Exposure to Chinese Famine during Early Life Increases the Risk of Fracture during Adulthood
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Sample
2.2. Outcome Variable: Fracture
2.3. Exposure Variable: Great Chinese Famine
Measures of Dietary Patterns and Nutrient Intake
2.4. Covariates
2.5. Statistical Analysis
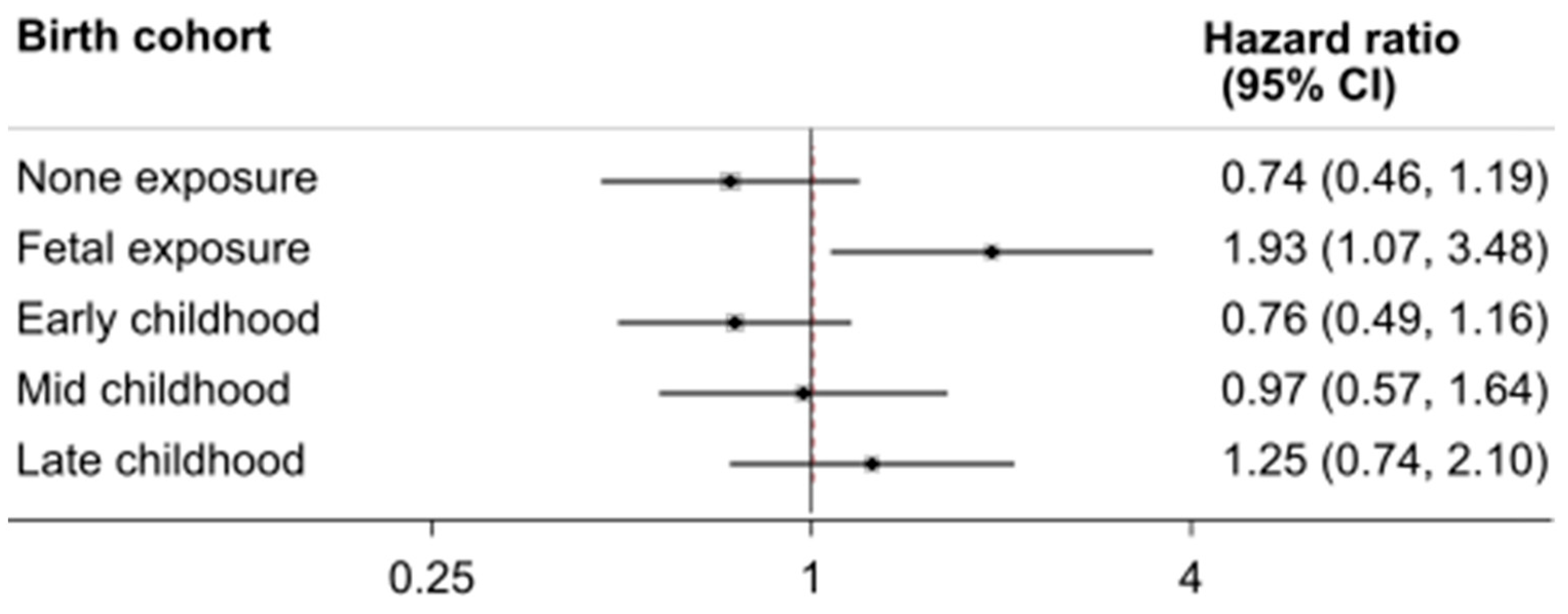
3. Results
Sample Description
4. Discussion
4.1. Comparison with Other Studies
4.2. Potential Mechanisms of Early Life Famine Exposure Related Adult Fracture
4.3. Limitations and Strengths
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, W.; Lv, H.; Liu, S.; Liu, B.; Zhu, Y.; Chen, X.; Yang, G.; Liu, L.; Zhang, T.; Wang, H.; et al. National incidence of traumatic fractures in China: —A Retrospective Survey of 512,187 Individuals. Lancet Glob. Health 2017, 5, e807–e817. [Google Scholar] [CrossRef]
- Yu, F.; Xia, W. The epidemiology of osteoporosis, associated fragility fractures, and management gap in China. Arch. Osteoporos. 2019, 14, 32. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.F.; Li, X.P.; Zhang, L.X.; Center, J.R.; Bliuc, D.; Shi, Y.; Wang, H.B.; He, L.; Wu, X.B. Current status and distribution of hip fractures among older adults in China. Osteoporos. Int. 2021, 32, 1785–1793. [Google Scholar] [CrossRef] [PubMed]
- Godfrey, K.M.; Barker, D.J. Fetal nutrition and adult disease. Am. J. Clin. Nutr. 2000, 71, 1344S–1352S. [Google Scholar] [CrossRef] [PubMed]
- Gluckman, P.D.; Hanson, M.A.; Cooper, C.; Thornburg, K.L. Effect of In Utero and Early-Life Conditions on Adult Health and Disease. N. Engl. J. Med. 2008, 359, 61–73. [Google Scholar] [CrossRef]
- Hales, C.N.; Barker, D.J.P. The thrifty phenotype hypothesis. Br. Med. Bull. 2001, 60, 5–20. [Google Scholar] [CrossRef] [PubMed]
- Roseboom, T.J.; Painter, R.C.; van Abeelen, A.F.; Veenendaal, M.V.; de Rooij, S.R. Hungry in the womb: —What are the Consequences? Lessons from the Dutch Famine. Maturitas 2011, 70, 141–145. [Google Scholar] [CrossRef]
- Smil, V. China’s great famine: 40 years later. BMJ 1999, 319, 1619–1621. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Mu, R.; Zhang, X. Famine and Overweight in China. Rev. Agric. Econ. 2006, 28, 296–304. [Google Scholar] [CrossRef]
- Huang, C.; Li, Z.; Wang, M.; Martorell, R. Early Life Exposure to the 1959–1961 Chinese Famine Has Long-Term Health Consequences. J. Nutr. 2010, 140, 1874–1878. [Google Scholar] [CrossRef] [PubMed]
- Meng, R.; Lv, J.; Yu, C.; Guo, Y.; Bian, Z.; Yang, L.; Chen, Y.; Zhang, H.; Chen, X.; Chen, J.; et al. Prenatal famine exposure, adulthood obesity patterns and risk of type 2 diabetes. Int. J. Epidemiol. 2018, 47, 399–408. [Google Scholar] [CrossRef]
