Methanolic Phoenix dactylifera L. Extract Ameliorates Cisplatin-Induced Hepatic Injury in Male Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Drug and Plant Extraction
2.2. Characterization of Compounds in the Methanolic Date Palm Extract Using LC-MS/MS Analysis
2.3. Animals
2.4. Experimental Design
2.5. Sampling
2.6. Relative Liver Weights
2.7. Serum Biochemical Analysis
2.8. Hepatic ADH and NADPH
2.9. Oxidative Stress Markers, Antioxidant Activity, Interleukin-12, and Interleukin-10
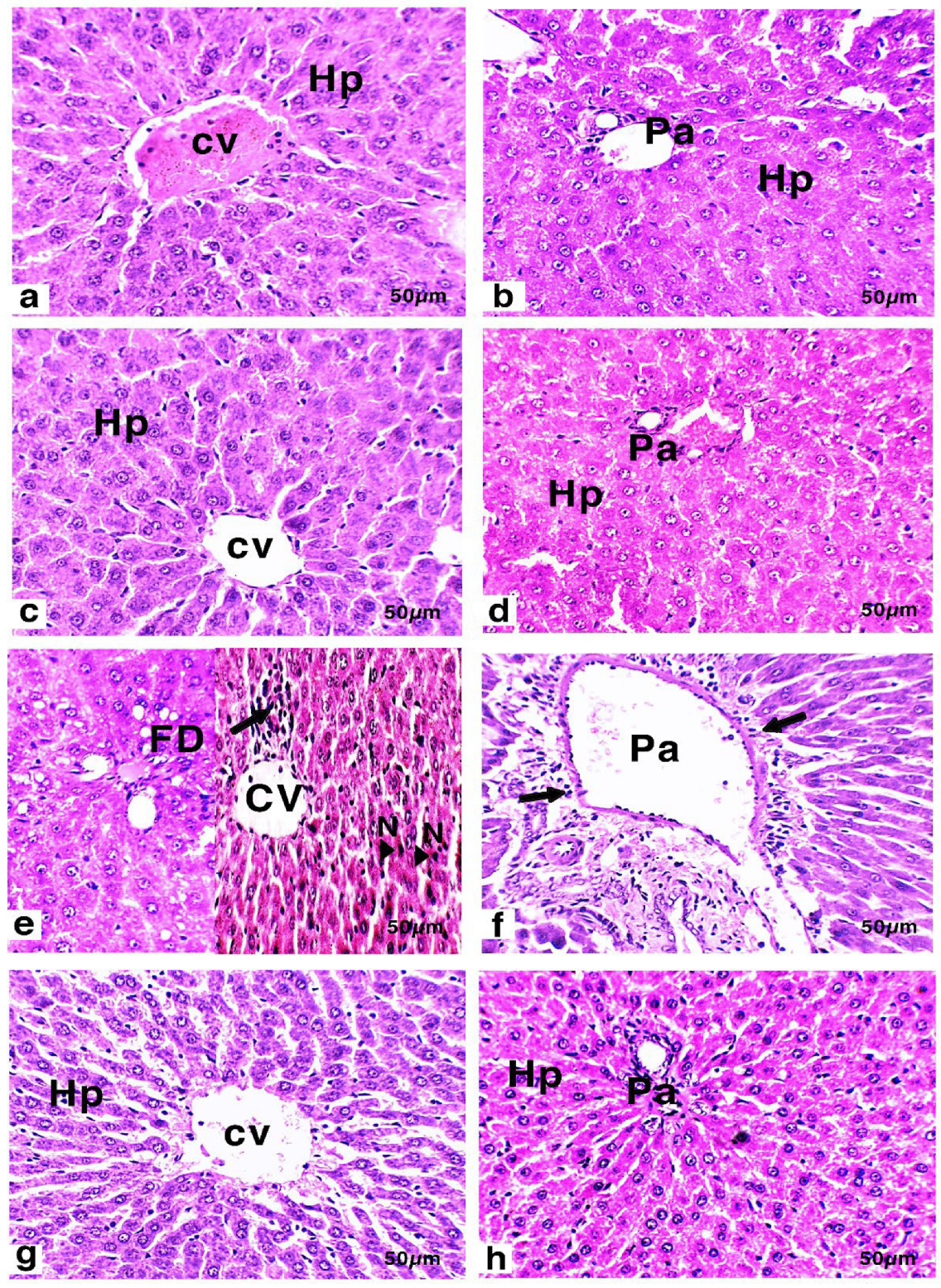
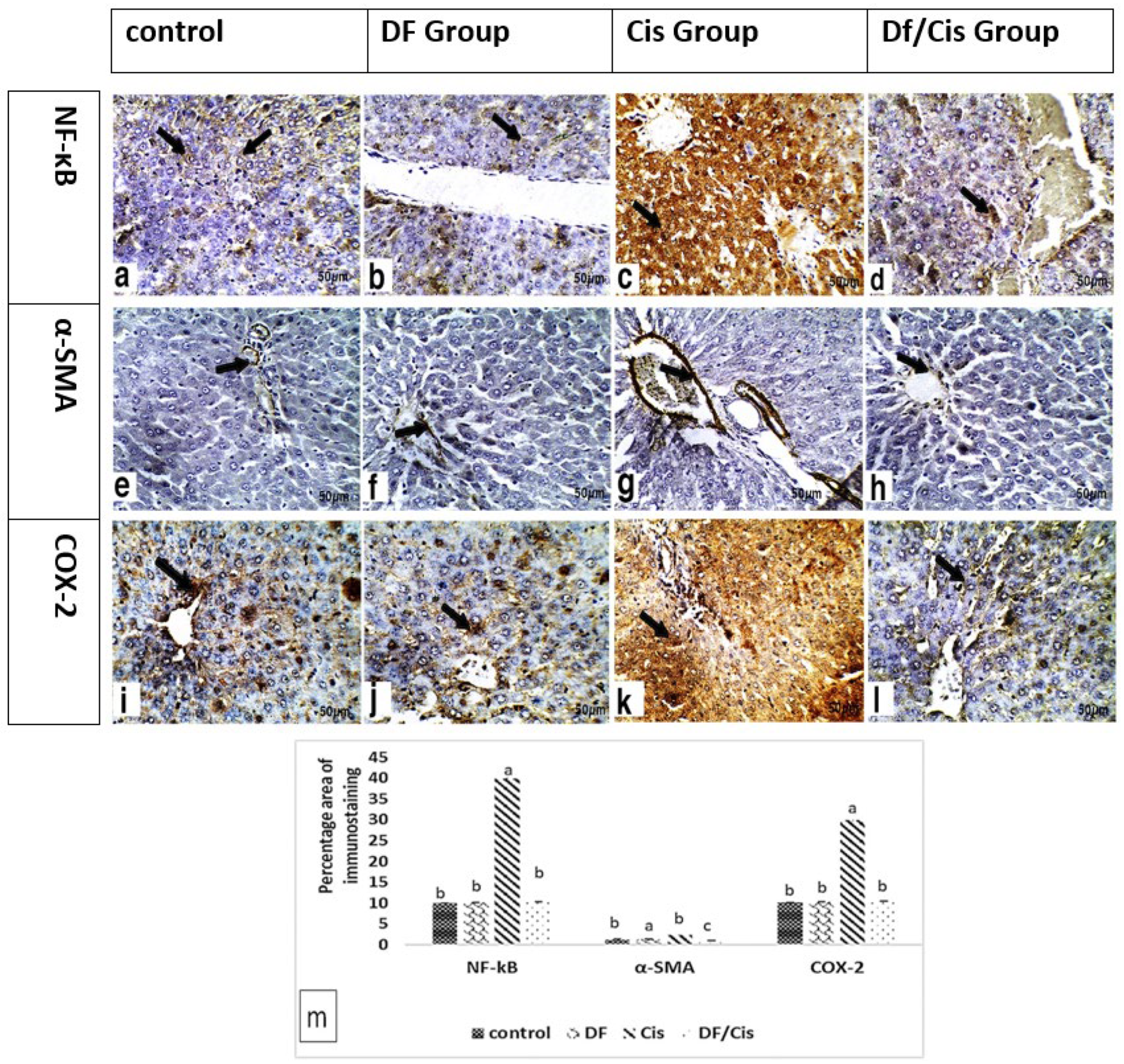
2.10. Hepatic Histology and Immunohistochemistry
2.11. Statistical Analysis
3. Results
3.1. LC-MS/MS Analysis
3.2. Relative Liver Weights
3.3. Serum Biochemical Analysis
3.4. Hepatic ADH and NADPH
3.5. Oxidative Stress Markers, Antioxidant Activity, Interleukin-12, and Interleukin-10
3.6. Histology and Immunohistochemistry of Liver
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- El-Sayyad, H.I.; Ismail, M.; Shalaby, F.M.; Abou-El-Magd, R.F.; Gaur, R.L.; Fernando, A.; Raj, M.H.G.; Ouhtit, A. Histopathological effects of cisplatin, doxorubicin and 5-flurouracil (5-FU) on the liver of male albino rats. Int. J. Biol. Sci. 2009, 5, 466–473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dasari, S.; Tchounwou, P.B. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zlatanova, J.; Yaneva, J.; Leuba, S. Proteins that specifically recognize cisplatin-damaged DNA: A clue to anticancer activity of cisplatin. FASEB J. 1998, 12, 791–799. [Google Scholar] [CrossRef] [PubMed]
- Weiss, R.B.; Christian, M.C. New Cisplatin Analogues in Development. Drugs 1993, 46, 360–377. [Google Scholar] [CrossRef] [PubMed]
- Shen, D.-W.; Pouliot, L.M.; Hall, M.D.; Gottesman, M.M. Cisplatin Resistance: A Cellular Self-Defense Mechanism Resulting from Multiple Epigenetic and Genetic Changes. Pharmacol. Rev. 2012, 64, 706–721. [Google Scholar] [CrossRef] [Green Version]
- Ishida, S.; Lee, J.; Thiele, D.J.; Herskowitz, I. Uptake of the anticancer drug cisplatin mediated by the copper transporter Ctr1 in yeast and mammals. Proc. Natl. Acad. Sci. USA 2002, 99, 14298–14302. [Google Scholar] [CrossRef] [Green Version]
- Saad, S.Y.; Najjar, T.A.; Alashari, M. Role of non-selective adenosine receptor blockade and phosphodiesterase inhibition in cisplatin-induced nephrogonadal toxicity in rats. Clin. Exp. Pharmacol. Physiol. 2004, 31, 862–867. [Google Scholar] [CrossRef]
- Cuadrado, A.; Lafarga, V.; Cheung, P.C.F.; Dolado, I.; Llanos, S.; Cohen, P.; Nebreda, A.R. A new p38 MAP kinase-regulated transcriptional coactivator that stimulates p53-dependent apoptosis. EMBO J. 2007, 26, 2115–2126. [Google Scholar] [CrossRef]
- Chant, I.D. Heat Shock Protein Expression and Apoptosis in Myeloid Leukaemias; University of Warwick: Coventry, UK, 1999. [Google Scholar]
