Bioavailability, Efficacy, Safety, and Regulatory Status of Creatine and Related Compounds: A Critical Review
Abstract
:1. Introduction
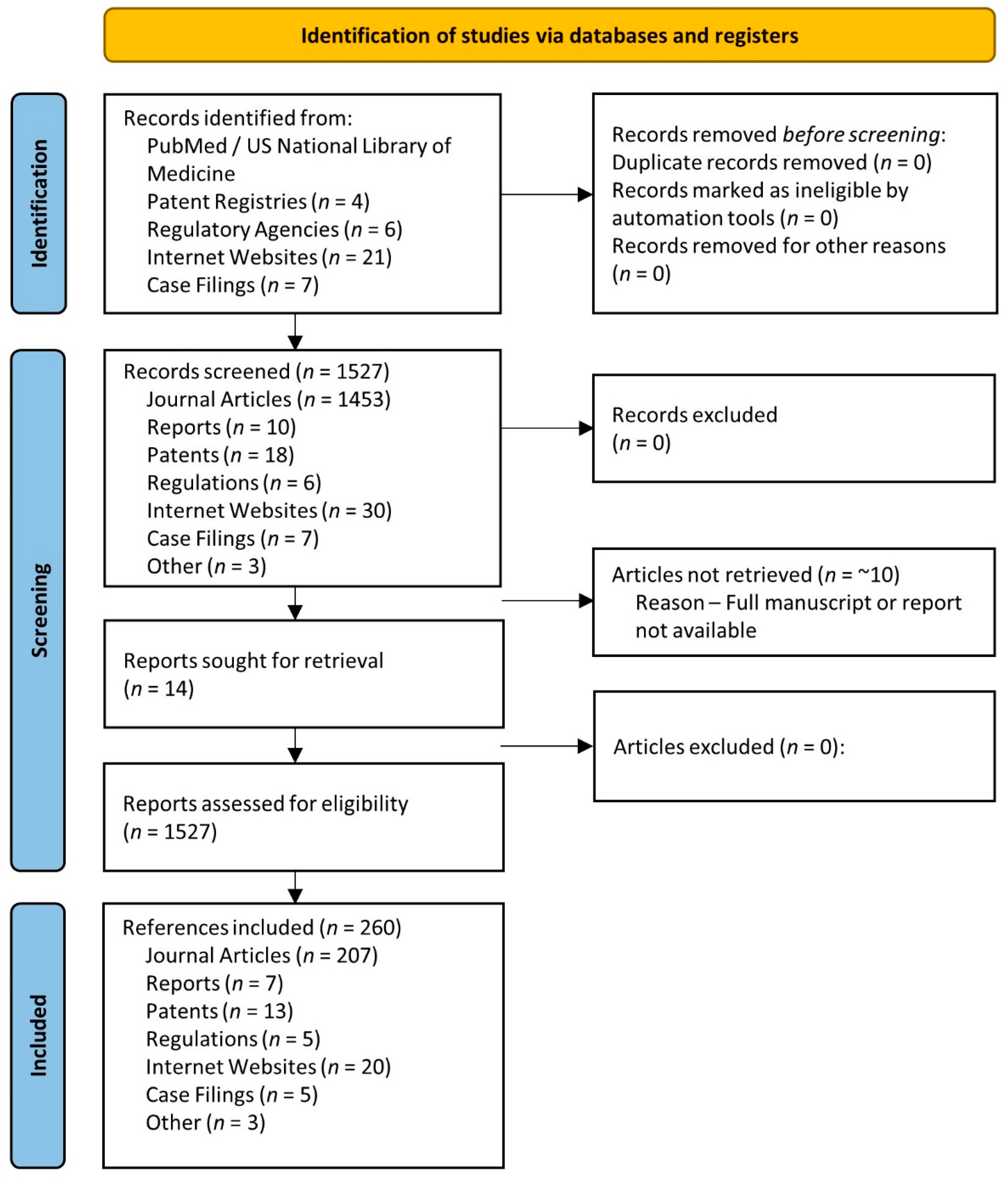
2. Methods
3. Bioavailability
3.1. Methods to Assess Bioavailability
3.1.1. Assess Chemical Structure
3.1.2. Assess Changes in Blood Creatine Content
3.1.3. Assess Changes in Tissue Creatine Content
4. Physio-Chemical Properties
5. Stability
6. Solubility
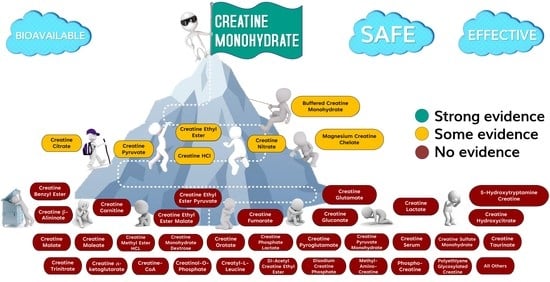
7. Purported Creatine Related Compounds
8. Strong Evidence to Support Bioavailability, Efficacy, and Safety
Creatine Monohydrate
9. Some Evidence to Support Bioavailability, Efficacy, and Safety
9.1. Creatine Salts
9.1.1. Creatine Citrate
9.1.2. Creatine Pyruvate
9.2. Magnesium Creatine Chelate
9.3. Creatine Ethyl Ester
9.4. Creatine HCl
9.5. Creatine Nitrate
9.6. Buffered Creatine Monohydrate
10. No Evidence to Support Bioavailability, Efficacy, and Safety
10.1. Other Creatine Salts
10.2. Creatine Serum
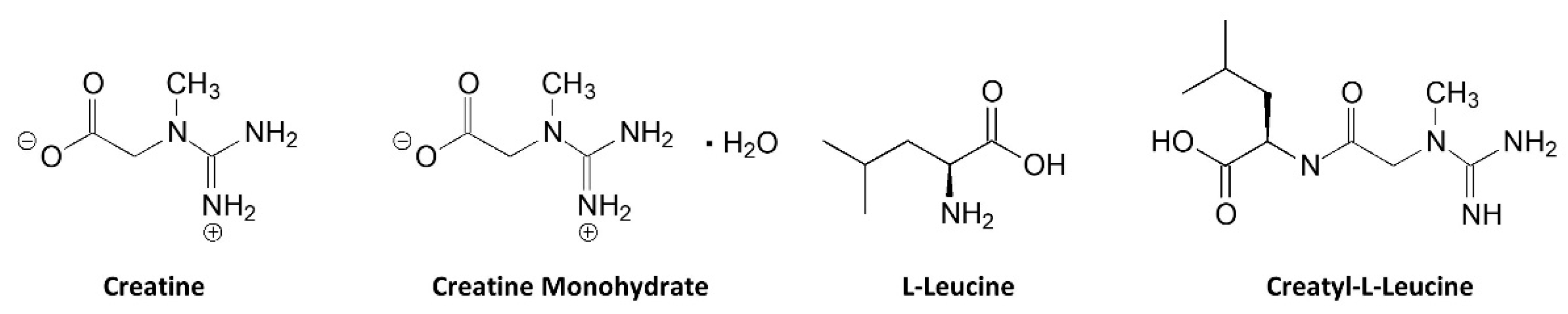
10.3. Creatyl-L-Leucine
10.4. Creatinol-O-Phosphate
11. Regulatory Status
11.1. United States
11.2. International Regulation
11.3. Assessment and Guidance for Industry
- (1)
- Only consider developing creatine supplements that contain a creatine molecule. Alteration of the chemical structure of creatine in any way is assumed to change the chemical activity and biological function and may negate any benefit of creatine supplementation. Additionally, binding creatine to other compounds may prevent creatine from being liberated in vivo, thereby making the form of creatine non-bioavailable or less bioavailable source of creatine.
- (2)
- Companies who develop new forms of creatine should conduct toxicology studies in animals to establish that high dose ingestion is safe and conduct clinical trials in humans to validate safety. We then recommend obtaining FDA GRAS status or Self-Affirming GRAS status.
- (3)
- Pharmacokinetic studies must be performed to show that the novel form of creatine is degraded into creatine and increases blood creatine levels to physiological levels necessary to promote creatine uptake into tissue (e.g., >200–500 µmol/L or 25–65 µg/L).
- (4)
- Bioavailability studies should be conducted to show recommended doses increase muscle and/or brain creatine content.
- (5)
- Placebo, double blind, and randomized clinical trials should be performed to substantiate that the form of creatine provides ergogenic benefit and does not cause any untoward side effects.
- (6)
- Comparative effectiveness trials at recommended and equivalent doses must be performed to show a new form of creatine increases muscle and/or brain creatine content to a greater degree than CrM to substantiate those claims.
- (7)
- Comparative effectiveness trials at recommended and equivalent doses must also be performed to determine if a new form of creatine is more effective and/or a safer alternative to CrM to substantiate those types of claims.
- (8)
- Supplement companies should clearly declare the source and amount of creatine contained in their products so consumers can know if they are taking effective doses.
- (9)
- Claims made about a form of creatine should be based on research conducted on that form of creatine at recommended doses, not untested hypotheses, speculation, assumptions, and/or marketing hyperbole. Such practices only undermine the scientific validity and consumer confidence about creatine supplementation.
- (10)
- Pure CrM is the only source of creatine with strong evidence of bioavailability, efficacy, and safety and considered as GRAS by the FDA, approved for use in the EU and Australia, and evaluated for safety by Health Canada.
- (11)
- Consumers should only consider taking supplements that contain sources of creatine that research has shown is bioavailable, effective, safe, and devoid of impurities.
12. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Paddon-Jones, D.; Borsheim, E.; Wolfe, R.R. Potential ergogenic effects of arginine and creatine supplementation. J. Nutr. 2004, 134, 2888S–2894S, discussion 2895S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brosnan, M.E.; Brosnan, J.T. The role of dietary creatine. Amino Acids 2016, 48, 1785–1791. [Google Scholar] [CrossRef]
- Da Silva, R.P.; Clow, K.; Brosnan, J.T.; Brosnan, M.E. Synthesis of guanidinoacetate and creatine from amino acids by rat pancreas. Br. J. Nutr. 2014, 111, 571–577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Da Silva, R.P.; Nissim, I.; Brosnan, M.E.; Brosnan, J.T. Creatine synthesis: Hepatic metabolism of guanidinoacetate and creatine in the rat in vitro and in vivo. Am. J. Physiol. Endocrinol. Metab. 2009, 296, E256–E261. [Google Scholar] [CrossRef]
- Kreider, R.B.; Stout, J.R. Creatine in Health and Disease. Nutrients 2021, 13, 447. [Google Scholar] [CrossRef]
- Wallimann, T.; Tokarska-Schlattner, M.; Schlattner, U. The creatine kinase system and pleiotropic effects of creatine. Amino Acids 2011, 40, 1271–1296. [Google Scholar] [CrossRef] [Green Version]
- Bonilla, D.A.; Kreider, R.B.; Stout, J.R.; Forero, D.A.; Kerksick, C.M.; Roberts, M.D.; Rawson, E.S. Metabolic Basis of Creatine in Health and Disease: A Bioinformatics-Assisted Review. Nutrients 2021, 13, 1238. [Google Scholar] [CrossRef] [PubMed]
- Buford, T.W.; Kreider, R.B.; Stout, J.R.; Greenwood, M.; Campbell, B.; Spano, M.; Ziegenfuss, T.; Lopez, H.; Landis, J.; Antonio, J. International Society of Sports Nutrition position stand: Creatine supplementation and exercise. J. Int. Soc. Sports Nutr. 2007, 4, 6. [Google Scholar] [CrossRef] [Green Version]
- Kreider, R.B.; Jung, Y.P. Creatine supplementation in exercise, sport, and medicine. J. Exerc. Nutr. Biochem. 2011, 15, 53–69. [Google Scholar] [CrossRef]
- Balsom, P.D.; Soderlund, K.; Ekblom, B. Creatine in humans with special reference to creatine supplementation. Sports Med. 1994, 18, 268–280. [Google Scholar] [CrossRef]
- Hultman, E.; Soderlund, K.; Timmons, J.A.; Cederblad, G.; Greenhaff, P.L. Muscle creatine loading in men. J. Appl. Physiol. 1996, 81, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Harris, R.C.; Soderlund, K.; Hultman, E. Elevation of creatine in resting and exercised muscle of normal subjects by creatine supplementation. Clin. Sci. 1992, 83, 367–374. [Google Scholar] [CrossRef] [Green Version]
- Kreider, R.B.; Melton, C.; Rasmussen, C.J.; Greenwood, M.; Lancaster, S.; Cantler, E.C.; Milnor, P.; Almada, A.L. Long-term creatine supplementation does not significantly affect clinical markers of health in athletes. Mol. Cell. Biochem. 2003, 244, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Kerksick, C.M.; Wilborn, C.D.; Roberts, M.D.; Smith-Ryan, A.; Kleiner, S.M.; Jager, R.; Collins, R.; Cooke, M.; Davis, J.N.; Galvan, E.; et al. ISSN exercise & sports nutrition review update: Research & recommendations. J. Int. Soc. Sports Nutr. 2018, 15, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kreider, R.B.; Kalman, D.S.; Antonio, J.; Ziegenfuss, T.N.; Wildman, R.; Collins, R.; Candow, D.G.; Kleiner, S.M.; Almada, A.L.; Lopez, H.L. International Society of Sports Nutrition position stand: Safety and efficacy of creatine supplementation in exercise, sport, and medicine. J. Int. Soc. Sports Nutr. 2017, 14, 18. [Google Scholar] [CrossRef] [PubMed]
- Meyers, S. Sports nutrition market growth watch. In Natural Products Insidier; Informa Exhibitions: Irving, TX, USA, 2016. [Google Scholar]
- Thomas, D.T.; Erdman, K.A.; Burke, L.M. Position of the Academy of Nutrition and Dietetics, Dietitians of Canada, and the American College of Sports Medicine: Nutrition and Athletic Performance. J. Acad. Nutr. Diet 2016, 116, 501–528. [Google Scholar] [CrossRef] [PubMed]
- Maughan, R.J.; Burke, L.M.; Dvorak, J.; Larson-Meyer, D.E.; Peeling, P.; Phillips, S.M.; Rawson, E.S.; Walsh, N.P.; Garthe, I.; Geyer, H.; et al. IOC Consensus Statement: Dietary Supplements and the High-Performance Athlete. Int. J. Sport Nutr. Exerc. Metab. 2018, 28, 104–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casey, A.; Constantin-Teodosiu, D.; Howell, S.; Hultman, E.; Greenhaff, P.L. Creatine ingestion favorably affects performance and muscle metabolism during maximal exercise in humans. Am. J. Physiol. 1996, 271, E31–E37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Green, A.L.; Hultman, E.; Macdonald, I.A.; Sewell, D.A.; Greenhaff, P.L. Carbohydrate ingestion augments skeletal muscle creatine accumulation during creatine supplementation in humans. Am. J. Physiol. 1996, 271, E821–E826. [Google Scholar] [CrossRef]
- Green, A.L.; Simpson, E.J.; Littlewood, J.J.; Macdonald, I.A.; Greenhaff, P. Carbohydrate ingestion augments creatine retention during creatine feeding in humans. Acta Physiol. Scand. 1996, 158, 195–202. [Google Scholar] [CrossRef]
- Greenwood, M.; Kreider, R.B.; Earnest, C.P.; Rasmussen, C.; Almada, A. Differences in creatine retention among three nutritional formulations of oral creatine supplements. J. Exerc. Physiol. Online 2003, 6, 37–43. [Google Scholar]
- Steenge, G.R.; Simpson, E.J.; Greenhaff, P.L. Protein- and carbohydrate-induced augmentation of whole body creatine retention in humans. J. Appl. Physiol. 2000, 89, 1165–1171. [Google Scholar] [CrossRef] [PubMed]
- Wax, B.; Kerksick, C.M.; Jagim, A.R.; Mayo, J.J.; Lyons, B.C.; Kreider, R.B. Creatine for Exercise and Sports Performance, with Recovery Considerations for Healthy Populations. Nutrients 2021, 13, 1915. [Google Scholar] [CrossRef]
- Jager, R.; Purpura, M.; Shao, A.; Inoue, T.; Kreider, R.B. Analysis of the efficacy, safety, and regulatory status of novel forms of creatine. Amino Acids 2011, 40, 1369–1383. [Google Scholar] [CrossRef] [Green Version]
