Abstract
Ready-meal consumption is increasing worldwide; however, its impact on human health remains unclear. We aimed to examine the association between processed food and beverage consumption during pregnancy and pregnancy outcomes. Pregnant women were recruited for the Japan Environment and Children’s Study (JECS), a nationwide, large-scale, prospective cohort study. This study included 104,102 registered children (including fetuses or embryos) and collected questionnaire-based data during the first and second/third trimester of pregnancy. Participants’ medical records were transcribed at pregnancy registration, immediately after delivery, and 1 month after delivery. Logistic regression analysis was used to estimate the association between processed food consumption and pregnancy outcomes. The incidence of stillbirth was higher in the group that consumed moderate (1–2 times per week) and high (≥3–7 times per week) amounts of ready-meals (adjusted odds ratio (aOR) = 2.054, 95% confidence interval (CI): 1.442–2.926, q = 0.002; aOR = 2.632, 95% CI: 1.507–4.597, q = 0.007, respectively) or frozen meals (aOR = 2.225, 95% CI: 1.679–2.949, q < 0.001; aOR = 2.170, 95% CI: 1.418–3.322, q = 0.005, respectively) than in the group that rarely consumed such foods. Processed food consumption during pregnancy should be carefully considered.
1. Introduction
Dietary habits have changed worldwide in the past few decades; the time spent cooking at home has decreased, with more people choosing to frequently dine out [1]. Moreover, the market for ready-made meals has grown, reflecting changes in the social landscape, such as the presence of more women in the workforce and the expanding range of modern life conveniences, which have reduced the time dedicated to domestic work. Concurrently, some studies have suggested that consuming processed foods might be desirable, as it increases the variety of food items consumed [2].
However, the impact of these new dietary choices, such as consuming beverages stored in plastic containers, on human health remains unclear due to inconclusive evidence. Some studies have reported that ready-made meal consumption is associated with an increased risk of overweight and obesity [3]; however, others have reported no such association [4]. The effects of consumption of processed foods during pregnancy on pregnancy outcomes have been largely unexplored; the evidence is confined to one study that suggests that consumption of meals not prepared at home may contribute to the risk of infertility [5]. The prospective birth cohort in Osaka, Japan, showed that the average intake of coffee in pregnant women is 0.14 cups/day [6]. Despite growing interest in the effects of processed food consumption on human health, few studies have examined the association between the intake of such meals and human health outcomes and specifically birth outcomes, such as stillbirth and pre-term birth, as well as on developmental measures, such as size (small for gestational age (SGA)) and low birth weight.
Elucidating the impact of processed food consumption on pregnancy-related out-comes is relevant to the formulation of public health policies, as evidence suggests that consumption of such foods is associated with exposure to chemicals, such as bisphenol A (BPA) and Di (2-ethylhexyl) phthalate (DEHP), that may disrupt endocrine function. Our previous study showed that serum BPA levels were significantly higher in women who had recurrent miscarriages than in controls [7]. Meanwhile, in a separate study, we found no evidence of an association between recurrent miscarriages and exposure to polychlorinated biphenyls, hexachlorobenzene, or 1,1,1-trichloro-2,2-bis (p-chlorophenyl) ethylene metabolite of 1,1-dichloro-2,2-bis (p-chlorophenyl) ethylene [8]. The present study aimed to examine the association between processed foods, including beverages stored in a can or plastic containers; their consumption during pregnancy; and pregnancy outcomes, using data from a large Japanese birth cohort study.
2. Materials and Methods
Pregnant women were recruited between January 2011 and March 2014 for the Japan Environment and Children’s Study (JECS), a nationwide, large-scale, prospective cohort study registered in the UMIN Clinical Trials Registry (number UMIN000030786). Expectant mothers were eligible for the present study if they resided in the study area at the time of recruitment, had their due date after 1 August 2011, and were fluent Japanese speakers who could understand and complete a set of self-administered questionnaires [9,10,11]. The sample size was calculated prospectively by the JECS Working Group and presented in the JECS protocols ahead of recruitment. Participants’ medical records were transcribed by physicians or Research Coordinators at registration, immediately after delivery, and at 1 month after delivery.