- Omar, H.M.; Ahmed, E.A.; Abdel-Ghafar, S.; Mohammed, S.; Nasser, A.Y. Hepatoprotective effects of vitamin C, DPPD, and L-cysteine against cisplatin-induced oxidative stress in male rats. J. Biol. Earth Sci. 2012, 2, 28–36. [Google Scholar]
- Brozovic, A.; Ambriović-Ristov, A.; Osmak, M. The relationship between cisplatin-induced reactive oxygen species, glutathione, and BCL-2 and resistance to cisplatin. Crit. Rev. Toxicol. 2010, 40, 347–359. [Google Scholar] [CrossRef]
- Bentli, R.; Parlakpinar, H.; Polat, A.; Samdanci, E.; Sarihan, M.E.; Sagir, M. Molsidomine prevents cisplatin-induced hepato-toxicity. Arch. Med. Res. 2013, 44, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Cersosimo, R.J. Hepatotoxicity Associated with Cisplatin Chemotherapy. Ann. Pharmacother. 1993, 27, 438–441. [Google Scholar] [CrossRef]
- Rashid, N.A.; Halim, S.A.S.A.; Teoh, S.L.; Budin, S.B.; Hussan, F.; Ridzuan, N.R.A.; Jalil, N.A.A. The role of natural antioxidants in cisplatin-induced hepatotoxicity. Biomed. Pharmacother. 2021, 144, 112328. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Liu, X.; Yang, X.; Jin, B.; Shao, C.; Kang, W.; Tang, R. Nanomaterial-Based Organelles Protect Normal Cells against Chemotherapy-Induced Cytotoxicity. Adv. Mater. 2018, 30, 1801304. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, J.K.; Panchal, H.; Saraf, P. Ameliorating Effects of Natural Antioxidant Compounds on Female Infertility: A Review. Reprod. Sci. 2021, 28, 1227–1256. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, H.I.; El-Beltagi, H.S.; Jain, S.M.; Al-Khayri, J.M. Date Palm (Phoenix dactylifera L.) Secondary Metabolites: Bioactivity and Pharmaceutical Potential. In Phytomedicine; Elsevier: Amsterdam, The Netherlands, 2021; pp. 483–531. [Google Scholar]
- Abuowf, I.A.A.; Abuowf, A. Hepatoprotective Activity of Date Palm (Phoenix dactylifera) Pollen Grains in Rats; University of Khartoum: Khartoum, Sudan, 2009. [Google Scholar]
- Younas, A.; Naqvi, S.A.; Khan, M.R.; Shabbir, M.A.; Jatoi, M.A.; Anwar, F.; Inam-Ur-Raheem, M.; Saari, N.; Aadil, R.M. Functional food and nutra-pharmaceutical perspectives of date (Phoenix dactylifera L.) fruit. J. Food Biochem. 2020, 44, e13332. [Google Scholar] [CrossRef]
- Okwuosa, C.N.; Udeani, T.K.; Umeifekwem, J.E.; Conuba, E.; Anioke, I.; Madubueze, R.E. Hepatoprotective effect of meth-anolic fruit extracts of Phoenix dactylifera (Arecaceae) on thioacetamide induced liver damage in rats. Am. J. Phytomed. Clinl. Ther. 2014, 2, 290–300. [Google Scholar]
- Al-Ghasham, A.; Ata, H.S.; El-Deep, S.; Meki, A.-R.; Shehada, S. Study of protective effect of date and Nigella sativa on af-latoxin B1 toxicity. Int. J. Health Sci. 2008, 2, 26. [Google Scholar]
