Towards a Common Definition for the Diagnosis of Iron Deficiency in Chronic Inflammatory Diseases
Abstract
:1. Introduction
2. Materials and Methods
2.1. The CARENFER Studies
2.2. Definitions and Statistics
3. Results
3.1. Study Populations
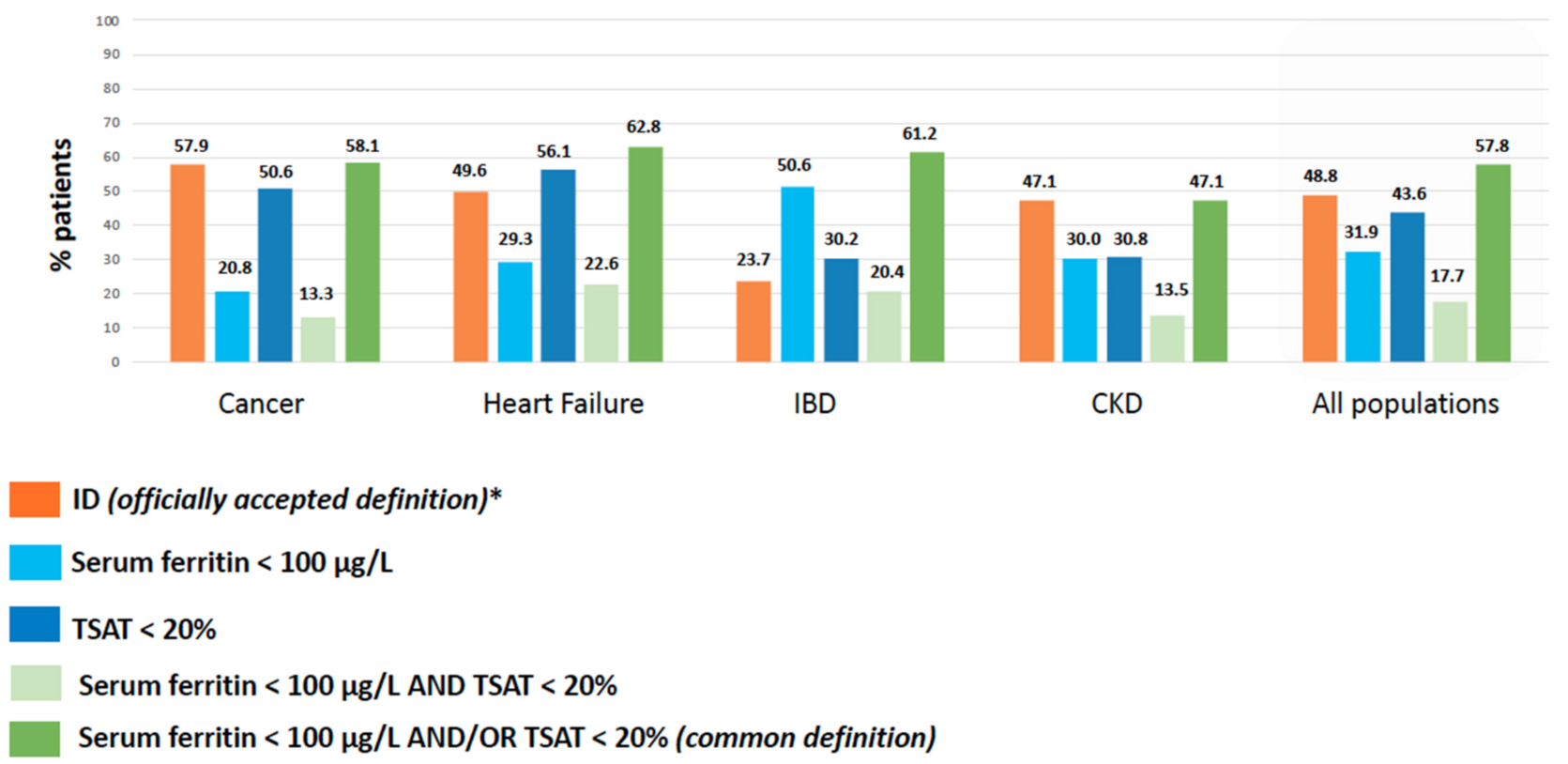
3.2. Prevalence of ID According to Officially Accepted vs. Common Definition of ID
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stevens, G.A.; Finucane, M.M.; De-Regil, L.M.; Paciorek, C.J.; Flaxman, S.R.; Branca, F.; Peña-Rosas, J.P.; Bhutta, Z.A.; Ezzati, M.; Nutrition Impact Model Study Group (Anaemia). Global, Regional, and National Trends in Haemoglobin Concentration and Prevalence of Total and Severe Anaemia in Children and Pregnant and Non-Pregnant Women for 1995–2011: A Systematic Analysis of Population-Representative Data. Lancet Glob. Health 2013, 1, e16–e25. [Google Scholar] [CrossRef] [Green Version]
- Peyrin-Biroulet, L.; Williet, N.; Cacoub, P. Guidelines on the Diagnosis and Treatment of Iron Deficiency across Indications: A Systematic Review. Am. J. Clin. Nutr. 2015, 102, 1585–1594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ludwig, H.; Müldür, E.; Endler, G.; Hübl, W. Prevalence of Iron Deficiency across Different Tumors and Its Association with Poor Performance Status, Disease Status and Anemia. Ann. Oncol. 2013, 24, 1886–1892. [Google Scholar] [CrossRef] [PubMed]
- Cappellini, M.D.; Comin-Colet, J.; de Francisco, A.; Dignass, A.; Doehner, W.; Lam, C.S.; Macdougall, I.C.; Rogler, G.; Camaschella, C.; Kadir, R.; et al. Iron Deficiency across Chronic Inflammatory Conditions: International Expert Opinion on Definition, Diagnosis, and Management: CAPPELLINI et Al. Am. J. Hematol. 2017, 92, 1068–1078. [Google Scholar] [CrossRef] [Green Version]
- Lopez, A.; Cacoub, P.; Macdougall, I.C.; Peyrin-Biroulet, L. Iron Deficiency Anaemia. Lancet 2016, 387, 907–916. [Google Scholar] [CrossRef]
- Camaschella, C. Iron Deficiency. Blood 2019, 133, 30–39. [Google Scholar] [CrossRef] [Green Version]
- Patterson, A.J.; Brown, W.J.; Powers, J.R.; Roberts, D.C. Iron Deficiency, General Health and Fatigue: Results from the Australian Longitudinal Study on Women’s Health. Qual. Life Res. 2000, 9, 491–497. [Google Scholar] [CrossRef]
- Jankowska, E.A.; Rozentryt, P.; Witkowska, A.; Nowak, J.; Hartmann, O.; Ponikowska, B.; Borodulin-Nadzieja, L.; von Haehling, S.; Doehner, W.; Banasiak, W.; et al. Iron Deficiency Predicts Impaired Exercise Capacity in Patients with Systolic Chronic Heart Failure. J. Card Fail. 2011, 17, 899–906. [Google Scholar] [CrossRef]
- Ludwig, H.; Aapro, M.; Bokemeyer, C.; Glaspy, J.; Hedenus, M.; Littlewood, T.J.; Österborg, A.; Rzychon, B.; Mitchell, D.; Beguin, Y. A European Patient Record Study on Diagnosis and Treatment of Chemotherapy-Induced Anaemia. Support. Care Cancer 2014, 22, 2197–2206. [Google Scholar] [CrossRef] [Green Version]
