ketogenic diet or to continue their usual ad libitum diet, in a random order for 3 months (n = 12 completed). | Training: subjects were instructed to maintain their normal training. Outcome: 1 RM for one or all of the subjects’ competition lifts. | Lower carbohydrate: A total of 2072 kcal CHO/protein/fat 41/119/159 g Higher carbohydrate: A total of 2058 kcal CHO/protein/fat 223/119/79 g | No significant difference between groups in changes in 1 RM. |
| Gregory et al. [62] | RCT: CrossFit athletes (n = 27) of both genders; low-carbohydrate ketogenic diet (n = 12) group or to maintain their normal dietary intake (control, n = 15) for 6 weeks. | Training: Four CrossFit training sessions per week. Outcome: changes in countermovement vertical jump height and standing long jump length and time-performance during a standardized CrossFit workout. | Lower carbohydrate: A total of 1581 kcal CHO/protein/fat 44/92/115 g Higher carbohydrate: A total of 1747 kcal CHO/protein/fat 187/80/73 g | No significant differences between groups in changes of any of the performance measurements. |
| Meirelles and Gomes [63] | CT: Overweight (≥25 BMI) but strength-trained males and females (n = 21) self-selected to follow a low-carbohydrate (n = 12) or a conventional/habitual diet (n = 9) for 8 weeks. | Training: full-body resistance training was performed three times per week, two sets of 11 exercises with 8–10 RM and 2-min rest-intervals. Outcome: 10 RM in the biceps pulldown, triceps pushdown and leg press. | Lower carbohydrate: A total of 1566 kcal CHO/protein/fat 83 g carbohydrates 1.5 g/kg/day protein Higher carbohydrate: A total of 1459 kcal CHO/protein/fat 171 g carbohydrates 1.6 g/kg/day of protein | No significant differences between groups in changes in any of the 10 RM tests. |
| Michalski et al. [75] | Female and male CrossFit athletes (n = 22); first 2 weeks of their usual diet, then a low-carbohydrate ketogenic diet for 4 weeks. | Training: maintaining their usual training. Outcome: as many repetitions as possible within a 17 min CrossFit workout. | Lower carbohydrate: A total of 2807 kcal CHO/protein/fat 33/125/238 g Higher carbohydrate: A total of 2565 kcal CHO/protein/fat 290/118/104 g | No significant differences between groups in CrossFit repetition performance. |
| Paoli et al. [76] | RCT: Male bodybuilders (n = 19); low-carbohydrate ketogenic diet (n = 9) or western diet (n = 10) for 8 weeks. | Training: maintaining their usual strength training (3–4 sessions per week). Outcome: 1 RM squat and bench press. | Lower carbohydrate: A total of 3444 kcal CHO/protein/fat 44/216/264 g Higher carbohydrate: A total of 3530 kcal CHO/protein/fat 488/223/79 g | No significant differences between groups in 1 RM changes. |
| Van Zant et al. [64] | Crossover: Strength-trained males (n = 6); high-carbohydrate or a moderate-carbohydrate diet for 3 weeks. | Training: maintaining their usual strength training. Outcome: knee- extension and flexion peak torque and total work performed during two sets of 30 isokinetic contractions, bench press 1 RM and bench press repetitions to failure at 80% 1 RM. | Lower carbohydrate: CHO: A total of 4.2 g/kg/day (~347 g) Higher carbohydrate: CHO: A total of 6.3 g/kg/day (~520 g) | No significant differences between groups in changes of any of the performance measurements. |
| Vidić et al. [77] | RCT: Strength-trained males (n = 18); low-carbohydrate ketogenic diet group (n = 9) or a non-ketogenic diet group (n = 9) for 8 weeks. | Training: resistance training was performed four times per week as a split-routine, unspecified load, three sets and 6–12 repetitions per set. Outcome: A total of 1 RM squat and bench press. | Lower carbohydrate: A total of 2156 kcal CHO/protein/fat 27/108/180 g Higher carbohydrate: A total of 2191 kcal CHO/protein/fat 82/110/158 g | No significant differences between groups in 1 RM changes. |