- Taleb, H.; Maddocks, S.E.; Morris, R.K.; Kanekanian, A.D. Chemical characterisation and the anti-inflammatory, anti-angiogenic and antibacterial properties of date fruit (Phoenix dactylifera L.). J. Ethnopharmacol. 2016, 194, 457–468. [Google Scholar] [CrossRef]
- Elsharkawy, A.M.; Mann, D.A. Nuclear factor-κB and the hepatic inflammation-fibrosis-cancer axis. Hepatology 2007, 46, 590–597. [Google Scholar] [CrossRef]
- Al-Rasheed, N.M.; Attia, H.A.; Mohamad, R.A.; Al-Rasheed, N.M.; Al-Amin, M.A.; Al-Onazi, A. Aqueous Date Flesh or Pits Extract Attenuates Liver Fibrosis via Suppression of Hepatic Stellate Cell Activation and Reduction of Inflammatory Cytokines, Transforming Growth Factor-β1 and Angiogenic Markers in Carbon Tetrachloride-Intoxicated Rats. Evid. Based Complement. Altern. Med. 2015, 2015, 1–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomes, L.R.; Terra, L.F.; Wailemann, R.A.; Labriola, L.; Sogayar, M.C. TGF-β1 modulates the homeostasis between MMPs and MMP inhibitors through p38 MAPK and ERK1/2 in highly invasive breast cancer cells. BMC Cancer 2012, 12, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Attia, H.; Al-Rasheed, N.; Mohamad, R.; Al-Rasheed, N.; Al-Amin, M. The antifibrotic and fibrolytic properties of date fruit extract via modulation of genotoxicity, tissue-inhibitor of metalloproteinases and nuclear factor- kappa B pathway in a rat model of hepatotoxicity. BMC Complement. Altern. Med. 2016, 16, 414. [Google Scholar] [CrossRef] [Green Version]
- El-Mousalamy, A.M.; Hussein, A.A.M.; Mahmoud, S.A.; Shaker, A.A.A.G. Aqueous and Methanolic Extracts of Palm Date Seeds and Fruits (Phoenix dactylifera) Protects against Diabetic Nephropathy in Type II Diabetic Rats. Biochem. Physiol. Open Access 2016, 5, 2–5. [Google Scholar] [CrossRef]
- Hegazy, M.M.; Metwaly, A.M.; Mostafa, A.E.; Radwan, M.M.; Mehany, A.B.M.; Ahmed, E.; Enany, S.; Magdeldin, S.; Afifi, W.M.; ElSohly, M.A. Biological and chemical evaluation of some African plants belonging to Kalanchoe species: Antitrypa-nosomal, cytotoxic, antitopoisomerase I activities and chemical profiling using ultra-performance liquid chromatog-raphy/quadrupole-time-of-flight mass spectrometer. Pharmacogn. Mag. 2021, 17, 6. [Google Scholar]
- Mohammed, H.A.; Khan, R.A.; Abdel-Hafez, A.A.; Abdel-Aziz, M.; Ahmed, E.; Enany, S.; Mahgoub, S.; Al-Rugaie, O.; Al-sharidah, M.; Aly, M.S. Phytochemical Profiling, In Vitro and In Silico Anti-Microbial and Anti-Cancer Activity Evaluations and Staph GyraseB and h-TOP-IIβ Receptor-Docking Studies of Major Constituents of Zygophyllum coccineum L. Aque-ous-Ethanolic Extract and Its Subsequent Fractions: An Approach to Validate Traditional Phytomedicinal Knowledge. Molecules 2021, 26, 577. [Google Scholar] [PubMed]
- National Research Council. Guide for the Care and use of Laboratory Animals; The National Academies Press: Washington, DC, USA, 2010.