- Comín-Colet, J.; Martín Lorenzo, T.; González-Domínguez, A.; Oliva, J.; Jiménez Merino, S. Impact of Non-Cardiovascular Comorbidities on the Quality of Life of Patients with Chronic Heart Failure: A Scoping Review. Health Qual. Life Outcomes 2020, 18, 329. [Google Scholar] [CrossRef]
- Klip, I.T.; Comin-Colet, J.; Voors, A.A.; Ponikowski, P.; Enjuanes, C.; Banasiak, W.; Lok, D.J.; Rosentryt, P.; Torrens, A.; Polonski, L.; et al. Iron Deficiency in Chronic Heart Failure: An International Pooled Analysis. Am. Heart J. 2013, 165, 575–582. [Google Scholar] [CrossRef]
- Cho, M.E.; Hansen, J.L.; Peters, C.B.; Cheung, A.K.; Greene, T.; Sauer, B.C. An Increased Mortality Risk Is Associated with Abnormal Iron Status in Diabetic and Non-Diabetic Veterans with Predialysis Chronic Kidney Disease. Kidney Int. 2019, 96, 750–760. [Google Scholar] [CrossRef] [PubMed]
- Eisenga, M.F.; Nolte, I.M.; van der Meer, P.; Bakker, S.J.L.; Gaillard, C.A.J.M. Association of Different Iron Deficiency Cutoffs with Adverse Outcomes in Chronic Kidney Disease. BMC Nephrol. 2018, 19, 225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, A.; Brookes, M.J. Iron Therapy in Inflammatory Bowel Disease. Nutrients 2020, 12, 3478. [Google Scholar] [CrossRef]
- Shah, Y.; Patel, D.; Khan, N. Iron Deficiency Anemia in IBD: An Overlooked Comorbidity. Expert Rev. Gastroenterol. Hepatol. 2021, 15, 771–781. [Google Scholar] [CrossRef]
- Jankowska, E.A.; Kasztura, M.; Sokolski, M.; Bronisz, M.; Nawrocka, S.; Oleśkowska-Florek, W.; Zymliński, R.; Biegus, J.; Siwołowski, P.; Banasiak, W.; et al. Iron Deficiency Defined as Depleted Iron Stores Accompanied by Unmet Cellular Iron Requirements Identifies Patients at the Highest Risk of Death after an Episode of Acute Heart Failure. Eur. Heart J. 2014, 35, 2468–2476. [Google Scholar] [CrossRef]
- Luporsi, E.; Turpin, A.; Massard, V.; Morin, S.; Chauffert, B.; Carnot, A.; Cacoub, P. Behalf of the CARENFER Study Group Iron Deficiency in Patients with Cancer: A Prospective Cross-Sectional Study. BMJ Support. Palliat. Care 2021. [Google Scholar] [CrossRef]
- Cohen-Solal, A.; Philip, J.-L.; Picard, F.; Delarche, N.; Taldir, G.; Gzara, H.; Korichi, A.; Trochu, J.-N.; Cacoub, P. CARENFER Study Group Iron Deficiency in Heart Failure Patients: The French CARENFER Prospective Study. ESC Heart Fail. 2022. [Google Scholar] [CrossRef]
- Choukroun, G.; Cacoub, P.; Trochu, J.-N. Objectifs et Rationnel de l’étude de Prévalence de La Carence Martiale Dans Une Population de Patients Insuffisants Rénaux Chroniques Non Dialysés: CARENFER. Néphrologie Thérapeutique 2020, 16, 294. [Google Scholar] [CrossRef]