This study was based on the jecs-ag-20160424 dataset, which included 104,102 registered children (fetuses and embryos), and was released to all researchers involved with the JECS in June 2016. In cases of multiple pregnancies, outcomes of the second and third pregnancies were excluded to remove duplicates (n = 1003 (0.96%)). Infants born with physical anomalies were excluded (n = 9008 (8.7%)). Twenty-nine (0.03%) participants withdrew from the study. Finally, data from 94,062 pregnancies were included in the main analysis (Figure 1). The mean (SD) age at registration was 30.7 (5.1) years. The mean (SD) gestational age at registration was 14.4 (5.6) weeks.
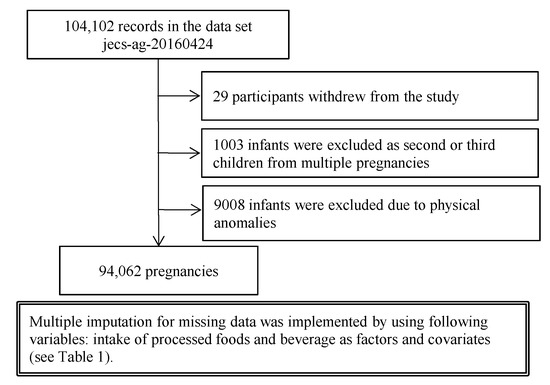
Figure 1.
Flow diagram summarizing the study recruitment process.
2.1. Ethical Approval
The JECS protocol was reviewed and approved by the Ministry of the Environment Institutional Review Board on Epidemiological Studies and by the Ethics Committees of all participating institutions. Written informed consent was obtained from all participants. The study was conducted in accordance with the Helsinki Declaration and other national regulations and guidelines.
2.2. Variables
Study participants completed questionnaires in the first and second/third trimesters of pregnancy, providing data on socio-demographic, socioeconomic, and life-style characteristics. Data transcribed from medical records at the time of study enrollment included information on maternal age, body weight, and height; use of in vitro fertilization and embryo transfer (IVF-ET) for the present pregnancy; and obstetric history. Data transcribed from medical records immediately after delivery included details of maternal and gestational age, single/multiple pregnancy status, and pregnancy outcomes, such as live/stillbirth, miscarriage/induced abortion, and the mode of delivery (vaginal delivery vs. cesarean section), pregnancy-related complications, perinatal outcomes, and infant sex and weight. Data transcribed from medical records 1 month after delivery included maternal age and information on birth defects.
2.3. Outcomes
The outcomes of interest were stillbirth, pre-term birth, SGA, and low birth weight. In this study, pre-term birth was defined as delivery between 22 and <37 weeks of gestation. SGA was defined as a birth weight below the 10th percentile, according to the new Japanese neonatal anthropometric charts for gestational age at birth.
2.4. Exposures and Covariates
The frequency of eating ready-made meals (pre-packed foods sold at convenience stores, supermarkets, or boxed lunch shops), frozen foods, retort pouch foods, convenience foods (instant noodles, soups, or other foods packed in plastic cups that can be cooked by pouring hot water), and canned foods during second/third trimesters was categorized as <1 time per week, 1–2 times per week, and 3–7 or more times per week based on self-reported lifestyle data [12]. In general, ready-made and frozen meals were defined as foods that require microwave preparation, while retort pouch and convenience foods were those that require cooking in boiling water either with or without packaging (e.g., curry or cup noodles, respectively).
In addition, we assessed the frequency of coffee consumption, including the type of container when purchased (e.g., a can or plastic bottle) and the mode of preparation by the consumer. The frequency of black, green, and oolong tea consumption was also evaluated, including whether it was in a can or plastic bottle and if the tea was made from tea leaves by the consumer. To estimate odds ratios (OR), beverage consumption was recalculated separately depending on the source of beverage (can or bottle vs. beans or leaves). Frequency categories were recorded as follows: “less than once a week” became “once a week”, “once or twice a week” became “twice a week”, “three to four times per week” became “four times per week”, “five to six times per week” became “six times per week”, “a cup daily” became “seven times per week”, “2–3 cups daily” became “21 times per week”, “4–6 cups daily” became “42 times per week”, “7–9 cups daily” became “63 times per week”, and “>10 cups daily” became “70 times per week”. Based on self-reported data, the total beverage consumption was defined as <7 times/week, 7–13 times/week, and ≥14 times/week.