- Afsar, T.; Razak, S.; Almajwal, A.; Khan, M.R. Modulatory influence of Acacia hydaspica R. Parker ethyl acetate extract against cisplatin inveigled hepatic injury and dyslipidemia in rats. BMC Complement. Altern. Med. 2017, 17, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Van Asselt, E.; Choudhary, M.; Clavica, F.; van Mastrigt, R. Urethane anesthesia in acute lower urinary tract studies in the male rat. Lab. Anim. 2017, 51, 256–263. [Google Scholar] [CrossRef]
- Mossa, A.-T.; Swelam, E.S.; Mohafrash, S. Sub-chronic exposure to fipronil induced oxidative stress, biochemical and histopathological changes in the liver and kidney of male albino rats. Toxicol. Rep. 2015, 2, 775–784. [Google Scholar] [CrossRef] [Green Version]
- Bergmeyer, H.U.; Hørder, M.; Rej, R. International Federation of Clinical Chemistry (IFCC) Scientific Committee, Analytical Section: Approved recommendation (1985) on IFCC methods for the measurement of catalytic concentration of enzymes. Part 2. IFCC method for aspartate aminotransferase (L-aspartate: 2-oxoglutarate aminotransferase, EC 2.6.1.1). J. Clin. Chem. Clin. Biochem./Z. Fur Klin. Chem. Und Klin. 1986, 24, 497–510. [Google Scholar]
- Liu, Y.; Zhao, P.; Cheng, M.; Yu, L.; Cheng, Z.; Fan, L.; Chen, C. AST to ALT ratio and arterial stiffness in non-fatty liver Japanese population:a secondary analysis based on a cross-sectional study. Lipids Health Dis. 2018, 17, 275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szasz, G. A kinetic photometric method for serum gamma-glutamyl transpeptidase. Clin. Chem. 1969, 15, 124–136. [Google Scholar] [CrossRef] [PubMed]
- De Leeuw-Israel, F.; Arp-Neefjes, J.; Hollander, C. Quantitative determination of albumin in microlitre amounts of rat serum: With a short note on serum albumin levels in ageing rats. Exp. Gerontol. 1967, 2, 255–260. [Google Scholar] [CrossRef]
- Debro, J.R.; Tarver, H.; Korner, A. The determination of serum albumin and globulin by a new method. J. Lab. Clin. Med. 1957, 50, 728–732. [Google Scholar] [PubMed]
- Skurský, L.; Kovář, J.; Štachová, M. A sensitive photometric assay for alcohol dehydrogenase activity in blood serum. Anal. Biochem. 1979, 99, 65–71. [Google Scholar] [CrossRef]
- Wagner, T.; Scott, M. Single Extraction Method for the Spectrophotometric Quantification of Oxidized and Reduced Pyridine Nucleotides in Erythrocytes. Anal. Biochem. 1994, 222, 417–426. [Google Scholar] [CrossRef]
- Richard, M.J.; Portal, B.; Meo, J.; Coudray, C.; Hadjian, A.; Favier, A. Malondialdehyde Kit Evaluated for Determining Plasma and Lipoprotein Fractions that React with Thiobarbituric Acid. Clin. Chem. 1992, 38, 704–709. [Google Scholar] [CrossRef]
- Mesquita, C.S.; Oliveira, R.; Bento, F.; Geraldo, D.; Rodrigues, J.V.; Marcos, J.C. Simplified 2, 4-dinitrophenylhydrazine spec-trophotometric assay for quantification of carbonyls in oxidized proteins. Anal. Biochem. 2014, 458, 69–71. [Google Scholar] [CrossRef]
- Yim, M.; Chock, P.; Stadtman, E. Enzyme function of copper, zinc superoxide dismutase as a free radical generator. J. Biol. Chem. 1993, 268, 4099–4105. [Google Scholar] [CrossRef]
- Brunt, E.M.; Janney, C.G.; Di Bisceglie, A.M.; Neuschwander-Tetri, B.A.; Bacon, B.R. Nonalcoholic steatohepatitis: A proposal for grading and staging the histological lesions. Am. J. Gastroenterol. 1999, 94, 2467–2474. [Google Scholar] [CrossRef]
- Shi, S.-R.; Liu, C.; Taylor, C.R. Standardization of Immunohistochemistry for Formalin-fixed, Paraffin-embedded Tissue Sections Based on the Antigen-retrieval Technique: From Experiments to Hypothesis. J. Histochem. Cytochem. 2006, 55, 105–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiong, Y.; Zhang, P.; Warner, R.D.; Fang, Z. Sorghum Grain: From Genotype, Nutrition, and Phenolic Profile to Its Health Benefits and Food Applications. Compr. Rev. Food Sci. Food Saf. 2019, 18, 2025–2046. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Djaoudene, O.; Mansinhos, I.; Gonçalves, S.; Jara-Palacios, M.J.; Bey, M.B.; Romano, A. Phenolic profile, antioxidant activity and enzyme inhibitory capacities of fruit and seed extracts from different Algerian cultivars of date (Phoenix dactylifera L.) were affected by in vitro simulated gastrointestinal digestion. South Afr. J. Bot. 2021, 137, 133–148. [Google Scholar] [CrossRef]