- Kreider, R.B.; Willoughby, D.S.; Greenwood, M.; Parise, G.; Payne, E.; Tarnopolsky, M.A. Effects of serum creatine supplementation on muscle creatine content. J. Exerc. Physiol. Online 2003, 6, 24–33. [Google Scholar]
- Spillane, M.; Schoch, R.; Cooke, M.; Harvey, T.; Greenwood, M.; Kreider, R.; Willoughby, D.S. The effects of creatine ethyl ester supplementation combined with heavy resistance training on body composition, muscle performance, and serum and muscle creatine levels. J. Int. Soc. Sports Nutr. 2009, 6, 6. [Google Scholar] [CrossRef] [Green Version]
- Jagim, A.R.; Oliver, J.M.; Sanchez, A.; Galvan, E.; Fluckey, J.; Riechman, S.; Greenwood, M.; Kelly, K.; Meininger, C.; Rasmussen, C.; et al. A buffered form of creatine does not promote greater changes in muscle creatine content, body composition, or training adaptations than creatine monohydrate. J. Int. Soc. Sports Nutr. 2012, 9, 43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galvan, E.; Walker, D.K.; Simbo, S.Y.; Dalton, R.; Levers, K.; O’Connor, A.; Goodenough, C.; Barringer, N.D.; Greenwood, M.; Rasmussen, C.; et al. Acute and chronic safety and efficacy of dose dependent creatine nitrate supplementation and exercise performance. J. Int. Soc. Sports Nutr. 2016, 13, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abraham, S.; Jiang, S. Process for Preparing a Creatine Heterocyclic Acid Salt and Method of Use. U.S. Patent No. 6,838,562, 4 January 2005. [Google Scholar]
- Negrisoli, G.; Del Corona, L. Hydrosoluble Organic Salts of Creatine. U.S. Patent No. 5,973,199, Application No. 08/649,620, 26 October 1999. [Google Scholar]
- Pischel, I.; Weiss, S. New Creatine Pyruvate Derivatives from Crystallisation in Polar Solvents. Germany Patent WO1998028263A1, 20 December 1996. [Google Scholar]
- Pischel, I.; Weiss, S.; Gloxhuber, C.; Mertschenk, B. Creatine Ascorbates and a Method of Producing Them. U.S. Patent No. 5,863,939, 26 January 1999. [Google Scholar]
- Child, R.; Tallon, M.J. Creatine ethyl ester rapidly degrades to creatinine in stomach acid. In Proceedings of the International Society of Sports Nutrition 4th Annual Meeting, Las Vegas, NV, USA, 12 June 2007. [Google Scholar]
- Giese, M.W.; Lecher, C.S. Non-enzymatic cyclization of creatine ethyl ester to creatinine. Biochem. Biophys. Res. Commun. 2009, 388, 252–255. [Google Scholar] [CrossRef]
- Dalton, R.L.; Sowinski, R.J.; Grubic, T.J.; Collins, P.B.; Coletta, A.M.; Reyes, A.G.; Sanchez, B.; Koozehchian, M.; Jung, Y.P.; Rasmussen, C.; et al. Hematological and Hemodynamic Responses to Acute and Short-Term Creatine Nitrate Supplementation. Nutrients 2017, 9, 1359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gill, N.D.; Hall, R.D.; Blazevich, A.J. Creatine serum is not as effective as creatine powder for improving cycle sprint performance in competitive male team-sport athletes. J. Strength Cond. Res. 2004, 18, 272–275. [Google Scholar] [CrossRef] [PubMed]
- Harris, R.C.; Almada, A.L.; Harris, D.B.; Dunnett, M.; Hespel, P. The creatine content of Creatine Serum and the change in the plasma concentration with ingestion of a single dose. J. Sports Sci. 2004, 22, 851–857. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Schumann, K.; Classen, H.G.; Hages, M.; Prinz-Langenohl, R.; Pietrzik, K.; Biesalski, H.K. Bioavailability of oral vitamins, minerals, and trace elements in perspective. Arzneimittelforschung 1997, 47, 369–380. [Google Scholar] [PubMed]
- Blancquaert, L.; Baguet, A.; Bex, T.; Volkaert, A.; Everaert, I.; Delanghe, J.; Petrovic, M.; Vervaet, C.; De Henauw, S.; Constantin-Teodosiu, D.; et al. Changing to a vegetarian diet reduces the body creatine pool in omnivorous women, but appears not to affect carnitine and carnosine homeostasis: A randomised trial. Br. J. Nutr. 2018, 119, 759–770. [Google Scholar] [CrossRef]
- Watt, K.K.; Garnham, A.P.; Snow, R.J. Skeletal muscle total creatine content and creatine transporter gene expression in vegetarians prior to and following creatine supplementation. Int. J. Sport Nutr. Exerc. Metab. 2004, 14, 517–531. [Google Scholar] [CrossRef] [PubMed]
- Forbes, S.C.; Candow, D.G.; Ostojic, S.M.; Roberts, M.D.; Chilibeck, P.D. Meta-Analysis Examining the Importance of Creatine Ingestion Strategies on Lean Tissue Mass and Strength in Older Adults. Nutrients 2021, 13, 1912. [Google Scholar] [CrossRef]
- Candow, D.G.; Forbes, S.C.; Kirk, B.; Duque, G. Current Evidence and Possible Future Applications of Creatine Supplementation for Older Adults. Nutrients 2021, 13, 745. [Google Scholar] [CrossRef] [PubMed]
- Harris, R.C.; Nevill, M.; Harris, D.B.; Fallowfield, J.L.; Bogdanis, G.C.; Wise, J.A. Absorption of creatine supplied as a drink, in meat or in solid form. J. Sports Sci. 2002, 20, 147–151. [Google Scholar] [CrossRef]
- Kaviani, M.; Shaw, K.; Chilibeck, P.D. Benefits of Creatine Supplementation for Vegetarians Compared to Omnivorous Athletes: A Systematic Review. Int. J. Environ. Res. Public Health 2020, 17, 3041. [Google Scholar] [CrossRef]
- Vandenberghe, K.; Goris, M.; Van Hecke, P.; Van Leemputte, M.; Vangerven, L.; Hespel, P. Long-term creatine intake is beneficial to muscle performance during resistance training. J. Appl. Physiol. 1997, 83, 2055–2063. [Google Scholar] [CrossRef]
- Jäger, R.; Purpura, M.; Harris, R.C. Reduction of plasma creatine concentrations as an indicator of improved bioavailability. J. Int. Soc. Sports Nutr. 2016, 13, 3–4. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez, N.R.; DiMarco, N.M.; Langley, S.; American Dietetic Association; Dietitians of Canada; American College of Sports Medicine: Nutrition and Athletic Performance. Position of the American Dietetic Association, Dietitians of Canada, and the American College of Sports Medicine: Nutrition and athletic performance. J. Am. Diet. Assoc. 2009, 109, 509–527. [Google Scholar] [PubMed]
- Pischel, I.; Gastner, T. Creatine--its chemical synthesis, chemistry, and legal status. Subcell. Biochem. 2007, 46, 291–307. [Google Scholar] [CrossRef]
- Jäger, R. The Use of Creatine Monohydrate in Sports Nutrition. Degussa BioActives Publications: Freising, Germany, 2003. [Google Scholar]
- Howard, A.N.; Harris, R.C. Compositions Containing Creatine. U.S. Patent No. 5,969,544, Application No. 08/866,517, 19 October 1999. [Google Scholar]
- Edgar, G.; Shiver, H.E. The equilibrium between creatine and creatinine, in aqueous solution: The effect of hydrogen ion. J. Am. Chem. Soc. 1925, 47, 1179–1188. [Google Scholar] [CrossRef]
- Cannon, J.G.; Orencole, S.F.; Fielding, R.A.; Meydani, M.; Meydani, S.N.; Fiatarone, M.A.; Blumberg, J.B.; Evans, W.J. Acute phase reponse in exercise: Interaction of age and vitamin E on neutrophils and muscle enzyme release. Am. J. Physiol. 1990, 259, R1214–R1219. [Google Scholar]
- Kreider, R.B.; Ferreira, M.; Wilson, M.; Grindstaff, P.; Plisk, S.; Reinardy, J.; Cantler, E.; Almada, A.L. Effects of creatine supplementation on body composition, strength, and sprint performance. Med. Sci. Sports Exerc. 1998, 30, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Parandak, K.; Petersen, B.L. Shelf-Stable Nitrogenous Organic Acid Compositions. U.S. Patent Application No. 16/164,762, 5 January 2019. [Google Scholar]
- CreaBev™: Soluble and Stable Creatine Monohydrate for Superior Performance Beverages. Available online: https://www.glanbianutritionals.com/en/nutri-knowledge-center/nutritional-resources/creabevtm (accessed on 5 January 2022).
- Persky, A.M.; Brazeau, G.A.; Hochhaus, G. Pharmacokinetics of the dietary supplement creatine. Clin. Pharmacokinet. 2003, 42, 557–574. [Google Scholar] [CrossRef] [PubMed]
- Deldicque, L.; Decombaz, J.; Zbinden Foncea, H.; Vuichoud, J.; Poortmans, J.R.; Francaux, M. Kinetics of creatine ingested as a food ingredient. Eur. J. Appl. Physiol. 2008, 102, 133–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greenhaff, P.; Bodin, K.; Harris, R.; Hultman, E.; Jones, D.G.; McIntyre, D.; Soderlund, K.; Turner, D.L. The influence of oral creatine supplementation on muscle phosphocreatine resynthesis following intenst contraction in man. J. Physiol. 1993, 467, 75P. [Google Scholar]
- Reddy, A.; Norris, D.F.; Momeni, S.S.; Waldo, B.; Ruby, J.D. The pH of beverages in the United States. J. Am. Dent. Assoc. 2016, 147, 255–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antonio, J.; Candow, D.G.; Forbes, S.C.; Gualano, B.; Jagim, A.R.; Kreider, R.B.; Rawson, E.S.; Smith-Ryan, A.E.; VanDusseldorp, T.A.; Willoughby, D.S.; et al. Common questions and misconceptions about creatine supplementation: What does the scientific evidence really show? J. Int. Soc. Sports Nutr. 2021, 18, 13. [Google Scholar] [CrossRef] [PubMed]
- Jäger, R.; Harris, R.C.; Purpura, M.; Francaux, M. Comparison of new forms of creatine in raising plasma creatine levels. J. Int. Soc. Sports Nutr. 2007, 4, 17. [Google Scholar] [CrossRef] [Green Version]
- Miller, D.W.; Vennerstrom, J.L.; Faulkner, M.C. Creatine Oral Supplementation Using Creatine Hydrochloride Salt. U.S. Patent No. 7,608,641 B2, Application No. 10/846,782, 27 October 2009. [Google Scholar]
- França, E.; Avelar, B.; Yoshioka, C.; Santana, J.; Madureira, D.; Rocha, L.; Zocoler, C.; Rossi, F.; Lira, F.; Rodrigues, B.; et al. Creatine HCl and Creatine Monohydrate Improve Strength but Only Creatine HCl Induced Changes on Body Composition in Recreational Weightlifters. Food Nutr. Sci. 2015, 6, 1624–1630. [Google Scholar] [CrossRef] [Green Version]
- Howard, A.N.; Harris, R. Compositions Containing Creatine and Aloe Vera Extract. U.S. Patent No. 6,168,802, 2 June 1999. [Google Scholar]
- Federal Food, Drug, and Cosmetic Act, 413(a) [21 U.S.C. 350b], United States Congress, 15 October 1994. Available online: https://uscode.house.gov/view.xhtml?req=(title:21%20section:350b%20edition:prelim (accessed on 7 January 2022).