The covariates of interest included maternal age at registration, body mass index, IVF-ET status, maternal smoking and drinking status, income level, maternal educational status, history of pregnancy loss, parity, maternal working hours, hypertensive disorders of pregnancy, gestational diabetes, maternal energy intake calculated using the Food Frequency Questionnaire [13] for pregnant women from the JECS data, and the consumption of processed foods and beverages that were beyond the focus of this analysis.
2.5. Statistical Analysis
Descriptive statistics were reported as frequencies. Logistic regression analysis was used to estimate the association between dietary habits and each pregnancy outcome. The OR of each pregnancy outcome was adjusted for covariates. Crude and adjusted odds ratios (aOR) or mean differences with 95% confidence intervals (95% CI) were reported, as suitable. Missing values were handled with multiple imputation methods. To correct the false discovery rate, the q-value was obtained using the Benjamini–Hochberg procedure in R statistical software (version 3.5.2). A q-value of <0.05 was considered statistically significant. Multiple correspondence analysis (MCA) and hierarchical cluster analysis (HCA; Ward’s method) were used to investigate the association between dietary habits and beverage intake. All analyses were conducted using SPSS version 23 (IBM Corp., Japan) except for q-value estimates.
3. Results
The participants’ maternal characteristics and pregnancy outcomes are presented in Table 1. Most participants reported consuming processed foods, such as ready-made meals (58.8%), frozen meals (62.9%), retort pouch (72.9%), convenience (74.8%), and canned (87.0%) foods, at a frequency of <1 time per week. In addition, most participants declared that they rarely (<7 times per week) drank beverages from a can or plastic bottle (60.0%) or those extracted from coffee beans or tea leaves (52.5%).

Table 1.
Maternal characteristics of participants and incidence of events (N = 94,062).
There was a significant association between the incidence of stillbirth and the consumption of processed foods in all categories except canned food (Table S1). The incidence of stillbirth increased in the group reporting moderate (1–2 times per week) consumption of ready-made (OR = 3.217, 95% CI: 2.371–4.364, q < 0·001) and frozen (OR = 3.404, 95% CI: 2.633–4.400, q < 0.001) meals and retort pouch (OR = 2.266, 95% CI: 1.801–2.851, q < 0.001) and convenience (OR = 2.369, 95% CI = 1.783–3.147, q < 0.001) foods. After adjusting for covariates, this association remained significant for ready-made and frozen meals but not for retort pouch or convenience foods (Table 2). The incidence of stillbirth was higher in the group reporting moderate (1–2 times per week) and high (≥ 3–7 times per week) consumption of ready-made (aOR = 2.054, 95% CI: 1.442–2.926, q = 0.002; aOR = 2.632, 95% CI: 1.507–4.597, q = 0.007, respectively) and moderate and high consumption of frozen (aOR = 2.225, 95% CI: 1.679–2.949, q < 0.001; aOR = 2.170, 95% CI: 1.418–3.322, q = 0.005, respectively) meals than in the group that consumed such foods less often than once per week. In addition, the incidence of pre-term birth was higher in the group reporting moderate (1–2 times per week) consumption of ready-made meals (aOR = 1.100, 95% CI: 1.024–1.181, q = 0.030), and the incidence of low birth weight was higher in the group reporting moderate consumption of retort pouch foods (aOR = 1.105, 95% CI: 1.035–1.180, q = 0.012) than that in the low consumption (less than once a week) group.

Table 2.
Maternal characteristics of participants and incidence of events (N = 94,062).
Beverage consumption was associated with pregnancy outcomes such as stillbirth, pre-term birth, SGA infant, and low birth weight. In particular, the incidence of stillbirth was higher in the group reporting moderate (7–13 times per week) and high (≥ 14 times per week) consumption of beverages (can or plastic bottle) (aOR = 3.484, 95% CI: 2.611–4.649, q < 0.001; aOR = 2.930, 95% CI: 1.837–4.673, q < 0.001, respectively) than that in the group reporting low consumption. In addition, the incidence of stillbirth increased in the group reporting moderate and high consumption of beverages extracted from beans or leaves (aOR = 3.752, 95% CI: 2.923–4.816, q < 0.001; aOR = 1.754, 95% CI: 1.192–2.581, q = 0.021, respectively). A similar trend was observed in the incidence of pre-term birth. The incidence of SGA infants increased in drinkers of coffee or tea from bean or leaves, and that of low-birth weight increased in drinkers of coffee or tea from cans or plastic bottles relative to the estimates of the low-consumption group.