- Babiaka, S.B.; Nia, R.; Abuga, K.O.; Mbah, J.A.; Nziko, V.D.P.N.; Paper, D.H.; Ntie-Kang, F. Antioxidant potential of flavonoid glycosides from Manniophyton fulvum Müll. (Euphorbiaceae): Identification and molecular modeling. Sci. Afr. 2020, 8, e00423. [Google Scholar] [CrossRef]
- Baldisserotto, A.; Malisardi, G.; Scalambra, E.; Andreotti, E.; Romagnoli, C.; Vicentini, C.B.; Manfredini, S.; Vertuani, S. Synthesis, Antioxidant and Antimicrobial Activity of a New Phloridzin Derivative for Dermo-Cosmetic Applications. Molecules 2012, 17, 13275–13289. [Google Scholar] [CrossRef] [Green Version]
- Sangha, G.; Kaur, K.; Khera, K.; Singh, B. Toxicological effects of cypermethrin on female albino rats. Toxicol. Int. 2011, 18, 5–8. [Google Scholar] [CrossRef] [Green Version]
- Hussain, M.I.; Farooq, M.; Syed, Q.A. Nutritional and biological characteristics of the date palm fruit (Phoenix dactylifera L.)—A review. Food Biosci. 2020, 34, 100509. [Google Scholar] [CrossRef]
- Guo, C.; Yang, J.; Wei, J.; Li, Y.; Xu, J.; Jiang, Y. Antioxidant activities of peel, pulp and seed fractions of common fruits as determined by FRAP assay. Nutr. Res. 2003, 23, 1719–1726. [Google Scholar] [CrossRef]
- Saleh, E.A.; Tawfik, M.S.; Abu-Tarboush, H.M. Phenolic contents and antioxidant activity of various date palm (Phoenix dactylifera L.) fruits from Saudi Arabia. Food Nutr. Sci. 2011, 2, 1134–1141. [Google Scholar]
- Giannini, E.G.; Testa, R.; Savarino, V. Liver enzyme alteration: A guide for clinicians. Can. Med Assoc. J. 2005, 172, 367–379. [Google Scholar] [CrossRef] [Green Version]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative stress and antioxidant defense. World Allergy Or-Ganization J. 2012, 5, 9–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cichoż-Lach, H.; Michalak, A. Oxidative stress as a crucial factor in liver diseases. World J. Gastroenterol. 2014, 20, 8082. [Google Scholar] [CrossRef] [PubMed]
- Palipoch, S.; Punsawad, C. Biochemical and Histological Study of Rat Liver and Kidney Injury Induced by Cisplatin. J. Toxicol. Pathol. 2013, 26, 293–299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Man, Q.; Deng, Y.; Li, P.; Ma, J.; Yang, Z.; Yang, X.; Zhou, Y.; Yan, X. Licorice Ameliorates Cisplatin-Induced Hepatotoxicity Through Antiapoptosis, Antioxidative Stress, Anti-Inflammation, and Acceleration of Metabolism. Front. Pharmacol. 2020, 11, 1702. [Google Scholar] [CrossRef]
- Bano, N.; Najam, R. Histopathological and biochemical assessment of liver damage in albino Wistar rats treated with cytotoxic platinum compounds in combination with 5-fluorouracil. Arch. Med. Sci. 2019, 15, 1092–1103. [Google Scholar] [CrossRef]
- Taghizadeh, F.; Hosseinimehr, S.J.; Zargari, M.; Malekshah, A.K.; Mirzaei, M.; Amiri, F.T. Alleviation of cisplatin-induced hepatotoxicity by gliclazide: Involvement of oxidative stress and caspase-3 activity. Pharmacol. Res. Perspect. 2021, 9, e00788. [Google Scholar] [CrossRef]
- Rush, G.F.; Gorski, J.R.; Ripple, M.G.; Sowinski, J.; Bugelski, P.; Hewitt, W.R. Organic hydroperoxide-induced lipid peroxidation and cell death in isolated hepatocytes. Toxicol. Appl. Pharmacol. 1985, 78, 473–483. [Google Scholar] [CrossRef]
- Xu, J.; Zhou, H.; Yu, H.; Deng, T.; Wang, Z.; Zhang, H.; Wu, J.; Yang, L. Computational design of highly stable and soluble alcohol dehydrogenase for NADPH regeneration. Bioresour. Bioprocess. 2021, 8, 12. [Google Scholar] [CrossRef]
- Oeckinghaus, A.; Ghosh, S. The NF-B Family of Transcription Factors and Its Regulation. Cold Spring Harb. Perspect. Biol. 2009, 1, a000034. [Google Scholar] [CrossRef]
- Mosser, D.M. The many faces of macrophage activation. J. Leukoc. Biol. 2003, 73, 209–212. [Google Scholar] [CrossRef]
- Haloul, M.; Oliveira, E.R.A.; Kader, M.; Wells, J.Z.; Tominello, T.R.; El Andaloussi, A.; Yates, C.C.; Ismail, N. mTORC1-mediated polarization of M1 macrophages and their accumulation in the liver correlate with immunopathology in fatal ehrlichiosis. Sci. Rep. 2019, 9, 14050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaker, M.E.; Shaaban, A.A.; El-Shafey, M.M.; El-Mesery, M.E. The selective c-Met inhibitor capmatinib offsets cispla-tin-nephrotoxicity and doxorubicin-cardiotoxicity and improves their anticancer efficacies. Toxicol. Appl. Pharmacol. 2020, 398, 115018. [Google Scholar] [CrossRef] [PubMed]