- Nelson, A.G.; Arnall, D.A.; Kokkonen, J.; Day, R.; Evans, J. Muscle glycogen supercompensation is enhanced by prior creatine supplementation. Med. Sci. Sports Exerc. 2001, 33, 1096–1100. [Google Scholar] [CrossRef]
- Tarnopolsky, M.A.; Parise, G. Direct measurement of high-energy phosphate compounds in patients with neuromuscular disease. Muscle Nerve 1999, 22, 1228–1233. [Google Scholar] [CrossRef]
- McKenna, M.J.; Morton, J.; Selig, S.E.; Snow, R.J. Creatine supplementation increases muscle total creatine but not maximal intermittent exercise performance. J. Appl. Physiol. 1999, 87, 2244–2252. [Google Scholar] [CrossRef] [PubMed]
- Greenhaff, P.L.; Bodin, K.; Soderlund, K.; Hultman, E. Effect of oral creatine supplementation on skeletal muscle phosphocreatine resynthesis. Am. J. Physiol. 1994, 266, E725–E730. [Google Scholar] [CrossRef]
- Choi, J.K.; Kustermann, E.; Dedeoglu, A.; Jenkins, B.G. Magnetic resonance spectroscopy of regional brain metabolite markers in FALS mice and the effects of dietary creatine supplementation. Eur. J. Neurosci. 2009, 30, 2143–2150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lyoo, I.K.; Kong, S.W.; Sung, S.M.; Hirashima, F.; Parow, A.; Hennen, J.; Cohen, B.M.; Renshaw, P.F. Multinuclear magnetic resonance spectroscopy of high-energy phosphate metabolites in human brain following oral supplementation of creatine-monohydrate. Psychiatry Res. 2003, 123, 87–100. [Google Scholar] [CrossRef]
- Roschel, H.; Gualano, B.; Ostojic, S.M.; Rawson, E.S. Creatine Supplementation and Brain Health. Nutrients 2021, 13, 586. [Google Scholar] [CrossRef]
- Dolan, E.; Gualano, B.; Rawson, E.S. Beyond muscle: The effects of creatine supplementation on brain creatine, cognitive processing, and traumatic brain injury. Eur. J. Sport Sci. 2019, 19, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Gualano, B.; Rawson, E.S.; Candow, D.G.; Chilibeck, P.D. Creatine supplementation in the aging population: Effects on skeletal muscle, bone and brain. Amino Acids 2016, 48, 1793–1805. [Google Scholar] [CrossRef] [PubMed]
- Rawson, E.S.; Venezia, A.C. Use of creatine in the elderly and evidence for effects on cognitive function in young and old. Amino Acids 2011, 40, 1349–1362. [Google Scholar] [CrossRef] [PubMed]
- Cornish, S.M.; Chilibeck, P.D.; Burke, D.G. The effect of creatine monohydrate supplementation on sprint skating in ice-hockey players. J. Sports Med. Phys. Fit. 2006, 46, 90–98. [Google Scholar]
- Dawson, B.; Vladich, T.; Blanksby, B.A. Effects of 4 weeks of creatine supplementation in junior swimmers on freestyle sprint and swim bench performance. J. Strength Cond. Res. 2002, 16, 485–490. [Google Scholar]
- Grindstaff, P.D.; Kreider, R.; Bishop, R.; Wilson, M.; Wood, L.; Alexander, C.; Almada, A. Effects of creatine supplementation on repetitive sprint performance and body composition in competitive swimmers. Int. J. Sport Nutr. 1997, 7, 330–346. [Google Scholar] [CrossRef]
- Juhasz, I.; Gyore, I.; Csende, Z.; Racz, L.; Tihanyi, J. Creatine supplementation improves the anaerobic performance of elite junior fin swimmers. Acta Physiol. Hung. 2009, 96, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.J.; Machado Reis, V.; Guidetti, L.; Bessone Alves, F.; Mota, P.; Freitas, J.; Baldari, C. Effect of creatine on swimming velocity, body composition and hydrodynamic variables. J. Sports Med. Phys. Fit. 2007, 47, 58–64. [Google Scholar]
- Stone, M.H.; Sanborn, K.; Smith, L.L.; O’Bryant, H.S.; Hoke, T.; Utter, A.C.; Johnson, R.L.; Boros, R.; Hruby, J.; Pierce, K.C.; et al. Effects of in-season (5 weeks) creatine and pyruvate supplementation on anaerobic performance and body composition in American football players. Int. J. Sport Nutr. 1999, 9, 146–165. [Google Scholar] [CrossRef] [PubMed]
- Bemben, M.G.; Bemben, D.A.; Loftiss, D.D.; Knehans, A.W. Creatine supplementation during resistance training in college football athletes. Med. Sci. Sports Exerc. 2001, 33, 1667–1673. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, J.; Ratamess, N.; Kang, J.; Mangine, G.; Faigenbaum, A.; Stout, J. Effect of creatine and beta-alanine supplementation on performance and endocrine responses in strength/power athletes. Int. J. Sport Nutr. Exerc. Metab. 2006, 16, 430–446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chilibeck, P.D.; Magnus, C.; Anderson, M. Effect of in-season creatine supplementation on body composition and performance in rugby union football players. Appl. Physiol. Nutr. Metab. 2007, 32, 1052–1057. [Google Scholar] [CrossRef] [PubMed]
- Claudino, J.G.; Mezencio, B.; Amaral, S.; Zanetti, V.; Benatti, F.; Roschel, H.; Gualano, B.; Amadio, A.C.; Serrao, J.C. Creatine monohydrate supplementation on lower-limb muscle power in Brazilian elite soccer players. J. Int. Soc. Sports Nutr. 2014, 11, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kerksick, C.M.; Rasmussen, C.; Lancaster, S.; Starks, M.; Smith, P.; Melton, C.; Greenwood, M.; Almada, A.; Kreider, R. Impact of differing protein sources and a creatine containing nutritional formula after 12 weeks of resistance training. Nutrition 2007, 23, 647–656. [Google Scholar] [CrossRef]
- Kerksick, C.M.; Wilborn, C.D.; Campbell, W.I.; Harvey, T.M.; Marcello, B.M.; Roberts, M.D.; Parker, A.G.; Byars, A.G.; Greenwood, L.D.; Almada, A.L.; et al. The effects of creatine monohydrate supplementation with and without D-pinitol on resistance training adaptations. J. Strength Cond. Res. 2009, 23, 2673–2682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Volek, J.S.; Kraemer, W.J.; Bush, J.A.; Boetes, M.; Incledon, T.; Clark, K.L.; Lynch, J.M. Creatine supplementation enhances muscular performance during high-intensity resistance exercise. J. Am. Diet. Assoc. 1997, 97, 765–770. [Google Scholar] [CrossRef]
- Volek, J.S.; Mazzetti, S.A.; Farquhar, W.B.; Barnes, B.R.; Gomez, A.L.; Kraemer, W.J. Physiological responses to short-term exercise in the heat after creatine loading. Med. Sci. Sports Exerc. 2001, 33, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Volek, J.S.; Ratamess, N.A.; Rubin, M.R.; Gomez, A.L.; French, D.N.; McGuigan, M.M.; Scheett, T.P.; Sharman, M.J.; Hakkinen, K.; Kraemer, W.J. The effects of creatine supplementation on muscular performance and body composition responses to short-term resistance training overreaching. Eur. J. Appl. Physiol. 2004, 91, 628–637. [Google Scholar] [CrossRef]
- Kreider, R.B.; Wilborn, C.D.; Taylor, L.; Campbell, B.; Almada, A.L.; Collins, R.; Cooke, M.; Earnest, C.P.; Greenwood, M.; Kalman, D.S.; et al. ISSN exercise & sport nutrition review: Research & recommendations. J. Int. Soc. Sports Nutr. 2010, 7, 7. [Google Scholar] [CrossRef] [Green Version]
- Branch, J.D. Effect of creatine supplementation on body composition and performance: A meta-analysis. Int. J. Sport Nutr. Exerc. Metab. 2003, 13, 198–226. [Google Scholar] [CrossRef] [PubMed]
- Devries, M.C.; Phillips, S.M. Creatine supplementation during resistance training in older adults-a meta-analysis. Med. Sci. Sports Exerc. 2014, 46, 1194–1203. [Google Scholar] [CrossRef]
- Lanhers, C.; Pereira, B.; Naughton, G.; Trousselard, M.; Lesage, F.X.; Dutheil, F. Creatine Supplementation and Lower Limb Strength Performance: A Systematic Review and Meta-Analyses. Sports Med. 2015, 45, 1285–1294. [Google Scholar] [CrossRef] [PubMed]
- Wiroth, J.B.; Bermon, S.; Andrei, S.; Dalloz, E.; Hebuterne, X.; Dolisi, C. Effects of oral creatine supplementation on maximal pedalling performance in older adults. Eur. J. Appl. Physiol. 2001, 84, 533–539. [Google Scholar] [CrossRef] [PubMed]
- McMorris, T.; Mielcarz, G.; Harris, R.C.; Swain, J.P.; Howard, A. Creatine supplementation and cognitive performance in elderly individuals. Neuropsychol. Dev. Cogn. B Aging Neuropsychol. Cogn. 2007, 14, 517–528. [Google Scholar] [CrossRef] [PubMed]
- Rawson, E.S.; Clarkson, P.M. Acute creatine supplementation in older men. Int. J. Sports Med. 2000, 21, 71–75. [Google Scholar] [CrossRef]
- Tarnopolsky, M.A. Potential benefits of creatine monohydrate supplementation in the elderly. Curr. Opin. Clin. Nutr. Metab. Care 2000, 3, 497–502. [Google Scholar] [CrossRef] [PubMed]
- Aguiar, A.F.; Januario, R.S.; Junior, R.P.; Gerage, A.M.; Pina, F.L.; do Nascimento, M.A.; Padovani, C.R.; Cyrino, E.S. Long-term creatine supplementation improves muscular performance during resistance training in older women. Eur. J. Appl. Physiol. 2013, 113, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Kreider, R.B. Effects of creatine supplementation on performance and training adaptations. Mol. Cell. Biochem. 2003, 244, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Gualano, B.; Macedo, A.R.; Alves, C.R.; Roschel, H.; Benatti, F.B.; Takayama, L.; de Sa Pinto, A.L.; Lima, F.R.; Pereira, R.M. Creatine supplementation and resistance training in vulnerable older women: A randomized double-blind placebo-controlled clinical trial. Exp. Gerontol. 2014, 53, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Candow, D.G.; Little, J.P.; Chilibeck, P.D.; Abeysekara, S.; Zello, G.A.; Kazachkov, M.; Cornish, S.M.; Yu, P.H. Low-dose creatine combined with protein during resistance training in older men. Med. Sci. Sports Exerc. 2008, 40, 1645–1652. [Google Scholar] [CrossRef]
- Hass, C.J.; Collins, M.A.; Juncos, J.L. Resistance training with creatine monohydrate improves upper-body strength in patients with Parkinson disease: A randomized trial. Neurorehabilit. Neural Repair 2007, 21, 107–115. [Google Scholar] [CrossRef]
- Candow, D.G.; Chilibeck, P.D. Effect of creatine supplementation during resistance training on muscle accretion in the elderly. J. Nutr. Health Aging 2007, 11, 185–188. [Google Scholar]
- Chilibeck, P.D.; Chrusch, M.J.; Chad, K.E.; Shawn Davison, K.; Burke, D.G. Creatine monohydrate and resistance training increase bone mineral content and density in older men. J. Nutr. Health Aging 2005, 9, 352–353. [Google Scholar]
- Burke, D.G.; Chilibeck, P.D.; Parise, G.; Candow, D.G.; Mahoney, D.; Tarnopolsky, M. Effect of creatine and weight training on muscle creatine and performance in vegetarians. Med. Sci. Sports Exerc. 2003, 35, 1946–1955. [Google Scholar] [CrossRef] [PubMed]
- Wilder, N.; Gilders, R.; Hagerman, F.; Deivert, R.G. The effects of a 10-week, periodized, off-season resistance-training program and creatine supplementation among collegiate football players. J. Strength Cond. Res. 2002, 16, 343–352. [Google Scholar] [PubMed]
- Izquierdo, M.; Ibanez, J.; Gonzalez-Badillo, J.J.; Gorostiaga, E.M. Effects of creatine supplementation on muscle power, endurance, and sprint performance. Med. Sci. Sports Exerc. 2002, 34, 332–343. [Google Scholar] [CrossRef] [PubMed]
- Chrusch, M.J.; Chilibeck, P.D.; Chad, K.E.; Davison, K.S.; Burke, D.G. Creatine supplementation combined with resistance training in older men. Med. Sci. Sports Exerc. 2001, 33, 2111–2117. [Google Scholar] [CrossRef] [PubMed]
- Becque, M.D.; Lochmann, J.D.; Melrose, D.R. Effects of oral creatine supplementation on muscular strength and body composition. Med. Sci. Sports Exerc. 2000, 32, 654–658. [Google Scholar] [CrossRef] [PubMed]
- Volek, J.S.; Duncan, N.D.; Mazzetti, S.A.; Staron, R.S.; Putukian, M.; Gomez, A.L.; Pearson, D.R.; Fink, W.J.; Kraemer, W.J. Performance and muscle fiber adaptations to creatine supplementation and heavy resistance training. Med. Sci. Sports Exerc. 1999, 31, 1147–1156. [Google Scholar] [CrossRef] [PubMed]
- Ahmun, R.P.; Tong, R.J.; Grimshaw, P.N. The effects of acute creatine supplementation on multiple sprint cycling and running performance in rugby players. J. Strength Cond. Res. 2005, 19, 92–97. [Google Scholar] [CrossRef]
- Cox, G.; Mujika, I.; Tumilty, D.; Burke, L. Acute creatine supplementation and performance during a field test simulating match play in elite female soccer players. Int. J. Sport Nutr. Exerc. Metab. 2002, 12, 33–46. [Google Scholar] [CrossRef]