Estimates could not be obtained for the high-consumption group due to the small sample size.
We examined dietary habits in MCA after dividing it into four clusters by HCA (Figure 2). Cluster A included high consumption of retort pouch, convenience, and canned foods. Cluster B included high consumption of ready-made and frozen meals. Cluster C included moderate consumption of ready-made, frozen meals, retort pouch, convenience, and canned foods. Cluster D included low consumption of all kinds of processed foods and all frequency groups of both beverages (in a can or plastic bottle and extracted from coffee bean or tea leaf). Clusters B and C represented the dietary pattern associated with a high risk of stillbirth, specifically moderate to high consumption of ready-made and frozen meals. The increase in the risk of stillbirth rates associated with consumption of ready-made and frozen meals was independent of the impact of beverage consumption in both cluster and covariate-adjusted analyses (Table 2).
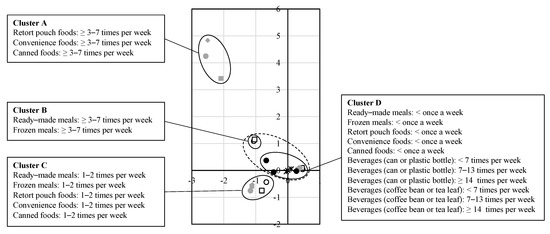
Figure 2.
The results of multiple correspondence analysis.
4. Discussion
To the best of our knowledge, this is the first large-scale birth cohort study to show that consumption of ready-made and frozen meals during pregnancy might increase the risk of stillbirth. Four kinds of processed foods were associated with an increased risk of stillbirth in the crude analysis (Table S1); however, consumption of foods that require microwave heating was significantly associated with a risk of stillbirth after adjusting for covariates (Table 2).
These findings suggest that food packaging and reheating methods may affect outcomes possibly through exposure to chemicals present in meal packaging that are released in the process of cooking in a microwave [14]. BPA, which is used in food packaging, may be a chemical particularly associated with the risk of stillbirth. BPA has a tolerable intake of 0.05 mg/kg b.w./day, as defined by the European Food Safety Authority [15]. However, microwave cooking has been reported to augment BPA migration [16]. The annual intake of BPA from canned foods among the Japanese population has been estimated as 644 ng/person/day in 2011–2012 [17]. BPA can be used as an antioxidant or as a plasticizer in the production of polypropylene, polyethylene, poly-vinyl chloride, and polycarbonate, which are often used in food packaging [18].
Previous studies have shown that BPA exposure may affect human reproductive health even at doses lower than the tolerable daily intake [19]. Several studies have replicated our previous findings on the association between serum BPA levels and the risk of recurrent miscarriage [7,20]. Moreover, Allard et al. showed that exposure to BPA increased the rate of sterility and embryonic death in a mammalian model, where it impaired chromosome synapsis and disrupted the meiotic double-strand break-repair progression [21]. In addition, our previous study showed evidence of a dose-response association between BPA concentration and miscarriage risk mediated by embryonic aneuploidy or antinuclear antibody positivity [7]. Lathi et al. suggested that BPA concentration values may be used to predict the risk of both embryonic euploid and aneuploid miscarriage [20].
In the present study, an association with stillbirths at ≥12 weeks’ gestation was found. It remains unclear how consumption of ready-made and frozen meals increases the risk of stillbirths and whether BPA exposure is involved; the risk of aneuploidy decreases with the increase in gestational age. Furthermore, ready-made meal consumption may increase exposure to phthalates, including DEHP [22], which has been weakly associated with an increased risk of adverse outcomes, such as miscarriage and pre-term birth [23]. Studies using animal models have recently shown the combined adverse effects of BPA and DEHP on gestational outcomes [24]. Styrene oligomers may also be released from food packaging; however, this substance has not been associated with human reproductive toxicity to date [25].