- Peyrin-Biroulet, L.; Bouguen, G.; Laharie, D.; Savoye, G.; Gilletta de Saint-Joseph, C.; Michiels, C.; Cacoub, P. Objectifs et Rationnel de l’étude de Prévalence de La Carence Martiale Dans Une Population de Patients Présentant Une Maladie Inflammatoire Chronique. Journées Francophones d’Hépato-Gastroentérologie et d’Oncologie Digestive, March 2021, Belgium. Available online: https://www.Snfge.Org/Resumes-2021/000538 (accessed on 29 January 2022).
- Ferraro, S.; Mozzi, R.; Panteghini, M. Revaluating Serum Ferritin as a Marker of Body Iron Stores in the Traceability Era. Clin. Chem. Lab. Med. 2012, 50, 1911–1916. [Google Scholar] [CrossRef] [PubMed]
- Wish, J.B. Assessing Iron Status: Beyond Serum Ferritin and Transferrin Saturation. Clin. J. Am. Soc. Nephrol. 2006, 1 (Suppl. 1), S4–S8. [Google Scholar] [CrossRef] [PubMed]
- Cacoub, P.; Vandewalle, C.; Peoc’h, K. Using Transferrin Saturation as a Diagnostic Criterion for Iron Deficiency: A Systematic Review. Crit. Rev. Clin. Lab. Sci. 2019, 56, 526–532. [Google Scholar] [CrossRef] [PubMed]
- Beverborg, N.G.; Klip, I.T.; Meijers, W.C.; Voors, A.A.; Vegter, E.L.; van der Wal, H.H.; Swinkels, D.W.; van Pelt, J.; Mulder, A.B.; Bulstra, S.K.; et al. Definition of Iron Deficiency Based on the Gold Standard of Bone Marrow Iron Staining in Heart Failure Patients. Circ. Heart Fail. 2018, 11, e004519. [Google Scholar] [CrossRef]
- Moliner, P.; Jankowska, E.A.; van Veldhuisen, D.J.; Farre, N.; Rozentryt, P.; Enjuanes, C.; Polonski, L.; Meroño, O.; Voors, A.A.; Ponikowski, P.; et al. Clinical Correlates and Prognostic Impact of Impaired Iron Storage versus Impaired Iron Transport in an International Cohort of 1821 Patients with Chronic Heart Failure. Int. J. Cardiol. 2017, 243, 360–366. [Google Scholar] [CrossRef]
- Bohm, N. Diagnosis and Management of Iron Deficiency Anemia in Inflammatory Bowel Disease. Am. J. Manag. Care 2021, 27, S211–S218. [Google Scholar] [CrossRef] [PubMed]
- Aapro, M.; Beguin, Y.; Bokemeyer, C.; Dicato, M.; Gascón, P.; Glaspy, J.; Hofmann, A.; Link, H.; Littlewood, T.; Ludwig, H.; et al. Management of Anaemia and Iron Deficiency in Patients with Cancer: ESMO Clinical Practice Guidelines. Ann. Oncol. 2018, 29, iv96–iv110. [Google Scholar] [CrossRef]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; González-Juanatey, J.R.; Harjola, V.-P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart Failure: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure of the European Society of Cardiology (ESC)Developed with the Special Contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2016, 37, 2129–2200. [Google Scholar] [CrossRef]
- Dignass, A.U.; Gasche, C.; Bettenworth, D.; Birgegård, G.; Danese, S.; Gisbert, J.P.; Gomollon, F.; Iqbal, T.; Katsanos, K.; Koutroubakis, I.; et al. European Consensus on the Diagnosis and Management of Iron Deficiency and Anaemia in Inflammatory Bowel Diseases. J. Crohn’s Colitis 2015, 9, 211–222. [Google Scholar] [CrossRef]