- Preen, D.; Dawson, B.; Goodman, C.; Lawrence, S.; Beilby, J.; Ching, S. Effect of creatine loading on long-term sprint exercise performance and metabolism. Med. Sci. Sports Exerc. 2001, 33, 814–821. [Google Scholar] [CrossRef]
- Aaserud, R.; Gramvik, P.; Olsen, S.R.; Jensen, J. Creatine supplementation delays onset of fatigue during repeated bouts of sprint running. Scand. J. Med. Sci. Sports 1998, 8, 247–251. [Google Scholar] [CrossRef]
- Bosco, C.; Tihanyi, J.; Pucspk, J.; Kovacs, I.; Gabossy, A.; Colli, R.; Pulvirenti, G.; Tranquilli, C.; Foti, C.; Viru, M.; et al. Effect of oral creatine supplementation on jumping and running performance. Int. J. Sports Med. 1997, 18, 369–372. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Campillo, R.; Gonzalez-Jurado, J.A.; Martinez, C.; Nakamura, F.Y.; Penailillo, L.; Meylan, C.M.; Caniuqueo, A.; Canas-Jamet, R.; Moran, J.; Alonso-Martinez, A.M.; et al. Effects of plyometric training and creatine supplementation on maximal-intensity exercise and endurance in female soccer players. J. Sci. Med. Sport 2016, 19, 682–687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yanez-Silva, A.; Buzzachera, C.F.; Picarro, I.D.; Januario, R.S.; Ferreira, L.H.; McAnulty, S.R.; Utter, A.C.; Souza-Junior, T.P. Effect of low dose, short-term creatine supplementation on muscle power output in elite youth soccer players. J. Int. Soc. Sports Nutr. 2017, 14, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dabidi Roshan, V.; Babaei, H.; Hosseinzadeh, M.; Arendt-Nielsen, L. The effect of creatine supplementation on muscle fatigue and physiological indices following intermittent swimming bouts. J. Sports Med. Phys. Fit. 2013, 53, 232–239. [Google Scholar]
- Selsby, J.T.; Beckett, K.D.; Kern, M.; Devor, S.T. Swim performance following creatine supplementation in Division III athletes. J. Strength Cond. Res. 2003, 17, 421–424. [Google Scholar] [PubMed]
- Leenders, N.M.; Lamb, D.R.; Nelson, T.E. Creatine supplementation and swimming performance. Int. J. Sport Nutr. 1999, 9, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Peyrebrune, M.C.; Nevill, M.E.; Donaldson, F.J.; Cosford, D.J. The effects of oral creatine supplementation on performance in single and repeated sprint swimming. J. Sports Sci. 1998, 16, 271–279. [Google Scholar] [CrossRef]
- Lamontagne-Lacasse, M.; Nadon, R.; Goulet, E.D. Effect of creatine supplementation on jumping performance in elite volleyball players. Int. J. Sports Physiol. Perform. 2011, 6, 525–533. [Google Scholar] [CrossRef]
- Ayoama, R.; Hiruma, E.; Sasaki, H. Effects of creatine loading on muscular strength and endurance of female softball players. J. Sports Med. Phys. Fit. 2003, 43, 481–487. [Google Scholar]
- Jones, A.M.; Atter, T.; Georg, K.P. Oral creatine supplementation improves multiple sprint performance in elite ice-hockey players. J. Sports Med. Phys. Fit. 1999, 39, 189–196. [Google Scholar] [CrossRef]
- Ziegenfuss, T.N.; Habowski, S.M.; Lemieux, R.; Sandrock, J.E.; Kedia, A.W.; Kerksick, C.M.; Lopez, H.L. Effects of a dietary supplement on golf drive distance and functional indices of golf performance. J. Int. Soc. Sports Nutr. 2015, 12, 4. [Google Scholar] [CrossRef] [Green Version]
- Tarnopolsky, M.A.; MacLennan, D.P. Creatine monohydrate supplementation enhances high-intensity exercise performance in males and females. Int. J. Sport Nutr. Exerc. Metab. 2000, 10, 452–463. [Google Scholar] [CrossRef]
- Ziegenfuss, T.N.; Rogers, M.; Lowery, L.; Mullins, N.; Mendel, R.; Antonio, J.; Lemon, P. Effect of creatine loading on anaerobic performance and skeletal muscle volume in NCAA Division I athletes. Nutrition 2002, 18, 397–402. [Google Scholar] [CrossRef]
- Benton, D.; Donohoe, R. The influence of creatine supplementation on the cognitive functioning of vegetarians and omnivores. Br. J. Nutr. 2011, 105, 1100–1105. [Google Scholar] [CrossRef] [Green Version]
- Johannsmeyer, S.; Candow, D.G.; Brahms, C.M.; Michel, D.; Zello, G.A. Effect of creatine supplementation and drop-set resistance training in untrained aging adults. Exp. Gerontol. 2016, 83, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Smith-Ryan, A.E.; Cabre, H.E.; Eckerson, J.M.; Candow, D.G. Creatine Supplementation in Women’s Health: A Lifespan Perspective. Nutrients 2021, 13, 877. [Google Scholar] [CrossRef]
- Greenwood, M.; Kreider, R.B.; Rasmussen, C.; Almada, A.L.; Earnest, C.P. D-Pinitol augments whole body creatine retention in man. J. Exerc. Physiol. Online 2001, 4, 41–47. [Google Scholar]
- Jäger, R.; Kendrick, I.; Purpura, M.; Harris, R.; Ribnicky, D.; Pischel, I. The effect of Russian Tarragon (Artemisia dracunculus L.) on the plasma creatine concentration with creatine monohydrate administration. J. Int. Soc. Sports Nutr. 2008, 5, P4. [Google Scholar] [CrossRef] [Green Version]
- Taylor, L.; Poole, C.; Pena, E.; Lewing, M.; Kreider, R.; Foster, C.; Wilborn, C. Effects of Combined Creatine Plus Fenugreek Extract vs. Creatine Plus Carbohydrate Supplementation on Resistance Training Adaptations. J. Sports Sci. Med. 2011, 10, 254–260. [Google Scholar] [PubMed]
- Oliver, J.M.; Jagim, A.R.; Pischel, I.; Jager, R.; Purpura, M.; Sanchez, A.; Fluckey, J.; Riechman, S.; Greenwood, M.; Kelly, K.; et al. Effects of short-term ingestion of Russian Tarragon prior to creatine monohydrate supplementation on whole body and muscle creatine retention and anaerobic sprint capacity: A preliminary investigation. J. Int. Soc. Sports Nutr. 2014, 11, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pakulak, A.; Candow, D.G.; Totosy de Zepetnek, J.; Forbes, S.C.; Basta, D. Effects of Creatine and Caffeine Supplementation During Resistance Training on Body Composition, Strength, Endurance, Rating of Perceived Exertion and Fatigue in Trained Young Adults. J. Diet. Suppl. 2021, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Van Bavel, D.; de Moraes, R.; Tibirica, E. Effects of dietary supplementation with creatine on homocysteinemia and systemic microvascular endothelial function in individuals adhering to vegan diets. Fundam. Clin. Pharmacol. 2019, 33, 428–440. [Google Scholar] [CrossRef]
- Moraes, R.; Van Bavel, D.; Moraes, B.S.; Tibirica, E. Effects of dietary creatine supplementation on systemic microvascular density and reactivity in healthy young adults. Nutr. J. 2014, 13, 115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Falk, D.J.; Heelan, K.A.; Thyfault, J.P.; Koch, A.J. Effects of effervescent creatine, ribose, and glutamine supplementation on muscular strength, muscular endurance, and body composition. J. Strength Cond. Res. 2003, 17, 810–816. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, M.; Farris, J.; Kreider, R.; Greenwood, L.; Byars, A. Creatine supplementation patterns and perceived effects in select division I collegiate athletes. Clin. J. Sport Med. 2000, 10, 191–194. [Google Scholar] [CrossRef]
- Greenwood, M.; Kreider, R.B.; Greenwood, L.; Byars, A. Cramping and Injury Incidence in Collegiate Football Players Are Reduced by Creatine Supplementation. J. Athl. Train. 2003, 38, 216–219. [Google Scholar] [PubMed]
- Greenwood, M.; Kreider, R.B.; Melton, C.; Rasmussen, C.; Lancaster, S.; Cantler, E.; Milnor, P.; Almada, A. Creatine supplementation during college football training does not increase the incidence of cramping or injury. Mol. Cell. Biochem. 2003, 244, 83–88. [Google Scholar] [CrossRef]
- National Institutes of Health; Office of Dietary Supplements. Dietary Supplement Fact Sheets: Dietary Supplements for Exercise and Athletic Performance. Available online: https://ods.od.nih.gov/factsheets/ExerciseAndAthleticPerformance-HealthProfessional/ (accessed on 5 January 2022).
- Muccini, A.M.; Tran, N.T.; de Guingand, D.L.; Philip, M.; Della Gatta, P.A.; Galinsky, R.; Sherman, L.S.; Kelleher, M.A.; Palmer, K.R.; Berry, M.J.; et al. Creatine Metabolism in Female Reproduction, Pregnancy and Newborn Health. Nutrients 2021, 13, 490. [Google Scholar] [CrossRef]
- Jagim, A.R.; Kerksick, C.M. Creatine Supplementation in Children and Adolescents. Nutrients 2021, 13, 664. [Google Scholar] [CrossRef]
- Dover, S.; Stephens, S.; Schneiderman, J.E.; Pullenayegum, E.; Wells, G.D.; Levy, D.M.; Marcuz, J.A.; Whitney, K.; Schulze, A.; Tein, I.; et al. The Effect of Creatine Supplementation on Muscle Function in Childhood Myositis: A Randomized, Double-blind, Placebo-controlled Feasibility Study. J. Rheumatol. 2021, 48, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Korovljev, D.; Todorovic, N.; Stajer, V.; Ostojic, S.M. Dietary Intake of Creatine in Children Aged 0-24 Months. Ann. Nutr. Metab. 2021, 77, 185–188. [Google Scholar] [CrossRef]
- Korovljev, D.; Todorovic, N.; Stajer, V.; Ostojic, S.M. Food Creatine and DXA-Derived Body Composition in Boys and Girls Aged 8 to 19 Years. Nutr. Metab. Insights 2021, 14, 11786388211059368. [Google Scholar] [CrossRef] [PubMed]
- Korovljev, D.; Stajer, V.; Ostojic, S.M. Relationship between Dietary Creatine and Growth Indicators in Children and Adolescents Aged 2-19 Years: A Cross-Sectional Study. Nutrients 2021, 13, 1027. [Google Scholar] [CrossRef]
- Harmon, K.K.; Stout, J.R.; Fukuda, D.H.; Pabian, P.S.; Rawson, E.S.; Stock, M.S. The Application of Creatine Supplementation in Medical Rehabilitation. Nutrients 2021, 13, 1825. [Google Scholar] [CrossRef] [PubMed]
- Solis, M.Y.; Artioli, G.G.; Gualano, B. Potential of Creatine in Glucose Management and Diabetes. Nutrients 2021, 13, 570. [Google Scholar] [CrossRef]
- Bredahl, E.C.; Eckerson, J.M.; Tracy, S.M.; McDonald, T.L.; Drescher, K.M. The Role of Creatine in the Development and Activation of Immune Responses. Nutrients 2021, 13, 751. [Google Scholar] [CrossRef]
- Li, B.; Yang, L. Creatine in T Cell Antitumor Immunity and Cancer Immunotherapy. Nutrients 2021, 13, 1633. [Google Scholar] [CrossRef] [PubMed]
- Balestrino, M. Role of Creatine in the Heart: Health and Disease. Nutrients 2021, 13, 1215. [Google Scholar] [CrossRef] [PubMed]
- Clarke, H.; Hickner, R.C.; Ormsbee, M.J. The Potential Role of Creatine in Vascular Health. Nutrients 2021, 13, 857. [Google Scholar] [CrossRef]
- Wallimann, T.; Hall, C.H.T.; Colgan, S.P.; Glover, L.E. Creatine Supplementation for Patients with Inflammatory Bowel Diseases: A Scientific Rationale for a Clinical Trial. Nutrients 2021, 13, 1429. [Google Scholar] [CrossRef]
- van der Veen, Y.; Post, A.; Kremer, D.; Koops, C.A.; Marsman, E.; Appeldoorn, T.Y.J.; Touw, D.J.; Westerhuis, R.; Heiner-Fokkema, M.R.; Franssen, C.F.M.; et al. Chronic Dialysis Patients Are Depleted of Creatine: Review and Rationale for Intradialytic Creatine Supplementation. Nutrients 2021, 13, 2709. [Google Scholar] [CrossRef]
- Ostojic, S.M. Diagnostic and Pharmacological Potency of Creatine in Post-Viral Fatigue Syndrome. Nutrients 2021, 13, 503. [Google Scholar] [CrossRef]
- Balsom, P.D.; Soderlund, K.; Sjodin, B.; Ekblom, B. Skeletal muscle metabolism during short duration high-intensity exercise: Influence of creatine supplementation. Acta Physiol. Scand. 1995, 154, 303–310. [Google Scholar] [CrossRef]
- Vandenberghe, K.; Van Hecke, P.; Van Leemputte, M.; Vanstapel, F.; Hespel, P. Phosphocreatine resynthesis is not affected by creatine loading. Med. Sci. Sports Exerc. 1999, 31, 236–242. [Google Scholar] [CrossRef]
- Bellinger, B.M.; Bold, A.; Wilson, G.R.; Noakes, T.D.; Myburgh, K.H. Oral creatine supplementation decreases plasma markers of adenine nucleotide degradation during a 1-h cycle test. Acta Physiol. Scand. 2000, 170, 217–224. [Google Scholar] [CrossRef]