This study revealed an association between beverage intake and the risk of stillbirth. Beverage intake was categorized as out of “a can or plastic bottle” and “extracted from coffee bean or tea leaf”; these categories were associated with ORs of 3.548 and 3.703, respectively. In addition, the consumption of these beverage types was associated with an increased risk of pre-term birth, SGA, and low birth weight. In general, canned and bottled beverages contain large amounts of sugar; consequently, our analyses were adjusted for energy consumption as a surrogate measure of sugar intake. Moreover, caffeine intake has been associated with pregnancy loss, pre-term birth, and low birth weight [26]; therefore, the World Health Organization recommends that daily coffee consumption not exceed 3–4 cups during pregnancy. A meta-analysis has shown that heavy caffeine intake increased the risk of pregnancy loss, including stillbirth [27]. The present findings are consistent with those of previous studies.
The present study has three primary strengths. First, it included a large sample of approximately 100,000 participants. Second, the participants were representative of the Japanese pregnant population since the JECS covered rural and urban areas across Japan [11]. Third, this study is potentially relevant to social policy; socioeconomic and environmental factors associated with processed food consumption should be considered alongside their health effects. It has been suggested that socioeconomic status is related to processed food consumption and resultant increased obesity rates; poor health literacy has been implicated as the source of unhealthy lifestyle choices, including poor diet [28]. Environmentally, the increase in processed food consumption has raised concerns [29]; in response, the concept of “ethical consumption” has been advocated, promoting sustainable consumption behavior. As a result, reducing processed food consumption falls within the scope of the Sustainable Development Goals [30]. Overall, the present study suggests that the development of a processed food and beverage consumption policy requires a comprehensive and multidisciplinary approach, as it has consequences for several fields outside of human health; the present study is one such contribution.
The main limitation of this study is that it did not identify underlying mechanisms or substances directly associated with the reported increases in risk; in addition, we did not measure biochemical parameters, such as BPA levels, which should be quantified in future studies. In addition, poor quality, such as excess sugar, fat, saturated fat intake, and lack of dietary fiber, is a problem in a processed-food-rich diet [31], and there is a possibility of residual confounding regarding the effect of this on outcomes. However, since energy intake is adjusted as confounding in this study, it is considered that the effect of food components that correlate with energy intake is adjusted. Finally, the incidence of early miscarriage could not be examined, as the mean gestational age at registration was 14.4 weeks.
5. Conclusions
Our findings suggest that processed food and beverage consumption during pregnancy increases the risk of adverse pregnancy outcomes, including stillbirth. This finding may result from exposure to chemicals contained in food packaging, which may increase with microwave cooking. The present findings suggest that dietary and nutritional advice should be included in prenatal counseling to help prevent serious adverse outcomes [32]. The environmental impact of processed food consumption should be examined, and future studies should examine biochemical parameters, including urine samples, and their impact on the risk of adverse pregnancy outcomes.
Supplementary Materials
The following are available online at: https://www.mdpi.com/article/10.3390/nu14040895/s1, Table S1: Association of perinatal abnormality and intake frequency of ready-cooked foods (Crude).
Author Contributions
H.T., Y.I., and M.S.-O. conceptualized the study and wrote the first draft of the manuscript. H.T. and T.M. verified the underlying data and performed the statistical analysis. T.E. organized the study team, assisted H.T. with statistical analysis, and helped in drafting the manuscript. S.K., H.S., Y.I., and S.S. were responsible for acquiring data, managing the cohort, and critically revising the manuscript. M.K. was responsible for supervision of the present study team. M.S.-O. corrected and finalized the manuscript. The JECS Group was involved in data collection and contributed to manuscript revision. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of the Environment, Government of Japan. The funder had no role in study design, data collection and analysis, decision to publish, or manuscript preparation. The corresponding author had full access to all study data and was responsible for the decision to submit for publication.