- Li, Y.; He, Y.; Qi, L.; Jaddoe, V.W.; Feskens, E.J.; Yang, X.; Ma, G.; Hu, F.B. Exposure to the Chinese Famine in Early Life and the Risk of Hyperglycemia and Type 2 Diabetes in Adulthood. Diabetes 2010, 59, 2400–2406. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, Y.; Han, X.; Liu, B.; Hu, H.; Wang, F.; Li, X.; Yang, K.; Yuan, J.; Yao, P.; et al. Exposure to the Chinese Famine in Childhood Increases Type 2 Diabetes Risk in Adults. J. Nutr. 2016, 146, 2289–2295. [Google Scholar] [CrossRef] [PubMed]
- Zimmet, P.; Shi, Z.; El-Osta, A.; Ji, L. Chinese Famine and the diabetes mellitus epidemic. Nat. Rev. Endocrinol. 2020, 16, 123. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, X.; Kong, Y.; Zhang, J.H.; Zeng, Q. The Great Chinese Famine Leads to Shorter and Overweight Females in Chongqing Chinese Population After 50 Years. Obesity 2010, 18, 588–592. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Jaddoe, V.W.; Qi, L.; He, Y.; Wang, D.; Lai, J.; Zhang, J.; Fu, P.; Yang, X.; Hu, F.B. Exposure to the Chinese Famine in Early Life and the Risk of Metabolic Syndrome in Adulthood. Diabetes Care 2011, 34, 1014–1018. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Wang, Y.; Ren, W.; Luo, R.; Zhang, S.; Zhang, J.H.; Zeng, Q. Risk of metabolic syndrome in adults exposed to the great Chinese famine during the fetal life and early childhood. Eur. J. Clin. Nutr. 2012, 66, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Nicholls, S.J.; Taylor, A.W.; Magliano, D.J.; Appleton, S.; Zimmet, P. Early life exposure to Chinese famine modifies the association between hypertension and cardiovascular disease. J. Hypertens. 2018, 36, 54–60. [Google Scholar] [CrossRef]
- Shi, Z.; Ji, L.; Ma, R.C.W.; Zimmet, P. Early life exposure to 1959–1961 Chinese famine exacerbates association between diabetes and cardiovascular disease. J. Diabetes 2020, 12, 134–141. [Google Scholar] [CrossRef]
- Weisz, G.M.; Albury, W.R. Hunger Whilst “In Utero” Programming Adult Osteoporosis. Rambam Maimonides Med. J. 2014, 5, e0004. [Google Scholar] [CrossRef]
- Zong, L.; Cai, L.; Liang, J.; Lin, W.; Yao, J.; Huang, H.; Tang, K.; Chen, L.; Li, L.; Lin, L.; et al. Exposure to Famine in Early Life and the Risk of Osteoporosis in Adulthood: A Prospective Study. Endocr. Pract. 2019, 25, 299–305. [Google Scholar] [CrossRef]
- Kin, C.F.W.; Shan, W.S.Y.; Shun, L.J.C.; Chung, L.P.; Jean, W. Experience of famine and bone health in post-menopausal women. Int. J. Epidemiol. 2007, 36, 1143–1150. [Google Scholar] [CrossRef] [PubMed][Green Version]