- Locatelli, F.; Bárány, P.; Covic, A.; de Francisco, A.; Del Vecchio, L.; Goldsmith, D.; Hörl, W.; London, G.; Vanholder, R.; van Biesen, W.; et al. Kidney Disease: Improving Global Outcomes Guidelines on Anaemia Management in Chronic Kidney Disease: A European Renal Best Practice Position Statement. Nephrol. Dial. Transplant. 2013, 28, 1346–1359. [Google Scholar] [CrossRef]
- Guibergia, C.; Choukroun, G. Algorithme de Prise En Charge de l’anémie et La Carence Martiale Des Patients IRC ND. 2020. Available online: https://www.sfndt.org/civicrm/mailing/view?Id=187&reset=1 (accessed on 20 February 2022).
- Fertrin, K.Y. Diagnosis and Management of Iron Deficiency in Chronic Inflammatory Conditions (CIC): Is Too Little Iron Making Your Patient Sick? Hematol. Am. Soc. Hematol. Educ. Program 2020, 2020, 478–486. [Google Scholar] [CrossRef]
- Gilreath, J.A.; Stenehjem, D.D.; Rodgers, G.M. Diagnosis and Treatment of Cancer-Related Anemia. Am. J. Hematol. 2014, 89, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Nanas, J.N.; Matsouka, C.; Karageorgopoulos, D.; Leonti, A.; Tsolakis, E.; Drakos, S.G.; Tsagalou, E.P.; Maroulidis, G.D.; Alexopoulos, G.P.; Kanakakis, J.E.; et al. Etiology of Anemia in Patients with Advanced Heart Failure. J. Am. Coll. Cardiol. 2006, 48, 2485–2489. [Google Scholar] [CrossRef] [Green Version]
- Locatelli, F.; Aljama, P.; Bárány, P.; Canaud, B.; Carrera, F.; Eckardt, K.-U.; Hörl, W.H.; Macdougal, I.C.; Macleod, A.; Wiecek, A.; et al. Revised European Best Practice Guidelines for the Management of Anaemia in Patients with Chronic Renal Failure. Nephrol. Dial. Transplant. 2004, 19 (Suppl. 2), ii1–ii47. [Google Scholar] [CrossRef] [PubMed]
- Martens, P.; Verbrugge, F.; Nijst, P.; Dupont, M.; Tang, W.H.W.; Mullens, W. Impact of Iron Deficiency on Response to and Remodeling After Cardiac Resynchronization Therapy. Am. J. Cardiol. 2017, 119, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Okonko, D.O.; Grzeslo, A.; Witkowski, T.; Mandal, A.K.J.; Slater, R.M.; Roughton, M.; Foldes, G.; Thum, T.; Majda, J.; Banasiak, W.; et al. Effect of Intravenous Iron Sucrose on Exercise Tolerance in Anemic and Nonanemic Patients with Symptomatic Chronic Heart Failure and Iron Deficiency FERRIC-HF: A Randomized, Controlled, Observer-Blinded Trial. J. Am. Coll. Cardiol. 2008, 51, 103–112. [Google Scholar] [CrossRef] [Green Version]