Institutional Review Board Statement
The JECS was approved by the Institutional Review Board of the Japan National Institute for Environmental Studies (no. 100910001) as well as by the ethics committees of all participating institutions.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data are unsuitable for public deposition due to ethical restrictions and legal framework of Japan. It is prohibited by the Act on the Protection of Personal Information (Act No. 57 of 30 May 2003, amendment on 9 September 2015) to publicly deposit data containing personal information. Ethical Guidelines for Medical and Health Research Involving Human Subjects enforced by the Japan Ministry of Education, Culture, Sports, Science, and Technology and the Ministry of Health, Labour, and Welfare also restrict the open sharing of epidemiologic data. All inquiries about access to data should be sent to jecs-en@nies.go.jp. The person responsible for handling enquiries sent to this e-mail address is Dr. Shoji F. Nakayama, JECS Program Office, National Institute for Environmental Studies.
Acknowledgments
The members of the Japan Environment and Children’s Study (JECS) Group as of 2021 include: Michihiro Kamijima (principal investigator, Nagoya City University, Nagoya, Japan), Shin Yamazaki (National Institute for Environmental Studies, Tsukuba, Japan), Yukihiro Ohya (National Centre for Child Health and Development, Tokyo, Japan), Reiko Kishi (Hokkaido University, Sapporo, Japan), Nobuo Yaegashi (Tohoku University, Sendai, Japan), Koichi Hashimoto (Fukushima Medical University, Fukushima, Japan), Chisato Mori (Chiba University, Chiba, Japan), Shuichi Ito (Yokohama City University, Yokohama, Japan), Zentaro Yamagata (University of Yamanashi, Chuo, Japan), Hidekuni Inadera (University of Toyama, Toyama, Japan), Takeo Nakayama (Kyoto University, Kyoto, Japan), Hiroyasu Iso (Osaka University, Suita, Japan), Masayuki Shima (Hyogo College of Medicine, Nishinomiya, Japan), Hiroshige Nakamura (Tottori University, Yonago, Japan), Narufumi Suganuma (Kochi University, Nankoku, Japan), Koichi Kusuhara (University of Occupational and Environmental Health, Kitakyushu, Japan), and Takahiko Katoh (Kumamoto University, Kumamoto, Japan). We thank all study participants and healthcare professionals who provided support for the JECS. The JECS was funded by the Ministry of the Environment of Japan. The findings and conclusions presented in this article are solely those of the authors and do not represent the official views of the Japanese Government.
Conflicts of Interest
The authors declare no conflict of interest associated with this manuscript.
References
- Smith, L.P.; Ng, S.W.; Popkin, B.M. Trends in US home food preparation and consumption: Analysis of national nutrition surveys and time use studies from 1965–1966 to 2007–2008. Nutr. J. 2013, 12, 45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lachat, C.; Khanh, L.N.B.; Khan, N.C.; Dung, N.Q.; van Anh, N.D.; Roberfroid, D.; Kolsteren, P. Eating out of home in Vietnamese adolescents: Socioeconomic factors and dietary associations. Am. J. Clin. Nutr. 2009, 90, 1648–1655. [Google Scholar] [CrossRef]
- Alkerwi, A.; Crichton, G.E.; Hebert, J.R. Consumption of ready-made meals and increased risk of obesity: Findings from the Observation of Cardiovascular Risk Factors in Luxembourg (ORISCAV-LUX) study. Br. J. Nutr. 2015, 113, 270–277. [Google Scholar] [CrossRef] [Green Version]
- Soriano, G.; de Barreto, P.S.; Rolland, Y.; Plessz, M.; Goisser, S.; Guyonnet, S.; Fougere, B.; Vellas, B.; Andrieu, S.; Sourdet, S. Ready-meal consumption in older people: Association with obesity and dietary intake. Aging Clin. Exp. Res. 2019, 31, 855–861. [Google Scholar] [CrossRef]