- Clària, J. Cyclooxygenase-2 biology. Curr. Pharm. Des. 2003, 9, 2177–2190. [Google Scholar] [CrossRef] [Green Version]
- Wen, S.-L.; Gao, J.-H.; Yang, W.-J.; Lu, Y.-Y.; Tong, H.; Huang, Z.-Y.; Liu, Z.-X.; Tang, C.-W. Celecoxib attenuates hepatic cirrhosis through inhibition of epithelial-to-mesenchymal transition of hepatocytes. J. Gastroenterol. Hepatol. 2014, 29, 1932–1942. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.M.; Park, K.C.; Kim, H.G.; Han, S.J. Effect of selective cyclooxygenase-2 inhibitor meloxicam on liver fibrosis in rats with ligated common bile ducts. Hepatol. Res. 2008, 38, 800–809. [Google Scholar] [CrossRef]
- Hassan, M.H.; Ghobara, M.M. Antifibrotic effect of meloxicam in rat liver: Role of nuclear factor kappa B, proinflammatory cytokines, and oxidative stress. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2016, 389, 971–983. [Google Scholar] [CrossRef]
- Khalid, S.; Khalid, N.; Khan, R.S.; Ahmed, H.; Ahmad, A. A review on chemistry and pharmacology of Ajwa date fruit and pit. Trends Food Sci. Technol. 2017, 63, 60–69. [Google Scholar] [CrossRef]
- El-Far, A.; Shaheen, H.; Abdel-Daim, M.; Al Jaouni, S.; Mousa, S. Date palm (Phoenix dactylifera): Protection and remedy food. Curr. Trends Nutraceuticals 2016, 1, 1–10. [Google Scholar]
- Gupta, M.; Mazumder, U.; Kumar, R.S.; Sivakumar, T.; Gomathi, P.; Rajeshwar, Y. Antioxidant Defense System Induced by a Methanol Extract of Caesalpinia bonducella. in Rat Liver. Pharm. Biol. 2005, 43, 411–419. [Google Scholar] [CrossRef]
- Omar, H.A.; Mohamed, W.; Arab, H.; Arafa, E.-S. Tangeretin Alleviates Cisplatin-Induced Acute Hepatic Injury in Rats: Targeting MAPKs and Apoptosis. PLoS ONE 2016, 11, e0151649. [Google Scholar] [CrossRef]
- Wang, Y.-P.; Cheng, M.-L.; Zhang, B.-F.; Mu, M.; Zhou, M.-Y.; Wu, J.; Li, C.-X. Effect of blueberry on hepatic and immunological functions in mice. Hepatobiliary Pancreat. Dis. Int. 2010, 9, 164–168. [Google Scholar] [PubMed]
- Singab, A.N.B.; Youssef, D.; Noaman, E.; Kotb, S. Hepatoprotective effect of flavonol glycosides rich fraction from Egyptian Vicia calcarata desf. Against CCI4-induced liver damage in rats. Arch. Pharmacal Res. 2005, 28, 791–798. [Google Scholar] [CrossRef] [PubMed]
- Babaeenezhad, E.; Nouryazdan, N.; Nasri, M.; Ahmadvand, H.; Sarabi, M.M. Cinnamic acid ameliorate gentamicin-induced liver dysfunctions and nephrotoxicity in rats through induction of antioxidant activities. Heliyon 2021, 7, e07465. [Google Scholar] [CrossRef] [PubMed]
- Arslan, Ö.; Ekinci Akdemir, F. Antioxidant effect of trans-3 hydroxycinnamic acid against liver damage methotrexate induced in rats. J. Anim. Plant Sci. 2018, 28, 1574–1578. [Google Scholar]
- Hussein, A.M.; El-Mousalamy, A.M.; Hussein, S.A.; Mahmoud, S.A. Effects of palm dates (Phoenix dactylifera L.) extracts on hepatic dysfunctions in Type 2 diabetic rat model. World J. Pharm. Pharm. Sci. 2015, 4, 62–79. [Google Scholar]
- Wali, A.F.; Rashid, S.; Rashid, S.M.; Ansari, M.A.; Khan, M.R.; Haq, N.; Alhareth, D.Y.; Ahmad, A.; Rehman, M.U. Naringenin Regulates Doxorubicin-Induced Liver Dysfunction: Impact on Oxidative Stress and Inflammation. Plants 2020, 9, 550. [Google Scholar] [CrossRef]
- Hakiminia, B.; Goudarzi, A.; Moghaddas, A. Has vitamin E any shreds of evidence in cisplatin-induced toxicity. J. Biochem. Mol. Toxicol. 2019, 33, e22349. [Google Scholar] [CrossRef]
- Rubin, R.; Strayer, D.S.; Rubin, E. Rubin’s Pathology: Clinicopathologic Foundations of Medicine; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2008. [Google Scholar]
- Paget, G.; Barnes, J. Toxicity Tests. In Evaluation of Drug Activities: Pharmacometrics; Elsevier: Amsterdam, The Netherlands, 1964; Volume 1, pp. 135–165. [Google Scholar]

| # | RT (min) | Name | Precursor m/z | Error ppm | Adduct | Formula | Class | MS/MS Spectrum |
|---|---|---|---|---|---|---|---|---|