- Francaux, M.; Demeure, R.; Goudemant, J.F.; Poortmans, J.R. Effect of exogenous creatine supplementation on muscle PCr metabolism. Int. J. Sports Med. 2000, 21, 139–145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burke, D.G.; Chilibeck, P.D.; Parise, G.; Tarnopolsky, M.A.; Candow, D.G. Effect of alpha-lipoic acid combined with creatine monohydrate on human skeletal muscle creatine and phosphagen concentration. Int. J. Sport Nutr. Exerc. Metab. 2003, 13, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Kreider, R.B.; Klesges, R.; Lotz, D.; Davis, M.; Cantler, E.; Grindstaff, P.; Ramsey, L.; Bullen, D.; Wood, L.; Almada, A. Effects of nutritional supplementation during off-season college football training on body composition and strength. J. Exerc. Physiol. Online 1999, 2, 24–39. [Google Scholar]
- Tarnopolsky, M.A.; Parise, G.; Yardley, N.J.; Ballantyne, C.S.; Olatinji, S.; Phillips, S.M. Creatine-dextrose and protein-dextrose induce similar strength gains during training. Med. Sci. Sports Exerc. 2001, 33, 2044–2052. [Google Scholar] [CrossRef] [PubMed]
- Willoughby, D.S.; Rosene, J. Effects of oral creatine and resistance training on myosin heavy chain expression. Med. Sci. Sports Exerc. 2001, 33, 1674–1681. [Google Scholar] [CrossRef] [PubMed]
- Newman, J.E.; Hargreaves, M.; Garnham, A.; Snow, R.J. Effect of creatine ingestion on glucose tolerance and insulin sensitivity in men. Med. Sci. Sports Exerc. 2003, 35, 69–74. [Google Scholar] [CrossRef]
- Tarnopolsky, M.; Parise, G.; Fu, M.H.; Brose, A.; Parshad, A.; Speer, O.; Wallimann, T. Acute and moderate-term creatine monohydrate supplementation does not affect creatine transporter mRNA or protein content in either young or elderly humans. Mol. Cell. Biochem. 2003, 244, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Willoughby, D.S.; Rosene, J.M. Effects of oral creatine and resistance training on myogenic regulatory factor expression. Med. Sci. Sports Exerc. 2003, 35, 923–929. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, A.E.; Fukuda, D.H.; Ryan, E.D.; Kendall, K.L.; Cramer, J.T.; Stout, J. Ergolytic/ergogenic effects of creatine on aerobic power. Int. J. Sports Med. 2011, 32, 975–981. [Google Scholar] [CrossRef] [PubMed]
- Horecky, J.; Gvozdjakova, A.; Kucharska, J.; Obrenovich, M.E.; Palacios, H.H.; Li, Y.; Vancova, O.; Aliev, G. Effects of coenzyme Q and creatine supplementation on brain energy metabolism in rats exposed to chronic cerebral hypoperfusion. Curr. Alzheimer Res. 2011, 8, 868–875. [Google Scholar] [CrossRef] [PubMed]
- Jäger, R.; Metzger, J.; Lautmann, K.; Shushakov, V.; Purpura, M.; Geiss, K.R.; Maassen, N. The effects of creatine pyruvate and creatine citrate on performance during high intensity exercise. J. Int. Soc. Sports Nutr. 2008, 5, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Schuylenbergh, R.; Van Leemputte, M.; Hespel, P. Effects of oral creatine-pyruvate supplementation in cycling performance. Int. J. Sports Med. 2003, 24, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Andres, S.; Ziegenhagen, R.; Trefflich, I.; Pevny, S.; Schultrich, K.; Braun, H.; Schanzer, W.; Hirsch-Ernst, K.I.; Schafer, B.; Lampen, A. Creatine and creatine forms intended for sports nutrition. Mol. Nutr. Food Res. 2017, 61, 1600772. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority. Orotic Acid Salts as Sources of Orotic Acid and Various Minerals Added for Nutritional Purposes to Food Supplements, Scientific Opinion of the Panel on Food Additives and Nutrient Sources Added to Food (ANS). EFSA J. 2009, 7, 1187. [Google Scholar] [CrossRef]
- Purpura, M.; Pischel, I.; Jäger, R.; Ortenburger, G. Solid and Stable Creatine/Citric Acid Composition(s) and Compositions Carbohydrate(s) or Hydrates Thereof, Method for the Production and Use Thereof. U.S. Patent Application No. 10/495,827, 17 February 2005. [Google Scholar]
- Gufford, B.T.; Sriraghavan, K.; Miller, N.J.; Miller, D.W.; Gu, X.; Vennerstrom, J.L.; Robinson, D.H. Physicochemical characterization of creatine N-methylguanidinium salts. J. Diet. Suppl. 2010, 7, 240–252. [Google Scholar] [CrossRef]
- Smith, A.E.; Walter, A.A.; Herda, T.J.; Ryan, E.D.; Moon, J.R.; Cramer, J.T.; Stout, J.R. Effects of creatine loading on electromyographic fatigue threshold during cycle ergometry in college-aged women. J. Int. Soc. Sports Nutr. 2007, 4, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Graef, J.L.; Smith, A.E.; Kendall, K.L.; Fukuda, D.H.; Moon, J.R.; Beck, T.W.; Cramer, J.T.; Stout, J.R. The effects of four weeks of creatine supplementation and high-intensity interval training on cardiorespiratory fitness: A randomized controlled trial. J. Int. Soc. Sports Nutr. 2009, 6, 18. [Google Scholar] [CrossRef] [Green Version]
- Fukuda, D.H.; Smith, A.E.; Kendall, K.L.; Dwyer, T.R.; Kerksick, C.M.; Beck, T.W.; Cramer, J.T.; Stout, J.R. The effects of creatine loading and gender on anaerobic running capacity. J. Strength Cond. Res. 2010, 24, 1826–1833. [Google Scholar] [CrossRef] [PubMed]
- Koh-Banerjee, P.K.; Ferreira, M.P.; Greenwood, M.; Bowden, R.G.; Cowan, P.N.; Almada, A.L.; Kreider, R.B. Effects of calcium pyruvate supplementation during training on body composition, exercise capacity, and metabolic responses to exercise. Nutrition 2005, 21, 312–319. [Google Scholar] [CrossRef] [Green Version]
- Constantin-Teodosiu, D.; Peirce, N.S.; Fox, J.; Greenhaff, P.L. Muscle pyruvate availability can limit the flux, but not activation, of the pyruvate dehydrogenase complex during submaximal exercise in humans. J. Physiol. 2004, 561, 647–655. [Google Scholar] [CrossRef] [PubMed]
- Ostojic, S.M.; Ahmetovic, Z. The effect of 4 weeks treatment with a 2-gram daily dose of pyruvate on body composition in healthy trained men. Int. J. Vitam. Nutr. Res. 2009, 79, 173–179. [Google Scholar] [CrossRef]
- Ivy, J.L. Effect of pyruvate and dihydroxyacetone on metabolism and aerobic endurance capacity. Med. Sci. Sports Exerc. 1998, 30, 837–843. [Google Scholar] [PubMed]
- Nuuttilla, S. Edustusmelojat testasivat kreatiinipyruvaatin. Suom. Urheilulehti 2000, 23. [Google Scholar]
- Wheelwright, D.C.; Ashmead, S.D. Bioavailable Chelates of Creatine and Essential Metals. U.S. Patent No. 6,114,379, 5 September 2000. [Google Scholar]
- Brilla, L.R.; Giroux, M.S.; Taylor, A.; Knutzen, K.M. Magnesium-creatine supplementation effects on body water. Metabolism 2003, 52, 1136–1140. [Google Scholar] [CrossRef]
- Selsby, J.T.; DiSilvestro, R.A.; Devor, S.T. Mg2+-creatine chelate and a low-dose creatine supplementation regimen improve exercise performance. J. Strength Cond. Res. 2004, 18, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Zajac, A.; Golas, A.; Chycki, J.; Halz, M.; Michalczyk, M.M. The Effects of Long-Term Magnesium Creatine Chelate Supplementation on Repeated Sprint Ability (RAST) in Elite Soccer Players. Nutrients 2020, 12, 2961. [Google Scholar] [CrossRef] [PubMed]
- Giese, M.W.; Lecher, C.S. Qualitative in vitro NMR analysis of creatine ethyl ester pronutrient in human plasma. Int. J. Sports Med. 2009, 30, 766–770. [Google Scholar] [CrossRef] [PubMed]
- Katseres, N.S.; Reading, D.W.; Shayya, L.; Dicesare, J.C.; Purser, G.H. Non-enzymatic hydrolysis of creatine ethyl ester. Biochem. Biophys. Res. Commun. 2009, 386, 363–367. [Google Scholar] [CrossRef] [PubMed]
- Gufford, B.T.; Ezell, E.L.; Robinson, D.H.; Miller, D.W.; Miller, N.J.; Gu, X.; Vennerstrom, J.L. pH-dependent stability of creatine ethyl ester: Relevance to oral absorption. J. Diet. Suppl. 2013, 10, 241–251. [Google Scholar] [CrossRef]
- Law, J.P.; Di Gerlando, S.; Pankhurst, T.; Kamesh, L. Elevation of serum creatinine in a renal transplant patient following oral creatine supplementation. Clin. Kidney J. 2019, 12, 600–601. [Google Scholar] [CrossRef] [Green Version]
- Velema, M.S.; de Ronde, W. Elevated plasma creatinine due to creatine ethyl ester use. Neth. J. Med. 2011, 69, 79–81. [Google Scholar]
- Arazi, H.; Eghbali, E.; Karimifard, M. Effect of creatine ethyl ester supplementation and resistance training on hormonal changes, body composition and muscle strength in underweight non-athlete men. Biomed. Hum. Kinet. 2019, 11, 158–166. [Google Scholar] [CrossRef] [Green Version]
- Stoppani, J. Supplement Breakdown: Creatine HCL; JS Stoppani Blog: Westlake Village, CA, USA, 24 September 2021. [Google Scholar]
- Alraddadi, E.A.; Lillico, R.; Vennerstrom, J.L.; Lakowski, T.M.; Miller, D.W. Absolute Oral Bioavailability of Creatine Monohydrate in Rats: Debunking a Myth. Pharmaceutics 2018, 10, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kreider, R.B. Species-specific responses to creatine supplementation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003, 285, R725–R726. [Google Scholar] [CrossRef] [Green Version]
- Yoshioka, C.A.F.; Madureira, D.; Carrara, P.; Gusmão, N.; Ressureição, K.S.; Santana, J.O.; Lamolha, M.A.; Viebig, R.F.; Sanches, I.C.; de Lira, F.S.; et al. Comparison between creatine monohydrate and creatine HCl on body composition and performance of the Brazilian Olympic team. Int. J. Food Nutr. Res. 2019, 3, 1–12. [Google Scholar]
- Tayebi, M.; Arazi, H. Is creatine hydrochloride better than creatine monohydrate for the improvement of physical performance and hormonal changes in young trained men? Sci. Sports 2020, 35, e135–e141. [Google Scholar] [CrossRef]
- Hlinský, T.; Kumstát, M.; Vajda, P. Effects of Dietary Nitrates on Time Trial Performance in Athletes with Different Training Status: Systematic Review. Nutrients 2020, 12, 2734. [Google Scholar] [CrossRef] [PubMed]
- Tan, R.; Cano, L.; Lago-Rodríguez, Á.; Domínguez, R. The Effects of Dietary Nitrate Supplementation on Explosive Exercise Performance: A Systematic Review. Int. J. Environ. Res. Public Health 2022, 19, 762. [Google Scholar] [CrossRef]
- Gao, C.; Gupta, S.; Adli, T.; Hou, W.; Coolsaet, R.; Hayes, A.; Kim, K.; Pandey, A.; Gordon, J.; Chahil, G.; et al. The effects of dietary nitrate supplementation on endurance exercise performance and cardiorespiratory measures in healthy adults: A systematic review and meta-analysis. J. Int. Soc. Sports Nutr. 2021, 18, 55. [Google Scholar] [CrossRef]
- Macuh, M.; Knap, B. Effects of Nitrate Supplementation on Exercise Performance in Humans: A Narrative Review. Nutrients 2021, 13, 3183. [Google Scholar] [CrossRef]
- Kramer, R.; Nikolaidis, A. Amino Acid Compositions. U.S. Patent No. 11155524 B2, Application No. 16/893,319, 26 October 2021. [Google Scholar]
- Yazdi, P. Creatine Nitrate: Benefits, Side Effects, Dosage, Reviews; SelfDecode: Miami, FL, USA, 9 September 2021. [Google Scholar]
- Ostojic, S.M.; Stajer, V.; Vranes, M.; Ostojic, J. Searching for a better formulation to enhance muscle bioenergetics: A randomized controlled trial of creatine nitrate plus creatinine vs. creatine nitrate vs. creatine monohydrate in healthy men. Food Sci. Nutr. 2019, 7, 3766–3773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, Y.P.; Earnest, C.P.; Koozehchian, M.; Galvan, E.; Dalton, R.; Walker, D.; Rasmussen, C.; Murano, P.S.; Greenwood, M.; Kreider, R.B. Effects of acute ingestion of a pre-workout dietary supplement with and without p-synephrine on resting energy expenditure, cognitive function and exercise performance. J. Int. Soc. Sports Nutr. 2017, 14, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, Y.P.; Earnest, C.P.; Koozehchian, M.; Cho, M.; Barringer, N.; Walker, D.; Rasmussen, C.; Greenwood, M.; Murano, P.S.; Kreider, R.B. Effects of ingesting a pre-workout dietary supplement with and without synephrine for 8 weeks on training adaptations in resistance-trained males. J. Int. Soc. Sports Nutr. 2017, 14, 1. [Google Scholar] [CrossRef] [Green Version]
- Joy, J.M.; Lowery, R.P.; Falcone, P.H.; Mosman, M.M.; Vogel, R.M.; Carson, L.R.; Tai, C.Y.; Choate, D.; Kimber, D.; Ormes, J.A.; et al. 28 days of creatine nitrate supplementation is apparently safe in healthy individuals. J. Int. Soc. Sports Nutr. 2014, 11, 60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- All American EFX. Kre-Alkalyn–The World’s Most Potent Creatine. Available online: http://krealkalyn.com/ (accessed on 5 January 2022).