- Bolger, A.P.; Bartlett, F.R.; Penston, H.S.; O’Leary, J.; Pollock, N.; Kaprielian, R.; Chapman, C.M. Intravenous Iron Alone for the Treatment of Anemia in Patients with Chronic Heart Failure. J. Am. Coll. Cardiol. 2006, 48, 1225–1227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anker, S.D.; Kirwan, B.-A.; van Veldhuisen, D.J.; Filippatos, G.; Comin-Colet, J.; Ruschitzka, F.; Lüscher, T.F.; Arutyunov, G.P.; Motro, M.; Mori, C.; et al. Effects of Ferric Carboxymaltose on Hospitalisations and Mortality Rates in Iron-Deficient Heart Failure Patients: An Individual Patient Data Meta-Analysis. Eur. J. Heart Fail. 2018, 20, 125–133. [Google Scholar] [CrossRef] [Green Version]
- Association Francophone Pour Les Soins Oncologiques de Support (AFSOS). Anémie. 2016. Available online: https://www.Afsos.Org/Fiche-Referentiel/Anemie-et-Cancer/Attachment/Anemie-Version-16-12-2016/ (accessed on 20 February 2022).
- National Comprehensive Cancer Network. NCCN Guidelines. Hematopoïetic Growth Factors. Management of Cancer- and Chemotherapy-Induced Anemia. 2020. Available online: https://www.Nccn.Org/Guidelines/Guidelines-Detail?Category=3&id=1493 (accessed on 20 February 2022).
- Anker, S.D.; Comin Colet, J.; Filippatos, G.; Willenheimer, R.; Dickstein, K.; Drexler, H.; Lüscher, T.F.; Bart, B.; Banasiak, W.; Niegowska, J.; et al. Ferric Carboxymaltose in Patients with Heart Failure and Iron Deficiency. N. Engl. J. Med. 2009, 361, 2436–2448. [Google Scholar] [CrossRef] [Green Version]
- McDonagh, T.; Damy, T.; Doehner, W.; Lam, C.S.P.; Sindone, A.; van der Meer, P.; Cohen-Solal, A.; Kindermann, I.; Manito, N.; Pfister, O.; et al. Screening, Diagnosis and Treatment of Iron Deficiency in Chronic Heart Failure: Putting the 2016 European Society of Cardiology Heart Failure Guidelines into Clinical Practice. Eur. J. Heart Fail. 2018, 20, 1664–1672. [Google Scholar] [CrossRef] [Green Version]
- Rosano, G.M.C. Preview of the 2021 ESC/HFA Heart Failure Guidelines. Available online: https://Pace-Cme.Org/2021/06/29/Preview-of-the-2021-Esc-Hfa-Heart-Failure-Guidelines/ (accessed on 20 February 2022).
- Fitzsimons, S.; Yeo, T.J.; Ling, L.H.; Sim, D.; Leong, K.T.G.; Yeo, P.S.D.; Ong, H.Y.; Jaufeerally, F.; Ng, T.P.; Poppe, K.; et al. Impact of Change in Iron Status over Time on Clinical Outcomes in Heart Failure According to Ejection Fraction Phenotype. ESC Heart Fail. 2021, 8, 4572–4583. [Google Scholar] [CrossRef]
- Thorp, M.L.; Johnson, E.S.; Yang, X.; Petrik, A.F.; Platt, R.; Smith, D.H. Effect of Anaemia on Mortality, Cardiovascular Hospitalizations and End-Stage Renal Disease among Patients with Chronic Kidney Disease. Nephrology 2009, 14, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Kidney Disease: Improving Global Outcomes (KDIGO) Working Group. Chapter 1: Diagnosis and Evaluation of Anemia in CKD. Kidney Int. Suppl. 2012, 2, 288–291. [Google Scholar] [CrossRef] [Green Version]
- National Institute for Health and Care Excellence. Chronic Kidney Disease: Managing Anaemia. NICE Guideline [NG8]. 2015. Available online: https://www.Nice.Org.Uk/Guidance/Ng8 (accessed on 20 February 2022).