- VanEvery, H.; Yang, W.-H.; Olsen, N.; Zhang, X.; Shu, R.; Lu, B.; Wu, S.; Cui, L.; Gao, X. In Utero and Early Life Exposure to the Great Chinese Famine and Risk of Rheumatoid Arthritis in Adulthood. Arthritis Rheumatol. 2021, 73, 596–603. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Meng, X.; Zhang, H.; Yang, F.; Pan, X.; Tang, K. Early-life famine exposure and rheumatoid arthritis in Chinese adult populations: —A Retrospective Cohort Study. BMJ Open 2021, 11, e043416. [Google Scholar] [CrossRef] [PubMed]
- Xue, A.-L.; Wu, S.-Y.; Jiang, L.; Feng, A.-M.; Guo, H.-F.; Zhao, P. Bone fracture risk in patients with rheumatoid arthritis. Medicine 2017, 96, e6983. [Google Scholar] [CrossRef] [PubMed]
- Popkin, B.M.; Du, S.; Zhai, F.; Zhang, B. Cohort Profile: The China Health and Nutrition Survey—Monitoring and understanding socio-economic and health change in China, 1989-2011. Int. J. Epidemiol. 2010, 39, 1435–1440. [Google Scholar] [CrossRef]
- Popkin, B.M.; Paeratakul, S.; Ge, K.; Zhai, F. Body weight patterns among the Chinese: Results from the 1989 and 1991 China Health and Nutrition Surveys. Am. J. Public Health 1995, 85, 690–694. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhai, F.Y.; Du, S.F.; Popkin, B.M. The China Health and Nutrition Survey, 1989–2011. Obes. Rev. 2014, 15, 2–7. [Google Scholar] [CrossRef]
- Zhai, F.Y.; Du, S.F.; Wang, Z.H.; Zhang, J.G.; Du, W.W.; Popkin, B.M. Dynamics of the Chinese diet and the role of urbanicity, 1991–2011. Obes. Rev. 2014, 15 (Suppl. 1), 16–26. [Google Scholar] [CrossRef]
- Melaku, Y.A.; Gill, T.K.; Appleton, S.L.; Taylor, A.W.; Adams, R.; Shi, Z. Prospective Associations of Dietary and Nutrient Patterns with Fracture Risk: A 20-Year Follow-Up Study. Nutrients 2017, 9, 1198. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Yu, Y.; Li, L.; Xu, W. Exposure to the Great Famine in Early Life and the Risk of Obesity in Adulthood: A Report Based on the China Health and Nutrition Survey. Nutrients 2021, 13, 1285. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zou, Z.; Wang, S.; Yang, Z.; Ma, J. Chinese famine exposure in infancy and metabolic syndrome in adulthood: —Results from the China Health and Retirement Longitudinal Study. Eur. J. Clin. Nutr. 2019, 73, 724–732. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Lumey, L.H. Exposure to the Chinese famine of 1959–61 in early life and long-term health conditions: A Systematic Review and Meta-Analysis. Int. J. Epidemiol. 2017, 46, 1157–1170. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Taylor, A.W.; Riley, M.; Byles, J.; Liu, J.; Noakes, M. Association between dietary patterns, cadmium intake and chronic kidney disease among adults. Clin. Nutr. 2018, 37, 276–284. [Google Scholar] [CrossRef]
- Ainsworth, B.E.; Haskell, W.L.; Whitt, M.C.; Irwin, M.L.; Swartz, A.M.; Strath, S.J.; O’Brien, W.L.; Bassett, D.R., Jr.; Schmitz, K.H.; Emplaincourt, P.O.; et al. Compendium of Physical Activities: An Update of Activity Codes and MET Intensities. Med. Sci. Sports Exerc. 2000, 32, S498–S516. [Google Scholar] [CrossRef]