- Gollini, J.M. Oral Creatine Supplement and Method for Making Same. U.S. Patent 6,399,661 B1, 4 June 2002. [Google Scholar]
- Affouras, A.; Vodenicharova, K.; Shishmanova, D.; Goranov, K.; Stroychev, K. Clinical Trial Comparing Kre-Alkalyn to Creatine Monohydrate; Medical Center: Sofia, Bulgaria, 2006; p. 3. [Google Scholar]
- Creatine Orotate. Available online: https://www.exercise.com/supplements/creatine-orotate/ (accessed on 5 January 2022).
- Muscle Marketing USA: XXTRA Powerlifter’s Creatine Serum. Available online: https://mmusa.com/ (accessed on 5 January 2022).
- Owac, J.H. Bang Revolution: Super Creatine, a Highly Significant Patent in History. 4 September 2019. Available online: https://www.youtube.com/watch?v=IRj2F-wsz5U&feature=youtu.be (accessed on 13 January 2022).
- Owac, J.H. Stable aqueous compositions comprising amide-protected bioactive creatine species and uses thereof. U.S. Patent 8,445,466 B2, 21 May 2013. [Google Scholar]
- Reddeman, R.A.; Glavits, R.; Endres, J.R.; Murbach, T.S.; Hirka, G.; Vertesi, A.; Beres, E.; Szakonyine, I.P. A Toxicological Assessment of Creatyl-l-Leucine. Int. J. Toxicol. 2018, 37, 171–187. [Google Scholar] [CrossRef] [PubMed]
- da Silva, R.P. The Dietary Supplement Creatyl-l-Leucine Does Not Bioaccumulate in Muscle, Brain or Plasma and Is Not a Significant Bioavailable Source of Creatine. Nutrients 2022, 14, 701. [Google Scholar] [CrossRef]
- Monster Energy Co., v. Vital Pharmaceuticals, Inc. et al. Case No. 5:18-cv-1882-JGB-SHK, Dkt. 434-46. C.D. Cal. Available online: https://www.law360.com/cases/5b8f06c4a5fd16342f7dd1b6 (accessed on 15 January 2022).
- Monster Energy Co., v. Vital Pharmaceuticals, Inc. et al. Case No. 5:18-cv-1882-JGB-SHK, Dkt. 434-53. C.D. Cal. Available online: https://www.law360.com/cases/5b8f06c4a5fd16342f7dd1b6 (accessed on 15 January 2022).
- Monster Energy Co., v. Vital Pharmaceuticals, Inc. et al. Case No. 5:18-cv-1882-JGB-SHK, Dkt. 434-50 (C.D. Cal.). Available online: https://www.law360.com/cases/5b8f06c4a5fd16342f7dd1b6 (accessed on 15 January 2022).
- Monster Energy Co., v. Vital Pharmaceuticals, Inc. et al. Case No. 5:18-cv-1882-JGB-SHK, Dkt. 434-51. (C.D. Cal.). Available online: https://www.law360.com/cases/5b8f06c4a5fd16342f7dd1b6 (accessed on 15 January 2022).
- Monster Energy Co., v. Vital Pharmaceuticals, Inc. et al. Case No. 5:18-cv-1882-JGB-SHK, Dkt. 434-133 (C.D. Cal.). Available online: https://www.law360.com/cases/5b8f06c4a5fd16342f7dd1b6 (accessed on 15 January 2022).
- Guglielmi, G.; Mammarella, A. A controlled clinical study of the use of creatinol-O-phosphate in subjects with deficient myocardial circulation. Clin. Ter. 1979, 91, 355–382. [Google Scholar] [PubMed]
- Melloni, G.F.; Minoja, G.M.; Lureti, G.F.; Merlo, L.; Pamparana, F.; Brusoni, B. Acute clinical tolerance of creatinol-O-phosphate. Arzneimittelforschung 1979, 29, 1477–1479. [Google Scholar]
- Godfraind, T.; Ghiradi, P.; Ferrari, G.; Cassagrande, C. Creatinol-O-Phopshate Having Therapeutical Action. U.S. Patent No. 4,376,117, Application No. 198,263, 8 March 1983. [Google Scholar]
- Marzo, A.; Ghirardi, P. Pharmacological and toxicological properties of creatinol O-phosphate. A review. Arzneimittelforschung 1979, 29, 1449–1452. [Google Scholar]
- Kreider, R.B.; Miller, G.W.; Williams, M.H.; Somma, C.T.; Nasser, T.A. Effects of phosphate loading on oxygen uptake, ventilatory anaerobic threshold, and run performance. Med. Sci. Sports Exerc. 1990, 22, 250–256. [Google Scholar] [PubMed]
- Kreider, R.B.; Miller, G.W.; Schenck, D.; Cortes, C.W.; Miriel, V.; Somma, C.T.; Rowland, P.; Turner, C.; Hill, D. Effects of phosphate loading on metabolic and myocardial responses to maximal and endurance exercise. Int. J. Sport Nutr. 1992, 2, 20–47. [Google Scholar] [CrossRef] [PubMed]
- Nicaise, J. Creatinol-O-phosphate (COP) and muscular performance: A controlled clinical trial. Curr. Ther. Res. Clin. Exp. 1975, 17, 531–534. [Google Scholar] [PubMed]
- New Dietary Ingredients in Dietary Supplements-Background for Industry. Available online: https://www.fda.gov/food/new-dietary-ingredients-ndi-notification-process/new-dietary-ingredients-dietary-supplements-background-industry (accessed on 26 October 2020).
- EAS. Product Phosphagen™ Marketed by Experimental and Applied Sciences (EAS™). Available online: https://www.eas.com (accessed on 7 February 2022).
- GRN, No. 931 Creatine Monohydrate; U.S. Food and Drug Administation. U.S. Department of Health and Human Services: Washington, DC USA, 2020.
- Carlson, S.J. GRAS Notice No. GRN 000931; Division of Food Ingredients; Office of Food Additive Safety; Center for Food Safety and Applied Nutrition: Washington, DC USA, 2021. [Google Scholar]
- U.S. Food & Drug Administration. GRAS Notices. Available online: https://www.cfsanappsexternal.fda.gov/scripts/fdcc/index.cfm?set=GRASNotices&sort=GRN_No&order=DESC&startrow=1&type=basic&search=CLL (accessed on 5 January 2022).
- Dwyer, J.T.; Coates, P.M.; Smith, M.J. Dietary Supplements: Regulatory Challenges and Research Resources. Nutrients 2018, 10, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- An Overview of the Regulation of Complementary Medicines in Australia. Available online: https://www.tga.gov.au/overview-regulation-complementary-medicines-australia (accessed on 5 January 2022).
- NNHPD. Natural Health Products Directorate; Health Canada: Ottawa, ON, Canada, 2013. [Google Scholar]
- NHPD. Natural Health Products Directorate; Health Canada: Ottawa, ON, Canada, 2018. [Google Scholar]
- Natural Health Products Ingredients Database. Available online: http://webprod.hc-sc.gc.ca/nhpid-bdipsn/ingredsReq.do?srchRchTxt=creatine&srchRchRole=-1&mthd=Search&lang=eng (accessed on 20 January 2022).
- European Commission on Food Safety: Food Supplements. Available online: https://ec.europa.eu/food/safety/labelling-and-nutrition/food-supplements_en (accessed on 20 January 2022).
- EPC. Commission Directive 2001/15/EC of 15 February 2001 on Substances That May be Added for Specific Nutritional Purposes in Foods for Particular Nutritional Uses. 2011. Available online: http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=CELEX:32001L0015:en:NOT (accessed on 20 January 2022).
- EPC. Directive 2002/46/EC of the European Parliament and of the Council of 10 June 2002 on the Approximation of the Laws of the Member States Relating to Food Supplements. 2002. Available online: https://eur-lex.europa.eu/eli/dir/2002/46/oj (accessed on 20 January 2022).
- EFSA. Opinion of the Scientific Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food (AFC) on a Request from the Commission Related to Creatine Monohydrate for Use in Foods for Particular Nutritional Uses; European Food Safey Authority: Parma, Italy, 2004. [Google Scholar]
- EFSA. Scientific Opinion on the substantiation of health claims related to creatine and increase in physical performance during short-term, high intensity, repeated exercise bouts (ID 739, 1520, 1521, 1522, 1523, 1525, 1526, 1531, 1532, 1533, 1534, 1922, 1923, 1924), increase in endurance capacity (ID 1527, 1535), and increase in endurance performance (ID 1521, 1963) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J. 2011, 9, 2303. [Google Scholar]
- EFSA. Scientific Opinion on the substantiation of health claims related to creatine and increased attention (ID 1524) and improvement of memory (ID 1528) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J. 2011, 9, 2216. [Google Scholar] [CrossRef] [Green Version]
- MHLW. List of the Ingredients (Raw Materials) Not Deemed to be Drugs Unless Medicinal Indications/Efficacies Are Noted (Translated). 2009. Available online: http://www.fukushihoken.metro.tokyo.jp/kenkou/kenko_shokuhin/ken_syoku/kanshi/seibun/index.html (accessed on 20 January 2022).
- MHLW. Director of Standards Division, Dept of Food Safety, Pharmaceutical and Food Safety Bureau; Ministry of Health, Labor and Welfare: Tokyo, Japan, 2001. [Google Scholar]
- MMHLW. Food for Specified Health Uses (FOSHU). 2022. Available online: https://www.mhlw.go.jp/english/topics/foodsafety/fhc/02.html (accessed on 20 January 2022).
- Shimizu, T. Korean latest conditions of health food labeling. Food Style 2008, 12, 54–56. [Google Scholar]
- KFDA. Functional Food Approval Status (Translated). 2009. Available online: http://hfoodi.kfda.go.kr/index.jsp (accessed on 20 January 2022).
- Federal Food, Drug and Cosmetic Act. In §413(a); United States Congress: Washington, DC, USA, 1938.