| 1 | 1.22 | 3′,4′,5,7-tetrahydroxyflavanone | 289.0819 | −2.2 | [M+H]+ | C15H12O6 | Flavanones | 127.04 [C6H6O3]+H+, 135.04 [C8H8O2-H]+, 149.06 [C9H7O2+H]+H+ |
| 2 | 1.22 | 4′,5,7-Trihydroxy-3-methoxyflavanone | 303.095 | 0.8 | [M+H]+ | C16H14O6 | Flavonoids | 122.1 [C7H6O2]+, 136.1 [C7H4O3]+, 166.1 [C9H10O3]+, 213.1 [C13H9O3]+, 256.1 [C15H10O4+H]+H+ |
| 3 | 1.27 | Kojibiose | 341.1921 | 22.7 | [M-H]− | C12H22O11 | Fatty Acyls | 89.1 [C3H4O3]+H+, 161.1 [C6H10O5-H]+, 179.1 [C6H11O6]+, 221.1 [C8H13O7]+ |
| 4 | 1.27 | trans-Cinnamate | 147.0445 | −97.6 | [M-H]− | C9H8O2 | Cinnamic acids | 87.1 [C7H6-2H]-H−, 103.028 [C8H7]−, 129.1 [C9H7O-H]-H− |
| 5 | 1.39 | Myricetin | 319.037 | 1.4 | [M+H]+ | C15H10O8 | Flavonols | 137.1 [C7H4O3]+H+, 200.1 [C11H5O4-H]+, 214.1 [C12H6O4]+, 229.1 [C12H6O5-H]+, 301.1 [C15H9O7]+ |
| 6 | 1.53 | Xanthine | 151.0233 | 12.4 | [M-H]− | C5H4N4O2 | Xanthines | 71.1 [C2HNO2]+, 108.1 [C4H3N3O-H]+ |
| 7 | 1.65 | Traumatic acid | 229.1516 | 8.2 | [M+H]+ | C12H20O4 | Fatty Acyls | 58.1 [C2H3O2-H]+, 114.1 [C6H9O2]+H+, 139.1 [C10H18]+H+, 142.1 [C8H13O2]+H+ |
| 8 | 1.84 | p-Coumaric acid | 163.0393 | 0 | [M-H]− | C9H8O3 | Hydroxycinnamic acids | 117.1 [C8H6O-H]+, 119.1 [C8H7O]+ |
| 9 | 1.97 | Piperidine | 86.06009 | 0.4 | [M+H]+ | C5H11N | Piperidines | 69.1 [C5H10-H]+ |
| 10 | 2.04 | Ferulic acid | 193.0497 | 2.1 | [M-H]− | C10H10O4 | Hydroxycinnamic acids | 134.1 [C8H6O2]−, 149.1 [C9H9O2]−, 178.1 [C9H7O4]-H− |
| 11 | 2.92 | Isookanin-7-glucoside | 449.0983 | 21.1 | [M-H]− | C21H22O11 | Flavonoid-7-O-glycosides | 259.1 [C14H11O5]−, 287.1 [C15H11O6]− |
| 12 | 5.59 | Kaempferol-3-O-alpha-L-rhamnoside | 431.1888 | 6 | [M-H]− | C21H20O10 | Flavonoid-3-O-glycosides | 179.1 [C9H5O4+2H]−, 223.1 [C10H5O6+2H]−, 294.01 [C14H16O7-H]-H−, 362.1 [C18H17O8+H]−, 385.1 [C20H17O8]− |
| 13 | 5.79 | Phlorizin | 435.1859 | 3.3 | [M-H]− | C21H24O10 | Flavonoid O-glycosides | 258.1 [C15H13O4+H]−, 298.1 [C13H14O8]−, 389.1 [C20H20O8+H]− |
| 14 | 6.44 | Delphinidin-3-O-beta-glucopyranoside | 463.0886 | −0.1 | [M-2H]− | C21H21O12 | Anthocyanidin-3-O-glycosides | 300.1 [C15H10O7]-2H−, 354.1 [C18H14O8-2H]-2H−, 394.1 [C18H18O10]− |
| 15 | 6.79 | Kaempferol-7-neohesperidoside | 593.151 | 0.3 | [M-H]− | C27H30O15 | Flavonoid-7-O-glycosides | 285.1 [C15H9O6]− |
| 16 | 6.86 | cyanidin-3-O-rutinoside | 595.1702 | −4.8 | [M]+ | C27H31O15 | Anthocyanidin-3-O-glycosides | 287.1 [C15H12O6-H]+, 449.1 [C21H22O11-H]+ |
| 17 | 6.94 | Hyperoside (Quercetin 3-galactoside) | 465.1897 | −3.1 | [M+H]+ | C21H20O12 | Flavonoid-3-O-glycosides | 85.028 [C4H7O2-H]-H−, 303.1 [C15H10O7+H]− |
| 18 | 6.94 | Isoquercitrin | 465.1053 | −2.4 | [M+H]+ | C21H20O12 | Flavonoid-3-O-glycosides | 145.1 [C6H10O4-H]+, 303.1 [C15H9O7+H]+H+ |
| 19 | 6.99 | Kuromanin (Cyanidin-3-glucoside) | 449.1058 | 5.2 | [M]+ | C21H21O11 | Anthocyanidin-3-O-glycosides | 275.1 [C14H9O6+2H]+, 287.1 [C15H10O6+H]+ |
| 20 | 7.26 | Isorhamnetin-3-O-glucoside | 477.1035 | −0.2 | [M-H]− | C22H22O12 | Flavonoid-3-O-glycosides | 314.1 [C16H11O7]-H−, 364.1 [C17H15O9+H]−, 392.1 [C18H17O10]-H−, 432.1 [C20H16O11]− |
| 21 | 7.51 | Diosmin | 609.1804 | 1.4 | [M+H]+ | C28H32O15 | Flavonoid-7-O-glycosides | 301.1 [C16H11O6+H]+H+, 463.1 [C22H21O11+H]+H+ |
| 22 | 7.53 | Rhoifolin | 577.1542 | 2.1 | [M-H]− | C27H30O14 | Flavonoid-7-O-glycosides | 269.1 [C15H9O5]−, 532.1 [C25H24O13]− |
| 23 | 7.72 | Petunidin-3-O-beta-glucopyranoside | 479.1093 | 15.9 | [M]+ | C22H23O12 | Anthocyanidin-3-O-glycosides | 302.1 [C15H9O7+H]+, 317.1 [C16H12O7+H]+, 371.1 [C19H16O8-H]+ |
| 24 | 7.97 | Peonidine-3-O-glucoside | 463.1237 | 1.4 | [M]+ | C22H23O11 | Anthocyanidin-3-O-glycosides | 301.1 [C16H12O6+H]+, 430.1 [C21H19O10-H]+, 446.1 [C22H22O10]+ |
| 25 | 8.05 | Daphnetin | 179.1064 | 0.8 | [M+H]+ | C9H6O4 | 7,8-dihydroxycoumarins | 91.1 [C6H3O]+, 105.1 [C7H4O]+H+, 133.1 [C8H6O2-H]+, 161.1 [C9H5O3]+ |
| 26 | 11.28 | Kaempferide | 301.0696 | 2.9 | [M+H]+ | C16H12O6 | Flavonols | 258.1 [C14H9O5]+H+, 286.1 [C15H9O6]+H+ |
| Serum Biochemical Parameters | Groups | ||||||
|---|---|---|---|---|---|---|---|
| Control | DF | Cis | DF/Cis | ||||
| Mean ± SE | Percentage Change | Mean ± SE | Percentage Change | Mean ± SE | Percentage Change | ||