- Marcus, E.-L.; Menczel, J. Experience of famine and bone health in post-menopausal women. Int. J. Epidemiol. 2009, 38, 886. [Google Scholar] [CrossRef][Green Version]
- Tarry-Adkins, J.L.; Ozanne, S.E. Nutrition in early life and age-associated diseases. Ageing Res. Rev. 2017, 39, 96–105. [Google Scholar] [CrossRef]
- Zheng, J.; Feng, Q.; Zheng, S.; Xiao, X. Maternal nutrition and the developmental origins of osteoporosis in offspring: Potential mechanisms and clinical implications. Exp. Biol. Med. 2018, 243, 836–842. [Google Scholar] [CrossRef]
- Cooper, C.; Fall, C.; Egger, P.; Hobbs, R.; Eastell, R.; Barker, D. Growth in infancy and bone mass in later life. Ann. Rheum. Dis. 1997, 56, 17–21. [Google Scholar] [CrossRef]
- Javaid, M.K.; Crozier, S.R.; Harvey, N.C.; Gale, C.R.; Dennison, E.M.; Boucher, B.J.; Arden, N.K.; Godfrey, K.M.; Cooper, C. Maternal vitamin D status during pregnancy and childhood bone mass at age 9 years: A Longitudinal Study. Lancet 2006, 367, 36–43. [Google Scholar] [CrossRef]
- Pettifor, J.M.; Moodley, G.P. Appendicular Bone Mass in Children with a High Prevalence of Low Dietary Calcium Intakes. J. Bone Miner. Res. 1997, 12, 1824–1832. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, S.; Huq, S.; Yatsunenko, T.; Haque, R.; Mahfuz, M.; Alam, M.A.; Benezra, A.; DeStefano, J.; Meier, M.F.; Muegge, B.D.; et al. Persistent gut microbiota immaturity in malnourished Bangladeshi children. Nature 2014, 510, 417–421. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z. Gut Microbiota: An Important Link between Western Diet and Chronic Diseases. Nutrients 2019, 11, 2287. [Google Scholar] [CrossRef] [PubMed]
- Das, M.; Cronin, O.; Keohane, D.M.; Cormac, E.M.; Nugent, H.; Nugent, M.; Molloy, C.; O’toole, P.W.; Shanahan, F.; Molloy, M.G.; et al. Gut microbiota alterations associated with reduced bone mineral density in older adults. Rheumatology 2019, 58, 2295–2304. [Google Scholar] [CrossRef] [PubMed]
- Savitsky, B.; Manor, O.; Lawrence, G.; Friedlander, Y.; Siscovick, D.S.; Hochner, H. Environmental mismatch and obesity in humans: The Jerusalem Perinatal Family Follow-Up Study. Int. J. Obes. 2021, 45, 1404–1417. [Google Scholar] [CrossRef] [PubMed]


| Factor | None Exposure (n = 1505) | Fetal Exposure (n = 840) | Early Childhood (n = 1204) | Mid-Childhood (n = 866) | Late Childhood (n = 820) | p-Value 1 |
|---|---|---|---|---|---|---|
| Birth year | 1962–1964 | 1959–1961 | 1956–1958 | 1954–1955 | 1952–1953 | |
| Age (years), mean (SD) | 37.7 (5.4) | 41.2 (5.7) | 44.3 (5.8) | 46.7 (5.9) | 48.7 (5.9) | <0.001 |
| Overweight | 24.7% | 30.2% | 28.9% | 26.3% | 28.9% | 0.043 |