| Wilson et al. [65] | RCT: Strength-trained men (n = 25); low-carbohydrate ketogenic diet group (n = 13) or a western diet group (n = 12) for 10 weeks (carbohydrates were then reintroduced in the ketogenic group in the 11th week). | Training: resistance training was performed three times per week as a split-routine, 65–95% of 1 RM, three to four sets per exercise and 1–15 repetitions per set. Outcome: bench press and back squat 1 RM, and 10 s Wingate cycle sprint (peak power). | Lower carbohydrate: 2617 kcal CHO/protein/fat 31/135/217 g Higher carbohydrate: A total of 2545 kcal CHO/protein/fat 317/131/84 g | No significant differences between groups in changes of any of the performance measurements. |
| Isocaloric, non-isonitrogenous studies | ||||
| De Oliveira et al. [71] | RCT: Male military police students (n = 16); protein supplement (4 g/kg/day, n = 8) or a carbohydrate supplement (225 g, n = 8) group for 8 weeks. | Training: resistance training three × per week, 80% 1 RM for eight repetitions × five sets. Exercises were arm curls, preacher curls, overhead triceps and lying down triceps extension. Outcome: maximal strength (1 RM) for all exercises, and peak torque from five repetitions of isokinetic elbow flexion and extension. | Lower carbohydrate: A total of 3710 kcal CHO/protein/fat 338/297/112 g Higher carbohydrate: A total of 3767 kcal CHO/protein/fat 581/130/100 g | No significant differences between groups in changes of 1 RM or peak torque. |
| Kreider et al. [66] | RCT: Obese women (n = 221); high-carbohydrate (n = 92) or a high-protein, low-carbohydrate diet (n = 129) for 10 weeks (diets consisted of 1200 kcal the first week, then 1600 kcals the next 9 weeks). | Training: supervised whole-body circuit resistance training three × per week. Outcome: bench press 1 RM and repetitions to failure at 70% of 1 RM. | Lower carbohydrate: 1411 kcal CHO/protein/fat 123/102/57 g Higher carbohydrate: 1379 kcal CHO/protein/fat 183/62/46 g | No significant differences between groups in changes in repetitions to failure or 1 RM. |
| Rhyu and Cho [67] | RCT: Male taekwondo athletes (n = 20); ketogenic diet group (n = 10) or a non-ketogenic diet group (n = 10) for 3 weeks in a 25% caloric deficit. | Training: resistance training and taekwondo training were performed 6 days per week. Outcome: grip strength, back strength and repetitions of sit ups performed in 60 s, 100 m sprint, Wingate peak and mean power and fatigue index and standing broad jump distance. | Lower carbohydrate: A total of CHO/protein/fat 4/41/55% 22 g CHO per day Higher carbohydrate: A total of CHO/protein/fat 40/30/30% | No significant differences between groups in changes in any performance outcomes, except for a significantly lower (better) anaerobic fatigue index during the Wingate test in the ketogenic group. |
| Non-isocaloric, protein equated studies | ||||
| Rozenek et al. [74] | RCT: Active males (n = 46); high-carbohydrate diet (n = 25) or a control group (n = 21) for 8 weeks. | Training: resistance training was performed four times per week as a 2-split routine, eight repetitions for four sets. Outcome: 1 RM in bench press, leg press, lat pull-down and in total. | Lower carbohydrate: A total of 2597 kcal CHO/Protein/Fat 337/107/84 g Higher carbohydrate: A total of 4339 kcal CHO/Protein/Fat 758/109/87 g | No significant differences between groups in changes of maximal strength. |
| Non-isocaloric, non-isonitrogenous studies | ||||
| Agee [68] | RCT: Male powerlifters (n = 12); ad libitum low-carbohydrate ketogenic diet (n = 4) or to maintain their habitual diet, control group (CON) (n = 8) for 6 weeks. | Training: resistance training was performed four times per week as a 2-split routine, 4–12 repetitions forthree to five sets. Outcome: 1 RM in bench press, squat and deadlift. | Lower carbohydrate: A total of 1918 kcal CHO/protein/fat: 107/136/106 g Higher carbohydrate: A total of 2862 kcal CHO/protein/fat: 268/166/121 g | No significant differences between groups in changes of maximal strength. |