- Choukroun, G.; Moulin, B.; Zaoui, P.; Chazot, C. Algorithme de Prise En Charge de l’anémie et La Carence Martiale Des Patients IRC ND. 2019. Available online: https://www.fondation-du-rein.org/wp-content/uploads/2019/12/Algorithme-1.pdf (accessed on 29 January 2022).
- Van Assche, G.; Dignass, A.; Bokemeyer, B.; Danese, S.; Gionchetti, P.; Moser, G.; Beaugerie, L.; Gomollón, F.; Häuser, W.; Herrlinger, K.; et al. Second European Evidence-Based Consensus on the Diagnosis and Management of Ulcerative Colitis Part 3: Special Situations. J. Crohn’s Colitis 2013, 7, 1–33. [Google Scholar] [CrossRef] [Green Version]
- French National Authority for Health (Haute Autorité de Santé, France). Choix Des Examens Du Métabolisme Du Fer En Cas de Suspicion de Carence En Fer—Rapport d’évaluation. 2011. Available online: https://www.has-sante.fr/upload/docs/application/pdf/2011-11/rapport_devaluation_bilan_martial_carence_2011-11-09_17-21-31_723.pdf (accessed on 30 January 2022).
- Cacoub, P.; Nicolas, G.; Peoc’h, K. Iron Deficiency Markers in Patients Undergoing Iron Replacement Therapy: A 9-Year Retrospective Real-World Evidence Study Using Healthcare Databases. Sci. Rep. 2020, 10, 14983. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Patel, D.; Shah, Y.; Yang, Y.-X. Factors Predicting Testing and Treatment of Iron Deficiency in a Nationwide Cohort of Anemic UC Patients. Inflamm. Bowel Dis. 2016, 22, 2894–2901. [Google Scholar] [CrossRef] [PubMed]

| International/National Guidelines | Common Definition | ||
|---|---|---|---|
| Cancer | ESMO 2018 | Serum ferritin < 100 μg/L or Serum ferritin ≥ 100 μg/L and TSAT < 20% | Serum ferritin < 100 μg/L and/or TSAT < 20% |
| Heart Failure | ESC 2016 | Serum ferritin < 100 μg/L or Serum ferritin (100 to 299) μg/L and TSAT < 20% | |
| IBD | ECCO 2015 | Non-inflammatory context (CRP < 5 mg/L): Serum ferritin < 30 μg/L Inflammatory context (CRP ≥ 5 mg/L): Serum ferritin ≤ 100 μg/L | |
| CKD | ERBP 2013/SFNDT 2020 | Serum ferritin < 100 μg/L and/or TSAT < 20% | |
| TSAT: iron-saturation of transferrin | |||
| ESMO: European Society of Medical Oncology [27] | |||
| ESC: European Society of Cardiology [28] | |||
| ECCO: European Crohn’s and Colitis Organization [29] | |||
| ERBP: European Renal Best Practice [30] | |||
| SFNDT: Société Francophone de Néphrologie Dialyse et Transplantation [31] | |||
| Characteristics | CARENFER Study Populations | |||||||
|---|---|---|---|---|---|---|---|---|
| Cancer | Heart Failure | IBD | CKD | |||||
| N = 1221 | N = 1661 | N = 1036 | N = 1211 | |||||
| General characteristics | ||||||||
| Gender | ||||||||
| Male, n (%) | 545 | (44.6) | 1023 | (61.6) | 496 | (47.9) | 737 | (60.9) |
| Female, n (%) | 676 | (55.4) | 638 | (38.4) | 540 | (52.1) | 474 | (39.1) |
| Age (years), median (IQR) | 64.0 | (55.0; 71.0) | 78.0 | (76.0; 86.0) | 39.0 | (29.0; 53.0) | 64.0 | (51.0; 74.0) |
| BMI (kg/m2), median (SD) | 24.4 | (21.6; 27.8) | 26.4 | (23.0; 30.4) | 23.9 | (21.3; 27.4) | 25.7 | (22.7; 29.6) |