- Lee, S.; Min, J.Y.; Kim, H.J.; Min, K.B. Association Between the Frequency of Eating Non-home-prepared Meals and Women Infertility in the United States. J. Prev. Med. Public Health 2020, 53, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Okubo, H.; Miyake, Y.; Tanaka, K.; Sasaki, S.; Hirota, Y. Maternal total caffeine intake, mainly from Japanese and Chinese tea, during pregnancy was associated with risk of preterm birth: The Osaka Maternal and Child Health Study. Nutr. Res. 2015, 35, 309–316. [Google Scholar] [CrossRef]
- Sugiura-Ogasawara, M.; Ozaki, Y.; Sonta, S.; Makino, T.; Suzumori, K. Exposure to bisphenol A is associated with recurrent miscarriage. Hum. Reprod. 2005, 20, 2325–2329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sugiura-Ogasawara, M.; Ozaki, Y.; Sonta, S.; Makino, T.; Suzumori, K. PCBs, hexachlorobenzene and DDE are not associated with recurrent miscarriage. Am. J. Reprod. Immunol. 2003, 50, 485–489. [Google Scholar] [CrossRef]
- Ishitsuka, K.; Nakayama, S.F.; Kishi, R.; Mori, C.; Yamagata, Z.; Ohya, Y.; Kawamoto, T.; Kamijima, M. Japan Environment and Children’s Study: Backgrounds, activities, and future directions in global perspectives. Environ. Health Prev. Med. 2017, 22, 61. [Google Scholar] [CrossRef]
- Kawamoto, T.; Nitta, H.; Murata, K.; Toda, E.; Tsukamoto, N.; Hasegawa, M.; Yamagata, Z.; Kayama, F.; Kishi, R.; Ohya, Y.; et al. Rationale and study design of the Japan environment and children’s study (JECS). BMC Public Health 2014, 14, 25. [Google Scholar] [CrossRef] [Green Version]
- Michikawa, T.; Nitta, H.; Nakayama, S.F.; Yamazaki, S.; Isobe, T.; Tamura, K.; Suda, E.; Ono, M.; Yonemoto, J.; Iwai-Shimada, M.; et al. Baseline Profile of Participants in the Japan Environment and Children’s Study (JECS). J. Epidemiol. 2018, 28, 99–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iwai-Shimada, M.; Nakayama, S.F.; Isobe, T.; Michikawa, T.; Yamazaki, S.; Nitta, H.; Takeuchi, A.; Kobayashi, Y.; Tamura, K.; Suda, E.; et al. Questionnaire results on exposure characteristics of pregnant women participating in the Japan Environment and Children Study (JECS). Environ. Health Prev. Med. 2018, 23, 45. [Google Scholar] [CrossRef]
- Yokoyama, Y.; Takachi, R.; Ishihara, J.; Ishii, Y.; Sasazuki, S.; Sawada, N.; Shinozawa, Y.; Tanaka, J.; Kato, E.; Kitamura, K.; et al. Validity of Short and Long Self-Administered Food Frequency Questionnaires in Ranking Dietary Intake in Middle-Aged and Elderly Japanese in the Japan Public Health Center-Based Prospective Study for the Next Generation (JPHC-NEXT) Protocol Area. J. Epidemiol. 2016, 26, 420–432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreira, M.A.; André, L.C.; Cardeal, Z.L. Analysis of phthalate migration to food simulants in plastic containers during microwave operations. Int. J. Environ. Res. Public Health 2013, 11, 507–526. [Google Scholar] [CrossRef] [PubMed]
- Mikolajewska, K.; Stragierowicz, J.; Gromadzinska, J. Bisphenol A—Application, sources of exposure and potential risks in infants, children and pregnant women. Int. J. Occup. Med. Environ. Health 2015, 28, 209–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, D.S.; Kwack, S.J.; Kim, K.B.; Kim, H.S.; Lee, B.M. Potential risk of bisphenol A migration from polycarbonate containers after heating, boiling, and microwaving. J. Toxicol. Environ. Health A 2009, 72, 1285–1291. [Google Scholar] [CrossRef]
- Kawamura, Y.; Etoh, M.; Hirakawa, Y.; Abe, Y.; Mutsuga, M. Bisphenol A in domestic and imported canned foods in Japan. Food Addit. Contam. Part A 2014, 31, 330–340. [Google Scholar] [CrossRef]