| Compound | Molecular Formula | Molecular Weight (g/mol) | Theoretical Percent Creatine by MW † | Difference from CrM (%) |
|---|---|---|---|---|
| Creatine (Creatine Anhydrous) | C4H9N3O2 | 131.13 | 100.0 | 13.8 |
| Creatine Monohydrate | C4H11N3O3 | 149.15 | 87.9 | 0.0 |
| Magnesium Creatine | C4H9MgN3O2 | 155.44 | 84.4 | −4.0 |
| Creatine Ethyl Ester | C6H13N3O2 | 159.19 | 82.4 | −6.3 |
| Methyl-Amino-Creatine | C5H12N4O2 | 160.17 | 81.9 | −6.9 |
| Creatine Hydrochloride | C4H10ClN3O | 167.59 | 78.2 | −11.0 |
| Creatine Methyl Ester Hydrochloride | C5H12ClN3O2 | 181.62 | 72.2 | −17.9 |
| Creatine Nitrate | C4H10N4O5 | 194.15 | 67.5 | −23.2 |
| Creatinol-O-Phosphate | C4H12N3O4P | 197.13 | 66.5 | −24.3 |
| Tri-Creatine Citrate | C14H26N6O11 | 585.50 | 67.2 | −23.5 |
| Phospho-Creatine | C4H10N3O5P | 211.11 | 62.1 | −29.3 |
| Creatine Pyruvate | C7H13N3O5 | 219.20 | 59.8 | −31.9 |
| Creatine Beta-Alaninate | C7H16N4O4 | 220.23 | 59.5 | −32.3 |
| Creatine Lactate | C7H15N3O5 | 221.21 | 59.3 | −32.6 |
| Creatine Benzyl Ester | C11H15N3O2 | 221.26 | 59.3 | −32.6 |
| Di-Creatine Citrate | C14H26N6O11 | 454.39 | 57.7 | −34.3 |
| Creatine Sulfate | C4H11N3O6S | 229.21 | 57.2 | −34.9 |
| Creatine Pyruvate Monohydrate | C7H15N3O6 | 237.21 | 55.3 | −37.1 |
| Di-Acetyl Creatine Ethyl Ester | C10H17N3O4 | 243.26 | 53.9 | −38.7 |
| Creatine Sulfate Monohydrate | C4H13N3O7S | 247.23 | 53.0 | −39.7 |
| Creatine Ethyl Ester Pyruvate | C9H17N3O5 | 247.25 | 53.0 | −39.7 |
| Sodium Creatine Phosphate | C4H8N3Na2O5P | 255.08 | 51.4 | −41.5 |
| Creatine Taurinate | C6H16N4O5S | 256.28 | 51.2 | −41.8 |
| Creatine Pyroglutamate | C9H16N4O5 | 260.25 | 50.4 | −42.7 |
| Creatine Malate | C8H15N3O7 | 265.22 | 49.4 | −43.8 |
| Creatine Glutamate | C9H16N4O6 | 276.25 | 47.5 | −46.0 |
| Creatine Orotate | C9H13N5O6 | 287.23 | 45.7 | −48.1 |
| Creatine Carnitine | C11H24N4O5 | 292.33 | 44.9 | −49.0 |
| Creatine Ethyl Ester Malate | C10H19N3O7 | 293.27 | 44.7 | −49.1 |
| 5-Hydroxytryptamine Creatine | C14H21N5O3 | 307.35 | 42.7 | −51.5 |
| Creatine Trinitrate | C4H12N6O11 | 320.17 | 41.0 | −53.4 |
| Creatine α-ketoglutarate | C11H20N4O7 | 320.30 | 40.9 | −53.4 |
| Creatine Citrate | C10H17N3O9 | 323.26 | 40.6 | −53.9 |
| D-Gluconic Acid Creatine Salt | C10H21N3O9 | 327.29 | 40.1 | −54.4 |
| Creatine Monohydrate Dextrose | C10H23N3O9 | 329.30 | 39.8 | −54.7 |
| Creatine Hydroxycitrate | C10H17N3O10 | 339.26 | 38.7 | −56.0 |
| Disodium Creatine Phosphate Tetrahydrate | C4H18N3Na2O10P | 345.15 | 76.0 | −13.6 |
| Creatine Phosphate Lactate | C13H22N3O15P | 491.30 | 26.7 | −69.6 |
| Creatine-CoA | C25H43N10O17P3S | 880.70 | 14.9 | −83.1 |
| Reference | Participants | Design | Duration | Dosing Protocol | Findings | Side Effects |
|---|---|---|---|---|---|---|
| Short-term Studies (<14 Days) | ||||||
| Greenhaff et al. [71] | 8 healthy males | SB | 5 days | 4 × 5 g CrM | CrM ↑ TCr by 25% and PCr resynthesis following electrically evoked isometric contractions. | None reported |
| Balsom et al. [161] | 7 males | SB | 6 days | 4 × 5 g CrM | ↑ in total muscle total creatine (18%), weight (1.1 kg), and 5 × 6 s cycling sprint performance and PCr recovery | None reported |
| Green et al. [20] | 24 healthy men | RDBP | 5 days | 4 × 5 g CrM followed by 93 g CHO or CHO | Ingesting CrM with CHO ↑ muscle TCr and glycogen | None reported |
| Vandenberghe et al. [162] | 9 healthy non-vegetarian males | RDBPC | 5 days with 5 week washout | 25 g/day CrM or PLA | CrM ↑ muscle PCr by 11% and 16% after 2 and 5 days. PCr resynthesis rate was not affected. | None reported |
| Bellinger et al. [163] | 20 endurance cyclists | RDBP | 7 days | 20 g/day CrM or PLA | CrM ↑ muscle creatine content by 30% and decreased TAN contribution to sprint | None reported |
| Francaux et al. [164] | 14 physically active males | RDBP | 14 days | 3 × 7 g of CrM or PLA | CrM ↑ MRS PCr by ~20% and PCr repletion by 15% and 10% during 40% and 70% MVCs. | None reported |
| Preen et al. [116] | 14 physically active men | RDBP | 5 days | 20 g/day CrM or PLA | CrM increase TCr stores and work during 80-min of repeated cycling sprint exercise. | None reported |
| Burke et al. [165] | 20 male resistance-trained athletes (18–32 years) | RDBP | 5 days | 4 × 5 g CrM, 4 × 5 g CrM + 25 g Sucrose, or 4 × 5 g CrM + 25 g Sucrose + 250 mg α-LA or PLA | CrM ↑ body weight (2.1 kg) with no differences among groups, TCr was ↑ more in the CrM + sucrose + α-LA group. | None reported |
| Longer-Term Studies (>14 days) | ||||||
| Vandenberghe et al. [47] | 19 young female volunteers | RDBP | 10 weeks phase I (n = 19); 10 weeks phase II (n = 13) | 4 × 5 g CrM for 4 days, 5 g/day thereafter or PLA | CrM ↑ muscle PCr, strength, and exercise capacity | None reported |
| Kreider et al. [55] | 25 American college football players during offseason resistance and agility training | RDBP | 28 days | CrM 15.75 g/day with glucose or glucose PLA | ↑ FFM, ↑ strength, ↑ muscular endurance, ↑ 6 × 6-s cycling sprint performance with 30-s rest | None reported |
| Volek et al. [113] | 19 healthy resistance-trained males | RDBP | 12 weeks | CrM 5 × 5 g for 7 days, 5 g/day for 11 weeks or PLA | ↑ FFM, strength, and muscle morphology | No differences |
| Kreider et al. [166] | 51 American college football players during offseason resistance and agility training and spring football | RDBP | 12 weeks | 20 g/day and 25 g/day of CrM with CHO and PRO; CHO only; or CHO + PRO only | CrM groups ↑ FFM, ↑ strength, ↑ muscular endurance. No changes in blood chemistry panels. | CrM groups had less GI complaints than those ingesting CHO and CHO + PRO. |
| Tarnopolsky et al. [167] | 23 young healthy but untrained males | RDBP | 8 weeks | 10 g/day CrM with 75 g CHO or PLA | CrM with CHO promoted greater ↑ in body mass and FFM during training. | None reported |
| Willoughby et al. [168] | 22 untrained males during resistance-training | RDBP | 12 weeks | CrM 6 g/day or PLA | CrM promoted > increases in body mass, FFM, thigh volume, muscle strength, myofibrillar protein content, and myosin heavy chain mRNA expression for Type I, IIa, and IIx fibers | None reported |
| Burke et al. [108] | 18 vegan and 24 non-vegan (20 men, 22 female) | RDBP | 56 days | 0.25 g/kg FFM/d of CrM for 7 days, 0.0625 g/kg FFM/d for 49 days or PLA | TCr content was lower in vegans. CrM ↑ PCr, TCr, and gains in bench press strength, isotonic work, Type II fiber area, and FFM during resistance training. | None reported |
| Lyoo et al. [73] | 15 males (23–35 years) | RDBP | 56 days | 2 × 0.15 g/kg CrM for 7 days, 2 × 0.015 g/kg CrM for 49 days or PLA | CrM ↑ brain PCr (3.4%), Pi (9.8%), and Cr (8.1%) while decreasing β-nucleoside triphosphate (NTP) by 7.8%. | None reported |
| Newman et al. [169] | 17 healthy active but untrained men | RDBP | 33 days | 4 × 5 g CrM + 3.75 glucose for 5-days, 3 g CrM + 3 g glucose thereafter or PLA | CrM ↑ muscle TCr after loading and maintenance doses. CrM had no effects on muscle glycogen, glucose tolerance or insulin sensitivity. | None reported |
| Tarnopolsky et al. [170] | Moderately active younger (13 men, 14 women; 19 resistance-trained men; Older resistance-trained men (15) and women (15) | RDBP | 5 days; 8 weeks; 14 weeks | 4 × 5 g CrM for 5 days; 10 g/day CrM with 75 g dextrose for 8 weeks during training; 5 g/day CrM + 2 g/day dextrose for 14 weeks during training or PLA | CrM ↑ muscle TCr in each study compared to placebo. CrM nor training influenced creatine transporter protein content. Citrate synthase was increased in older participants. | None reported |
| Willoughby et al. [171] | 22 untrained males during resistance-training | RDBP | 12 weeks | 6 g/day CrM or PLA | CrM promoted > ↑ in muscle CK, myogenin, and MRF-4. | None reported |
| Reference | Participants | Design | Duration | Dosing Protocol | Findings | Side Effects |
|---|---|---|---|---|---|---|
| Creatine Salts | ||||||
| Jäger et al. [63] | 3 females and 3 males | RDBPC | 1 oral dose with 7 day washout | 5 g CrM 6.7 g CC 7.3 g CPY | Creatine peak AUC was higher with CPY with no differences in absorption kinetics | None reported |
| Smith et al. [180] | 15 recreationally active women (22.3 ± 0.6 yrs) | RDBP | 5 days | 20 g/day of CC | CC loading delayed the onset of neuromuscular fatigue during cycle ergometry. | None reported |
| Jäger et al. [174] | 49 healthy males (26.5 ± 4 yrs) | RDBP | 28 days | 5 g/day of CC, CPY, or PLA | CPY and CC ↑ intermittent handgrip exercise of maximal intensity. Some evidence CPY might benefit endurance exercise. | None reported |
| Graef and coworkers [181] | 43 recreationally active men (22.6 ± 5 yrs) | RDBP | 5 days/week for 6-weeks | 2 × 5 g/day of PLA or CC on training days | CC increases ventilatory anaerobic threshold (PLA 10%, CC 16%). No differences in time to exhaustion or total work. | None reported |
| Smith et al. [172] | 55 active men (27) and women (28) | RDBP | 5 days | 4 × 5 g/day of CC or PLA | CC did not positively or negatively affect maximal aerobic capacity, critical velocity, time to exhaustion, or body mass. | None reported |
| Fukuda et al. [182] | 50 recreationally active men (24) and women (26) 22 ± 3 yrs | RDBP | 5 days | 4 × 5 g/day of CC or PLA | CC loading ↑ anaerobic running capacity (+23%) with no effect in PLA group in men but not women. | None reported |
| Stone et al. [83] | 42 American football players | RDBP | 5 weeks | 0.22 g/kg/day of PLA, CrM, caPYR, or CrM + caPYR | CrM and CrM + caPYR ↑ strength, FFM, and power output. No difference from PLA or caPYR alone. | GI issues with caPYR. None reported with CrM |
| Van Schuylenbergh et al. [175] | 14 well-trained male endurance athletes (4 cyclists, 10 triathletes) | RDBP | 7 days | 2 × 3.5 g of CPY with 8 g CHO or PLA | CYP had no effects on 1-h time trial steady-state power output, interval sprints, total work lactate, or heart rate. | None reported |
| Nuuttilla et al. [187] | Olympic canoeists | RDBP | 7 days | 7.5 g/day of CPY or PLA | CPY improved paddle rate and lowered blood lactate suggesting an improvement in aerobic exercise efficiency. | None reported |
| Magnesium Creatine Chelate | ||||||
| Brilla et al. [189] | 35 recreationally active men | RDBP | 14 days | 800 mg/day magnesium (Mg) and 5 g/day Cr as Mg oxide plus Cr or MgCr-C | Body mass and power ↑ in both Cr groups while intracellular and extracellular water and peak torque only increased in the MgCr-C group | None reported |
| Selsby et al. [190] | 31 resistance-trained men | RDBP | 10 days | 2.5 g/day of PLA, Cr or Mg-Cr | Both Cr groups improved bench press total work compared to PLA. No differences between groups. | None reported |
| Zajac et al. [191] | 20 elite soccer players | RDBP | 16 weeks | 5.5 g/day of r MgCr-C or PLA | MgCr-C ↑ 35 m repeated sprint performance, total time, average power, and peak power with no changes in PLA group. | MgCr-C ↑ serum creatinine compared to PLA |
| Creatine Ethyl Ester | ||||||
| Spillane et al. [27] | 30 healthy males (20.4 ± 1.7 yrs) | RDBP | 47 days | 0.30 g/kg FFM for 5-days, 0.075 g/kg FFM for 42 days of PLA, CrM, or CEE | CEE ↑ in muscle TCr after 27-days compared to PLA. However, CrM observed significantly greater ↑ in TCr compared to PLA and CEE. CEE did not promote > training adaptations. | CEE ↑ serum creatinine twofold > than PLA and CrM. None reported with CrM. |
| Arazi et al. [197] | 16 resistance trained males | RDBP | 42 days | 4 × 5 g/day of PLA or CEE for 5 days, 5 g/day for 37 days | CEE during resistance-training ↑ body weight and leg press strength while percent body fat ↓ with some evidence of an ↑ in testosterone and growth hormone. | None reported. |
| Creatine HCl | ||||||
| de França et al. [65] | 40 healthy males and females | RDBP | 28 days | 5 g/day PLA, 1.5 g/day of Cr-HCl, 5 g/day of Cr-HCl, or 5 g/day CrM | Reported some effects on skinfold determined fat mass and FFM and leg press strength but gains in CrM were greater than Cr-HCl | None reported. |
| Yoshioka et al. [201] | 11 healthy elite Brazilian gymnasts | RDBP | 30 days | 5 g/dayay of CrM or 1.5 g/dayay of Cr-HCL with 3.5 g/dayay of resistant starch | Skinfold caliper determined FFM, strength, and BIA determined total body water was increased to a greater degree in the CrM group (CrM + 1.81 L vs. Cr-HCL +0.24 L). | None reported. |
| Tayebi et al. [202] | 36 resistance trained men | RDBP | 7 days | 20 g/day CrM, 3 g/day CrM, 3 g/day Cr-HCL, or PLA | 3 g/day of Cr-HCl did not promote greater gains in performance or hormonal responses than 3 or 20 g/day of CrM. | None reported. |
| Creatine Nitrate | ||||||
| Ostojic et al. [209] | 10 healthy men | RDBPC | 1 oral dose | 3 g CrN + 3 g CNN, 3 g CrN, 3 g CrM | CrN + CNN ingestion promoted a greater increase in serum creatine AUC levels (183.7 ± 15.5, 163.8 ± 12.9, and 118.6 ± 12.9 µmol/L, respectively). | None reported. |
| Ostojic et al. [209] | 10 healthy men | RDBPC | 5 days | 3 g/day CrN + 3 g/day CNN, 3 g/day CrN, 3 g/day CrM | MRS determined muscle creatine content increased to a greater degree with CrN + CNN (9.6%, 8.0%, 2.1%, respectively) | Irregular bowel movement (1 CrN and CrN + CNN), Excessive sleepiness (1 CrN), Seldom stomach bloating (1 CrM). CrN + CNN decrease eGFR determined kidney function. |
| Galvan et al. [29] | 13 males | RDBPC | 1 oral dose with 7 day washout | 1.5 g CrN (CrN-Low), 3 g CrN (CrN-High), 5 g CrM or a placebo | CrM ↑ plasma Cr AUC to a greater degree than PLA, CrN-Low, and CrN-High while plasma nitrate ↑ in CrN treatments. | None reported. |