| Kephart et al. [69] | CT: Male (n = 9) and female (n = 3) CrossFit athletes (n = 12); self-selected to either continue their normal diet (n = 5) or follow a ketogenic diet (n = 7) for 12 weeks. | Training: continued CrossFit workouts (ketogenic diet group completed 27 workouts, whereas the control completed 20 workouts). Outcome: back squat and power clean 1 RM, one set of push-up repetitions to failure and 400-m running time. | Lower carbohydrate: 1948 kcal CHO/protein/fat: 15/89/170 g Higher carbohydrate: Not reported. | No significant differences between groups in changes of any of the performance measurements. |
| LaFountain et al. [72] | CT: Healthy military men (n = 25) and women (n = 4); self-selected to follow an ad libitum ketogenic diet or to continue their normal mixed diet for 12 weeks. | Training: supervised full-body resistance training 2 × per week. three to four sets, 4–12 repetitions at 60–95% 1 RM. Outcome: countermovement vertical jump power, 1 RM squat and bench press, 10 sprint intervals and obstacle course performance. | Lower carbohydrate: <50 g/day carbohydrates Control diet: >40% carbohydrates | No significant differences between groups in changes of any of the performance measurements. |
| Paoli et al. [70] | Crossover: Elite male gymnasts (n = 8); ad libitum very-low-carbohydrate ketogenic diet for the first 30 days, and then 30 days with a Western diet 3 months later. | Training: instructed to continue their normal training schedule of approx. 30 h per week. Outcome: One set of pushups, pull ups, dips, hanging straight and bodyweight leg raises until failure, in addition to squat- and countermovement jumps. | Lower carbohydrate: A total of 1973 kcal CHO/Protein/Fat: 22/201/120 g Higher carbohydrate: A total of 2276 kcal CHO/protein/fat: 264/84/97 g | No significant differences between groups in changes of any of the performance tests. |
| Vargas-Molina et al. [73] | RCT: Strength-trained women (n = 21); non-ketogenic diet (n = 11) or a ketogenic diet (n = 10) for 8 weeks. | Training: supervised 2-split resistance training four times per week (strength, hypertrophy and muscle endurance phases: 3–25 repetitions × 3 sets). Outcome: 1 RM squat and bench press, and countermovement jump height. | Lower carbohydrate: A total of 1710 kcal CHO/protein/fat: 39/115/122 g Higher carbohydrate: A total of 1980 kcal CHO/protein/fat: 282/97/51 g | Significantly greater increase in changes of 1 RM for squat and bench press in the non-ketogenic diet group (10- and 3.3 kg difference, respectively), with no group differences in CMJ performance. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Henselmans, M.; Bjørnsen, T.; Hedderman, R.; Vårvik, F.T. The Effect of Carbohydrate Intake on Strength and Resistance Training Performance: A Systematic Review. Nutrients 2022, 14, 856. https://doi.org/10.3390/nu14040856
Henselmans M, Bjørnsen T, Hedderman R, Vårvik FT. The Effect of Carbohydrate Intake on Strength and Resistance Training Performance: A Systematic Review. Nutrients. 2022; 14(4):856. https://doi.org/10.3390/nu14040856
Chicago/Turabian StyleHenselmans, Menno, Thomas Bjørnsen, Richie Hedderman, and Fredrik Tonstad Vårvik. 2022. "The Effect of Carbohydrate Intake on Strength and Resistance Training Performance: A Systematic Review" Nutrients 14, no. 4: 856. https://doi.org/10.3390/nu14040856
APA StyleHenselmans, M., Bjørnsen, T., Hedderman, R., & Vårvik, F. T. (2022). The Effect of Carbohydrate Intake on Strength and Resistance Training Performance: A Systematic Review. Nutrients, 14(4), 856. https://doi.org/10.3390/nu14040856