| BMI (kg/m2), mean (SD) | 23.2 (3.1) | 23.5 (3.3) | 23.4 (3.2) | 23.2 (3.1) | 23.5 (3.1) | 0.093 |
| Height (cm), mean (SD) | 162.5 (7.9) | 161.9 (8.0) | 161.3 (8.1) | 161.0 (8.1) | 160.7 (8.3) | <0.001 |
| Weight (kg), mean (SD) | 61.5 (10.7) | 61.8 (10.9) | 61.1 (10.3) | 60.3 (10.2) | 60.9 (10.0) | 0.043 |
| Women | 51.2% | 51.0% | 51.3% | 49.8% | 52.0% | 0.927 |
| Income | 0.594 | |||||
| Low | 26.9% | 24.7% | 27.2% | 26.9% | 26.3% | |
| Medium | 34.6% | 35.6% | 32.9% | 32.8% | 31.4% | |
| High | 38.5% | 39.7% | 39.9% | 40.3% | 42.4% | |
| Education | <0.001 | |||||
| Low | 19.4% | 23.0% | 32.7% | 44.0% | 51.7% | |
| Medium | 42.2% | 32.0% | 35.0% | 31.2% | 30.6% | |
| High | 38.4% | 45.0% | 32.3% | 24.8% | 17.7% | |
| Rural residence | 59.3% | 59.4% | 58.2% | 58.0% | 61.4% | 0.625 |
| Severe famine area | 85.0% | 79.3% | 80.7% | 80.8% | 80.9% | 0.003 |
| Smoking | 0.161 | |||||
| Non smoker | 66.4% | 63.8% | 65.4% | 65.0% | 62.7% | |
| Ex-smokers | 0.8% | 0.9% | 1.6% | 1.7% | 2.1% | |
| Current smokers | 32.8% | 35.3% | 33.0% | 33.3% | 35.1% | |
| Physical activity (MET-hours/week), mean (SD) | 149.3 (112.0) | 157.1 (117.0) | 144.0 (109.5) | 149.6 (116.1) | 141.7 (118.6) | 0.063 |
| Energy intake (kcal/d), mean (SD) | 2235.1 (756.0) | 2241.4 (692.1) | 2241.6 (1062.4) | 2213.5 (734.7) | 2218.2 (705.4) | 0.923 |
| Fat intake (g/d), mean (SD) | 68.9 (36.9) | 71.0 (35.5) | 72.8 (100.3) | 70.6 (52.9) | 68.4 (35.5) | 0.481 |
| Protein intake (g/d), mean (SD) | 70.3 (43.8) | 71.3 (22.6) | 70.1 (23.6) | 68.3 (23.7) | 69.6 (24.8) | 0.375 |
| Carbohydrate intake (g/d), mean (SD) | 328.4 (135.9) | 324.4 (132.1) | 319.8 (127.2) | 320.6 (126.5) | 325.3 (133.0) | 0.520 |
| Calcium intake (mg/d), mean (SD) | 372.8 (339.6) | 381.3 (205.3) | 392.0 (228.8) | 376.8 (215.4) | 405.1 (349.8) | 0.090 |
| Traditional south dietary pattern, mean (SD) | 0.0 (1.0) | −0.0 (0.9) | 0.0 (1.0) | 0.0 (1.0) | 0.1 (1.0) | 0.463 |
| Modern dietary pattern, mean (SD) | −0.1 (0.9) | 0.0 (1.1) | −0.0 (1.1) | −0.1 (1.1) | −0.1 (1.0) | 0.037 |
| Survey year | <0.001 | |||||
| 1997 | 54.2% | 52.1% | 53.4% | 55.5% | 55.2% | |
| 2000 | 14.0% | 14.0% | 11.9% | 11.3% | 10.6% | |
| 2004 | 6.6% | 3.9% | 5.5% | 5.1% | 5.5% | |
| 2006 | 4.9% | 5.8% | 3.3% | 2.4% | 2.1% | |
| 2009 | 6.7% | 7.7% | 7.1% | 5.0% | 5.1% | |
| 2011 | 13.6% | 16.3% | 18.9% | 20.7% | 21.5% |
| None Exposure (n = 1505) | Fetal Exposure (n = 840) | Early Childhood (n = 1204) | Mid-Childhood (n = 866) | Late Childhood (n = 820) | |
|---|---|---|---|---|---|
| No. of cases | 91 | 64 | 108 | 78 | 77 |
| Person-years | 16,858 | 9111 | 12,976 | 9640 | 8884 |
| Incidence rate (per 1000 person-year) | 5.4 | 7.0 | 8.3 | 8.1 | 8.7 |