| ALT (U/l) | 23.09 ± 0.60 c | 21.30 ± 0.40 c | −7.80% | 31.75 ± 2.30 a | 37.50% | 27.80 ± 2.00 b | 20.30% |
| AST (U/l) | 16.95 ± 2.00 c | 13.87 ± 3.00 c | −18.10% | 27.57 ± 1.90 a | 62.65% | 21.79 ± 0.50 b | 28.50% |
| AST/ALT | 0.74 ± 0.60 a | 0.65 ± 0.40 a | −12.10% | 0.86 ± 0.050 a | 16.20% | 0.79 ± 0.33 a | 6.75% |
| GGT (IU/L) | 15.12 ± 1.20 c | 14.20 ± 1.20 c | −6.00% | 30.90 ± 0.50 a | 104.30% | 23.75 ± 0.80 b | 57.00% |
| Total protein (mg/dL) | 6.69 ± 0.34 ab | 7.11 ± 0.08 a | 6.20% | 5.44 ± 0.41 b | −18.60% | 6.23 ± 0.25 ab | −6.87% |
| Albumin (mg/dL) | 4.03 ± 0.17 ab | 4.26 ± 0.18a | 5.70% | 2.82 ± 0.13 c | −30.00% | 3.44 ± 0.18 bc | −14.60% |
| Globulin (mg/dL) | 2.66 ± 0.10 a | 2.62 ± 0.20 a | −1.50% | 2.62 ± 0.40 a | −1.50% | 2.72 ± 0.30 a | 2.25% |
| ADH (U/g protein) | 29.44 ± 0.40 a | 29.24 ± 1.40 a | −0.67% | 17.27 ± 0.10 c | −41.30% | 26.24 ± 1.40 b | −0.10% |
| NADPH (nmol/mg protein) | 10.80 ± 0.30 a | 11.54 ± 0.70 a | 6.90% | 6.00 ± 0.30 c | −44.40% | 8.78 ± 0.30 b | −1.73% |
| Liver Biochemical Parameters | Groups | ||||||
|---|---|---|---|---|---|---|---|
| Control | DF | Cis | DF/Cis | ||||
| Mean ± SE | Percentage Change | Mean ± SE | Percentage Change | Mean ± SE | Percentage Change | ||
| MDA (nmol/g) | 0.85 ± 0.16 c | 0.73 ± 0.20 c | −14.10% | 2.37 ± 0.20 a | 178.80% | 1.49 ± 0.10 b | 75.20% |
| PCC (nmol/gtissue) | 1.73 ± 0.02 c | 1.58 ± 0.20 c | −8.60% | 4.05 ± 0.10 a | 134.10% | 2.54 ± 0.30 b | 46.80% |
| SOD (U/g protein) | 11.21 ± 0.30 b | 12.89 ± 0.50 a | 14.90% | 7.79 ± 0.02 d | −30.50% | 9.82 ± 0.20 c | −12.30% |
| GSH (mg/g tissue) | 18.95 ± 0.11 a | 20.46 ± 0.70 a | 7.90% | 13.50 ± 0.80 c | −28.70% | 16.53 ± 0.40 b | −12.70% |
| IL-10 (pg/mL) | 57.75 ± 1.20 a | 58.26 ± 0.50 a | 0.80% | 38.87 ± 1.70 c | −0.56% | 43.94 ± 3.10 c | −23.90% |
| IL-12 (pg/mL) | 34.65 ± 1.17 c | 32.24 ± 1.81 c | −6.90% | 71.97 ± 2.10 a | 107.70% | 53.10 ± 2.60 b | 53.20% |
| Groups | |||||
|---|---|---|---|---|---|
| Histological Changes | Degree | Control | DF | Cis | DF/Cis |
| Necrosis | 1 | 0.00 ± 0.00 | 0.00 ± 0.00 | 3.10 ± 0.30 * | 1.10 ± 0.01 * |
| 2 | 0.00 ± 0.00 | 0.00 ± 0.00 | 6.40 ± 0.50 * | 0.00 ± 0.00 | |
| 3 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | |
| Hydropic degeneration | 1 | 0.00 ± 0.00 | 0.00 ± 0.00 | 2.20 ± 2.20 * | 1.00 ± 0.01 * |
| 2 | 0.00 ± 0.00 | 0.00 ± 0.00 | 11.60 ± 0.20 * | 0.00 ± 0.00 | |
| 3 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | |
| Fatty degeneration | 1 | 0.00 ± 0.00 | 0.00 ± 0.00 | 5.80 ± 1.50 * | 0.00 ± 0.00 |
| 2 | 0.00 ± 0.00 | 0.00 ± 0.00 | 1.66 ± 0.70 * | 0.00 ± 0.00 | |
| 3 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | |
| Infiltration of inflammatory cells | 1 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 1.08 ± 0.01 * |
| 2 | 0.00 ± 0.00 | 0.00 ± 0.00 | 2.68 ± 0.80 * | 0.00 ± 0.00 | |
| 3 | 0.00 ± 0.00 | 0.00 ± 0.00 | 8.20 ± 1.11 * | 0.00 ± 0.00 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gad El-Hak, H.N.; Mahmoud, H.S.; Ahmed, E.A.; Elnegris, H.M.; Aldayel, T.S.; Abdelrazek, H.M.A.; Soliman, M.T.A.; El-Menyawy, M.A.I. Methanolic Phoenix dactylifera L. Extract Ameliorates Cisplatin-Induced Hepatic Injury in Male Rats. Nutrients 2022, 14, 1025. https://doi.org/10.3390/nu14051025
Gad El-Hak HN, Mahmoud HS, Ahmed EA, Elnegris HM, Aldayel TS, Abdelrazek HMA, Soliman MTA, El-Menyawy MAI. Methanolic Phoenix dactylifera L. Extract Ameliorates Cisplatin-Induced Hepatic Injury in Male Rats. Nutrients. 2022; 14(5):1025. https://doi.org/10.3390/nu14051025
Chicago/Turabian StyleGad El-Hak, Heba Nageh, Hany Salah Mahmoud, Eman A. Ahmed, Heba M. Elnegris, Tahany Saleh Aldayel, Heba M. A. Abdelrazek, Mohamed T. A. Soliman, and Menna Allah I. El-Menyawy. 2022. "Methanolic Phoenix dactylifera L. Extract Ameliorates Cisplatin-Induced Hepatic Injury in Male Rats" Nutrients 14, no. 5: 1025. https://doi.org/10.3390/nu14051025
APA StyleGad El-Hak, H. N., Mahmoud, H. S., Ahmed, E. A., Elnegris, H. M., Aldayel, T. S., Abdelrazek, H. M. A., Soliman, M. T. A., & El-Menyawy, M. A. I. (2022). Methanolic Phoenix dactylifera L. Extract Ameliorates Cisplatin-Induced Hepatic Injury in Male Rats. Nutrients, 14(5), 1025. https://doi.org/10.3390/nu14051025