| Galvan et al. [29] | 48 active males | RDBP | 28 days | 4 × 5 g PLA, 4 × 1.5 g/day of CrN (CrN-Low), 4 × 3 g/day CrN (CrN-High), 4 × 3 g/day CrM for 7 days and 1 dose/d for 21 days | Creatine loading (12 g/day of CrM and CrN) ↑ muscle TCr in the CrM (7.1 mmol/kg DW) and CrN-High (4.6 mmol/kg DW) groups. CrM maintained ↑ muscle TCr. CrN-Low had no effects on TCr compared to PLA after 7 and 28 days. 3 g/day of CrN was not sufficient to maintain elevated muscle TCr after 28 days. | None reported. |
| Dalton et al. [36] | 28 participants (18 men, 10 women) | RDBPC | 6 days | 3 g/day of PLA. 3 g/day CrN, 6 g/day CrN. | Up to 6 g of CrN for 6-days does not negatively affect resting hemodynamics, response to a postural challenge, the ability to perform high-intensity exercise, or clinical chemistry profiles. | None reported. |
| Joy et al. [212] | 58 young males and females (24.3 ± 4 yrs) | R | 28 days | Control group, 1 g/day CrN, 2 g/day CrN with other nutrients | 1–2 g/day of CrN supplementation during training had no adverse effects on clinical blood chemistries compared to a non-supplemented group. | None reported. |
| Buffered Creatine | ||||||
| Jagim et al. [28] | 36 resistance trained males | RDBP | 28 days | CrM (4 × 5 g/day for 7 days, 5 g/day for 21 days); CrM-Alk at recommended doses (1.5 g/day for 28 days); or CrM-Alk with equivalent doses to CrM (4 × 5 g/day for 7 days, 5 g/day for 21 days). | Neither recommended doses nor loading and maintenance equivalent doses of CrM-Alk promoted greater changes in muscle TCr, body composition, strength, or anaerobic capacity compared to CrM. Recommended doses did not ↑ TCr. | None reported. |
| Reference | Participants | Design | Duration | Dosing Protocol | Findings | Side Effects |
|---|---|---|---|---|---|---|
| Creatine Serum | ||||||
| Harris et al. [38] | 6 males | R | 1 oral dose with 7 day washout | Water Control, 5 mL CS (purportedly delivering 2.5 g CrM), 2.5 g CrM | CrM ↑ plasma Cr while CS had no effects and was similar to water. Analytic chemistry analysis showed < 10 mg of creatine and 90 mg creatinine in CS sample. | None reported. |
| Kreider et al. [27] | 40 males (18–30 years) | RDBP | 5 days | 5 mL PLA, 5 mL of CS (recommended dose purportedly providing 2.5 g of CrM); 8 × 5 mL/day CS (purportedly providing 20 g/day of CrM); and 4 × 5 g doses of CrM (20 g/day) | CrM increased muscle creatine stores. Consumption of CS at recommended and 8× recommended levels had no effect. | None reported. |
| Creatyl-L-Leucine | ||||||
| Reddeman et al. [189] | Rats | Open Label | 90 days | Repeated-dose oral gavage toxicity study at doses of 1250, 2500, and 5000 mg/kg body weight per day. | There was no genotoxic activity observed in an in vivo mammalian micronucleus test at concentrations up to the limit dose of 2000 mg/kg body weight per day. The no observed adverse effect level from the 90-day study was determined to be 5000 mg/kg body weight per day, which was the highest dose tested for male and female rats. | None reported. |
| da Silva [221] | 24 rats | R | 7 days | Control diet, a diet containing 4.0 g/kg/day CrM, or a diet containing 6.56 g/kg/day CLL | CrM ↑ [creatine] in arterial plasma (+7-fold), portal vein plasma (+10-fold), muscle TCr (+1.63-fold from control, and +1.53-fold from CLL) while tending to increase brain creatine content compared to controls. CLL did not increase blood, muscle, and brain creatine content above rats fed a control diet with values lower than CrM. | None reported. |
| Creatinol-O-Phosphate | ||||||
| Nicaise et al. [233] | - | - | - | Intramuscular and intravenous injection | COP↑ handgrip performance. | None reported. |
| FDA Generally Recognized As Safe (GRAS) | ||||
|---|---|---|---|---|
| Purported Creatine Source Submitted | FDA Report Number | Submission Year | Intended Dosage | FDA Response |
| Creatine Monohydrate (Creapure®) | GRN 931 | 2020 | 1 g creatine (1.12 g creatine monohydrate) as an ingredient in “energy” drinks, protein bars and powders, milk shakes, meal replacement powders and bars, meat analogs, and powdered drink mixes (excluding infant formula. | FDA has no questions at this time. |
| Self-Affirmed Generally Recognized As Safe (GRAS) | ||||
| Purported Creatine Source Submitted | FDA Report Number | Submission Year | Intended Dosage | FDA Response |
| Creatine chelated with Mg (Creatine MagnaPower®) | 2013 | |||
| New Dietary Ingredient Notifications (NDIN) * | ||||
| Purported Creatine Source Submitted | FDA Report Number | Submission Year | Intended Dosage | FDA Response |
| Creatine Pyruvate | RPT28 | 1998 | 5–10 g/day in 2 equal doses | Filed by FDA without substantive comments. |
| Creatine Ethylesters [Brand: Cre-Ester™] | RPT154 | 2002 | Maximum daily dose of 30 g | Objected by the FDA. |
| Creatine Ethylesters [Brand: Cre-Ester™] | RPT190 | 2002 | 0.5–5.0 g/day | Objected by the FDA. |
| Tricreatine Orotate | RTP201 | 2003 | 1–2 g 3 ×/day (3–6 g/day) | Objected by the FDA. |
| Creatine ethyl ester HCL [Brand: CE2™] | RTP249 | 2004 | 500 mg–5 g/day | Objected by the FDA. |
| Creatine from creatine ethyl ester HCL [Brand: CE2™] | RTP264 | 2004 | 500 mg–3 g/day | Objected by the FDA. |
| Beta Creatine | RPT660 | 2010 | 4.5–7.5 g/day creatine 3–6 g/day beta-alanine | Objected by the FDA. |
| Creatine Nitrate | RTP696 | 2011 | 1.5 g serving, maximum dose 3 g/day | Objected by the FDA. |
| Creatine Nitrate | RPT993 | 2017 | 750 mg per day | Acknowledged with no objections by FDA. |
| Creatine acesulfame | RPT1064 | 2018 | 10 g per day | Objected by the FDA. |
| Country | Responsible Agency | Primary Regulations/Statutes | Regulatory Status of Creatine |
|---|---|---|---|
| Australia | Department of Health, Therapeutic Goods Administration (TGA). | Dietary supplements are considered complimentary medicines and regulated under the Therapeutic Goods Act (TGA) of 1989 [241] and 1990 TGA regulations [198]. Medicinal products are categorized as lower risk medicines that can be listed on the Australian Register of Therapeutic Goods (ARTG) [198] while higher risks medicines must be registered with the ARTG [241]. | As of this writing, of the 90,988 products listed in the ARTG database, only 25 products contain creatine. Of these, CrM is the only source of creatine listed as an ingredient. |
| Canada | Health Canada [242] | Natural and Non-prescription Health Products Directorate (NNHPD) of Health Canada [242]. The NNHPD maintains a compendium of articles that reviews the safety and efficacy of licensed NHP’s [243]. CrM was assigned a monograph by the NNHPD that overviews research on CrM to substantiate safety and efficacy. Only products containing CrM can benefit from an abbreviated licensing process by referencing the monograph. Applicants using all other creatine forms are required to submit their own evidence of safety and efficacy for review as part of the pre-market licensing process. | As of this writing, 20 compounds purported to contain creatine are included in the NHP Ingredient Database [244] including creatine, creatine-alpha-ketoglutarate, creatine ethyl ester, creatine ethyl ester HCl, creatine gluconate, creatine HCl, creatine hydroxycitrate, creatine monohydrate, creatine nitrate, creatine orotate, creatine phosphate, creatine pyroglutamate, creatine pyruvate, creatine taurinate, dicreatine malate, disodium creatine phosphate, magnesium creatine chelate, polyethylene glycosylated creatine, polyethylene glycosylated creatine HCl, and tricreatine citrate. Creatinol-O-phosphate is listed as a medicinal product in the NHP database [244]. |
| China | New Food Safety Law of the People’s Republic of China and the Administrative Permission Law of the People’s Republic of China, CFDA [198]. | Nutritional supplements in China must be orally ingested, have at least one of 22 preventive functions as recognized by the Ministry of Health, and cannot be a curative drug [198]. Imported supplements must be approved by the National Medical Product Administration while foods are supervised by the State Administration for Market Regulation) [198]. | Importers of dietary supplements and foods containing creatine must submit notification materials for review and approval before being allowed to be sold in China. Since CrM and other forms of creatine are produced in China, they would seemingly be legal to consume. However, it is unclear which forms of creatine are allowed to be imported into China. |
| European Union (EU) | European Commission Directive on Food Supplements [245,246,247] | The European Food Safety Authority (EFSA) evaluates scientific health claims. Creatine is considered a substance that may be added for specific nutritional purposes in foods for particular nutritional uses (FPNU) [246]. | In 2004, EFSA indicated that the use of creatine in foods for nutritional use was not a matter of concern provided that the source had high purity (99.95%), did not contain impurities, and that dose of up to 3 g/day of supplemental creatine which is similar to the normal daily turnover rate of creatine was unlikely to pose any risk [248]. EFSA substantiated scientific health claims of CrM include: (1) CrM increases physical performance during short-term, high intensity, repeated exercise bouts, endurance capacity, and endurance performance [249], (2) CrM increases attention and improves memory [250], and, (3) CrM (at least 3 g/day) in combination with resistance training and improved muscle strength. All studies cited were performance on pure CrM so the regulatory status of other “forms” of creatine in the EU are less clear. |
| Japan | Ministry of Health, Labor and Welfare (MHLW) | Dietary substances in Japan are legally classified as food, food additives or “non-drug” (food). The Consumer Affairs Agency started “Foods with Function Claims” which reviews and approves health claims related to dietary supplements. | CrM is considered a “non-drug” [251] that is allowed to be sold as a food ingredient and additive under the Food Sanitation Law [252]. Health claims of CrM for muscle maintenance with exercise was accepted in 2019. Thus, CrM can be imported, distributed, and produced in Japan. CEE has been included on the “non-drug” list. In order for other forms to be imported, distributed, and/or produced in Japan, safety data and similarity of the proposed form to CrM must be submitted and approved by the MHLW [253]. In addition to CrM, creatine citrate and creatine pyruvate have been approved to be imported into Japan. It is unclear whether other forms of creatine can be imported into Japan and sold as dietary supplements. |
| South Korea | Ministry of Food and Drug Safety (MFDS) [254]. | Similar to the U.S., new dietary ingredients must have sufficient safety data including toxicology studies in animals and supporting safety and efficacy data from human clinical trials to support efficacy at the recommended daily doses marketed. | An application to register CrM as a dietary supplement was filed in 2005 and approved by the MFDA for use as a dietary supplement 2008 with an accompanying health claim [255]. Given these requirements, forms of creatine reviewed above that have bioavailability data at recommended doses substantiating efficacy and safety seemingly be eligible for approval while those that do not have that data would likely experience more difficulty obtaining approval to sell their form of creatine in South Korea. |
| Strong Evidence | Some Evidence | No Evidence |
|---|---|---|
| Creatine Monohydrate | Creatine Citrate | 5-Hydroxytryptamine Creatine |
| Creatine Pyruvate | Creatine Benzyl Ester | |
| Magnesium Creatine Chelate | Creatine Beta-Alaninate | |
| Creatine Ethyl Ester | Creatine Carnitine | |
| Creatine HCl | Creatine Ethyl Ester Malate | |
| Creatine Nitrate | Creatine Ethyl Ester Pyruvate | |
| Buffered Creatine Monohydrate | Creatine Fumarate | |
| Creatine Gluconate | ||
| Creatine Glutamate | ||
| Creatine Hydroxycitrate | ||
| Creatine Lactate | ||
| Creatine Malate | ||
| Creatine Maleate | ||
| Creatine Methyl Ester HCL | ||
| Creatine Monohydrate Dextrose | ||
| Creatine Orotate | ||
| Creatine Phosphate Lactate | ||
| Creatine Pyroglutamate | ||
| Creatine Pyruvate Monohydrate | ||
| Creatine Serum | ||
| Creatine Sulfate Monohydrate | ||
| Creatine Taurinate | ||
| Creatine Trinitrate | ||
| Creatine α-ketoglutarate | ||
| Creatine-CoA | ||
| Creatinol-0-Phosphate | ||
| Creatyl-L-Leucine | ||
| Di-Acetyl Creatine Ethyl Ester | ||
| Disodium Creatine Phosphate | ||
| Methyl-Amino-Creatine | ||
| Phospho-Creatine | ||
| Polyethylene Glycosylated Creatine |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kreider, R.B.; Jäger, R.; Purpura, M. Bioavailability, Efficacy, Safety, and Regulatory Status of Creatine and Related Compounds: A Critical Review. Nutrients 2022, 14, 1035. https://doi.org/10.3390/nu14051035
Kreider RB, Jäger R, Purpura M. Bioavailability, Efficacy, Safety, and Regulatory Status of Creatine and Related Compounds: A Critical Review. Nutrients. 2022; 14(5):1035. https://doi.org/10.3390/nu14051035
Chicago/Turabian StyleKreider, Richard B., Ralf Jäger, and Martin Purpura. 2022. "Bioavailability, Efficacy, Safety, and Regulatory Status of Creatine and Related Compounds: A Critical Review" Nutrients 14, no. 5: 1035. https://doi.org/10.3390/nu14051035
APA StyleKreider, R. B., Jäger, R., & Purpura, M. (2022). Bioavailability, Efficacy, Safety, and Regulatory Status of Creatine and Related Compounds: A Critical Review. Nutrients, 14(5), 1035. https://doi.org/10.3390/nu14051035