| Model 1 1 | 1.00 | 1.31 (0.95–1.81) | 1.56 (1.18–2.06) | 1.49 (1.10–2.02) | 1.61 (1.19–2.18) |
| Model 2 2 | 1.00 | 1.29 (0.90–1.85) | 1.48 (1.08–2.03) | 1.45 (1.02–2.06) | 1.53 (1.07–2.19) |
| Model 3 3 | 1.00 | 1.29 (0.90–1.85) | 1.48 (1.08–2.03) | 1.45 (1.02–2.06) | 1.54 (1.08–2.20) |
| Model 3 + age ≥ 35 years | 1.00 | 1.47 (1.01–2.15) | 1.71 (1.22–2.40) | 1.68 (1.16–2.43) | 1.79 (1.23–2.60) |
| None Exposure (n = 1505) | Fetal Exposure (n = 840) | Early Childhood (n = 1204) | Mid-Childhood (n = 866) | Late Childhood (n = 820) | |
|---|---|---|---|---|---|
| Residence | |||||
| Urban | 1.00 | 2.29 (1.18–4.48) | 2.59 (1.40–4.80) | 2.8 (1.47–5.31) | 2.41 (1.47–5.31) |
| Rural | 1.00 | 1.06 (0.69–1.64) | 1.2 (0.83–1.75) | 1.05 (0.68–1.61) | 1.24 (0.68–1.61) |
| p for interaction | 0.076 | 0.078 | 0.026 | 0.140 | |
| Modern dietary pattern | |||||
| High modern diet | 1.00 | 1.99 (1.20–3.32) | 1.91 (1.18–3.08) | 1.96 (1.18–3.28) | 1.96 (1.18–3.28) |
| Low modern diet | 1.00 | 0.88 (0.51–1.50) | 1.21 (0.79–1.86) | 1.09 (0.67–1.77) | 1.26 (0.67–1.77) |
| p for interaction | 0.030 | 0.148 | 0.059 | 0.111 | |
| Famine severity | |||||
| Less severe | 1.00 | 2.77 (0.89–8.65) | 2.41 (0.79–7.35) | 3.1 (0.99–9.70) | 1.14 (0.99–9.70) |
| Severe | 1.00 | 1.21 (0.83–1.78) | 1.43 (1.03–2.00) | 1.34 (0.93–1.94) | 1.58 (0.93–1.94) |
| p for interaction | 0.277 | 0.518 | 0.281 | 0.585 | |
| Overweight | |||||
| No | 1.00 | 1.54 (1.02–2.35) | 1.41 (0.96–2.08) | 1.32 (0.86–2.02) | 1.69 (0.86–2.02) |
| Yes | 1.00 | 0.85 (0.41–1.77) | 1.64 (0.93–2.90) | 1.8 (0.97–3.33) | 1.25 (0.97–3.33) |
| p for interaction | 0.320 | 0.451 | 0.216 | 0.268 | |
| Sex | |||||
| Men | 1.00 | 1.14 (0.71–1.85) | 1.33 (0.88–2.00) | 1.20 (0.75–1.90) | 1.30 (0.75–1.90) |
| Women | 1.00 | 1.57 (0.90–2.73) | 1.65 (0.99–2.75) | 1.83 (1.07–3.14) | 1.86 (1.07–3.14) |
| p for interaction | 0.320 | 0.451 | 0.216 | 0.268 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, Z.; Shi, X.; Yan, A.F. Exposure to Chinese Famine during Early Life Increases the Risk of Fracture during Adulthood. Nutrients 2022, 14, 1060. https://doi.org/10.3390/nu14051060
Shi Z, Shi X, Yan AF. Exposure to Chinese Famine during Early Life Increases the Risk of Fracture during Adulthood. Nutrients. 2022; 14(5):1060. https://doi.org/10.3390/nu14051060
Chicago/Turabian StyleShi, Zumin, Xinyu Shi, and Alice F. Yan. 2022. "Exposure to Chinese Famine during Early Life Increases the Risk of Fracture during Adulthood" Nutrients 14, no. 5: 1060. https://doi.org/10.3390/nu14051060
APA StyleShi, Z., Shi, X., & Yan, A. F. (2022). Exposure to Chinese Famine during Early Life Increases the Risk of Fracture during Adulthood. Nutrients, 14(5), 1060. https://doi.org/10.3390/nu14051060