| Overweight/obesity, n (%) # | 548 | (44.9) | 972/1616 | (60.1) | 407/1021 | (39.9) | 597/1065 | (56.0) |
| Disease characteristics | ||||||||
| Type | ||||||||
| Solid or hematological tumor & | 1221 | (100.0) | ||||||
| Acute decompensated HF * | 887/1475 | (60.1) | ||||||
| Chronic HF | 588/1475 | (39.9) | ||||||
| Crohn’s disease | 685 | (66.1) | ||||||
| Ulcerative colitis | 351 | (33.9) | ||||||
| Kidney transplantation | 616/1211 | (50.9) | ||||||
| Reason for admission | ||||||||
| Decompensation | NA | 887/1475 | (60.1) | 44 | (4.2) | NA | ||
| Scheduled follow-up $ | NA | 588/1475 | (39.9) | 992 | (95.8) | NA | ||
| Severity | ||||||||
| Cancer—Metastatic treatment | 626/1199 | (52.2) | ||||||
| HF—NYHA III-IV | 801/1601 | (50.0) | ||||||
| HF—LVEF < 40% | 664/1502 | (44.2) | ||||||
| IBD—Disease remission | 504/987 | (51.1) | ||||||
| IBD—Mild/Moderate activity | 433/483 | (89.6) | ||||||
| CKD—Stage 3B and 4 | 640/1208 | (53.0) | ||||||
| Time from disease diagnosis | ||||||||
| Median (IQR), in years | 1.0 | (0.0; 3.0) | NA | NA | 5.9 | (2.0; 14.7) | ||
| Ongoing treatment | ||||||||
| Disease-specific | 1091 | (89.4) | ||||||
| Chemotherapy, n (%) | 823/1091 | (75.4) | ||||||
| Proton pump inhibitors, n (%) | 741/1660 | (44.6) | ||||||
| Aspirin, n (%) | 566/1660 | (34.1) | ||||||
| Oral anticoagulant, n (%) | 864/1660 | (52.0) | ||||||
| Anti-TNF | 622/1035 | (64.1) | ||||||
| Immunosuppressive drug, n (%) | 234/1035 | (24.1) | ||||||
| Current/completed treatment for ID | ||||||||
| Oral iron, n (%) | 20/1215 | (1.6) | 81/1660 | (4.9) | 36 | (3.5) | 112 | (9.2) |
| Intravenous iron, n (%) | 49/1175 | (4.2) | 188/1660 | (11.3) | 218 | (21.0) | 33 | (2.7) |
| Erythropoietin, n (%) | 24/1213 | (2.0) | NA | NA | 241 | (19.9) | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cacoub, P.; Choukroun, G.; Cohen-Solal, A.; Luporsi, E.; Peyrin-Biroulet, L.; Peoc’h, K.; Andrieu, V.; Lasocki, S.; Puy, H.; Trochu, J.-N. Towards a Common Definition for the Diagnosis of Iron Deficiency in Chronic Inflammatory Diseases. Nutrients 2022, 14, 1039. https://doi.org/10.3390/nu14051039
Cacoub P, Choukroun G, Cohen-Solal A, Luporsi E, Peyrin-Biroulet L, Peoc’h K, Andrieu V, Lasocki S, Puy H, Trochu J-N. Towards a Common Definition for the Diagnosis of Iron Deficiency in Chronic Inflammatory Diseases. Nutrients. 2022; 14(5):1039. https://doi.org/10.3390/nu14051039
Chicago/Turabian StyleCacoub, Patrice, Gabriel Choukroun, Alain Cohen-Solal, Elisabeth Luporsi, Laurent Peyrin-Biroulet, Katell Peoc’h, Valérie Andrieu, Sigismond Lasocki, Hervé Puy, and Jean-Noël Trochu. 2022. "Towards a Common Definition for the Diagnosis of Iron Deficiency in Chronic Inflammatory Diseases" Nutrients 14, no. 5: 1039. https://doi.org/10.3390/nu14051039
APA StyleCacoub, P., Choukroun, G., Cohen-Solal, A., Luporsi, E., Peyrin-Biroulet, L., Peoc’h, K., Andrieu, V., Lasocki, S., Puy, H., & Trochu, J.-N. (2022). Towards a Common Definition for the Diagnosis of Iron Deficiency in Chronic Inflammatory Diseases. Nutrients, 14(5), 1039. https://doi.org/10.3390/nu14051039