- Hermabessiere, L.; Dehaut, A.; Paul-Pont, I.; Lacroix, C.; Jezequel, R.; Soudant, P.; Duflos, G. Occurrence and effects of plastic additives on marine environments and organisms: A review. Chemosphere 2017, 182, 781–793. [Google Scholar] [CrossRef] [Green Version]
- Vom Saal, F.S.; Welshons, W.V. Large effects from small exposures. II. The importance of positive controls in low-dose research on bisphenol A. Environ. Res. 2006, 100, 50–76. [Google Scholar] [CrossRef]
- Lathi, R.B.; Liebert, C.A.; Brookfield, K.F.; Taylor, J.A.; vom Saal, F.S.; Fujimoto, V.Y.; Baker, V.L. Conjugated bisphenol A in maternal serum in relation to miscarriage risk. Fertil. Steril. 2014, 102, 123–128. [Google Scholar] [CrossRef] [Green Version]
- Allard, P.; Colaiácovo, M.P. Bisphenol A impairs the double-strand break repair machinery in the germline and causes chromosome abnormalities. Proc. Natl. Acad. Sci. USA 2010, 107, 20405–20410. [Google Scholar] [CrossRef] [Green Version]
- Martinez, M.A.; Rovira, J.; Sharma, R.P.; Nadal, M.; Schuhmacher, M.; Kumar, V. Prenatal exposure estimation of BPA and DEHP using integrated external and internal dosimetry: A case study. Environ. Res. 2017, 158, 566–575. [Google Scholar] [CrossRef] [Green Version]
- Radke, E.G.; Glenn, B.S.; Braun, J.M.; Cooper, G.S. Phthalate exposure and female reproductive and developmental outcomes: A systematic review of the human epidemiological evidence. Environ. Int. 2019, 130, 104580. [Google Scholar] [CrossRef] [PubMed]
- Dagher, J.B.; Hahn-Townsend, C.K.; Kaimal, A.; Mansi, M.A.; Henriquez, J.E.; Tran, D.G.; Laurent, C.R.; Bacak, C.J.; Buechter, H.E.; Cambric, C.; et al. Independent and combined effects of Bisphenol A and Diethylhexyl Phthalate on gestational outcomes and offspring development in Sprague-Dawley rats. Chemosphere 2021, 263, 128307. [Google Scholar] [CrossRef] [PubMed]
- Gelbke, H.P.; Banton, M.; Block, C.; Dawkins, G.; Eisert, R.; Leibold, E.; Pemberton, M.; Puijk, I.M.; Sakoda, A.; Yasukawa, A. Risk assessment for migration of styrene oligomers into food from polystyrene food containers. Food Chem. Toxicol. 2019, 124, 151–167. [Google Scholar] [CrossRef]
- Poole, R.; Kennedy, O.J.; Roderick, P.; Fallowfield, J.A.; Hayes, P.C.; Parkes, J. Coffee consumption and health: Umbrella review of meta-analyses of multiple health outcomes. BMJ 2017, 359, j5024. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.W.; Wu, Y.; Neelakantan, N.; Chong, M.F.; Pan, A.; van Dam, R.M. Maternal caffeine intake during pregnancy and risk of pregnancy loss: A categorical and dose-response meta-analysis of prospective studies. Public Health Nutr. 2016, 19, 1233–1244. [Google Scholar] [CrossRef] [Green Version]
- Janssen, H.G.; Davies, I.G.; Richardson, L.D.; Stevenson, L. Determinants of takeaway and fast food consumption: A narrative review. Nutr. Res. Rev. 2018, 31, 16–34. [Google Scholar] [CrossRef] [PubMed]
- Rivera, X.C.S.; Azapagic, A. Life cycle environmental impacts of ready-made meals considering different cuisines and recipes. Sci. Total Environ. 2019, 660, 1168–1181. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, N.; Vivekadhish, S. Millennium development goals (MDGS) to sustainable development goals (SDGS): Addressing unfinished agenda and strengthening sustainable development and partnership. Indian J. Community Med. 2016, 41, 1. [Google Scholar] [CrossRef]
- Marino, M.; Puppo, F.; Del Bo, C.; Vinelli, V.; Riso, P.; Porrini, M.; Martini, D. A Systematic Review of Worldwide Consumption of Ultra-Processed Foods: Findings and Criticisms. Nutrients 2021, 13, 2778. [Google Scholar] [CrossRef] [PubMed]
- Peters, M.D.; Lisy, K.; Riitano, D.; Jordan, Z.; Aromataris, E. Caring for families experiencing stillbirth: Evidence-based guidance for maternity care providers. Women Birth 2015, 28, 272–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).