Red and Processed Meat Intake, Polygenic Risk Score, and Colorectal Cancer Risk
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Study Population
2.2. Data Collection
2.3. Dietary Assessment
2.4. Derivation of Polygenic Risk Score
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics of the Study Population
3.2. Association of Red and Processed Meat Intake and PRS with CRC Risk
3.3. Genetic Risk Equivalents for Red and Processed Meat Intake Categories
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bouvard, V.; Loomis, D.; Guyton, K.Z.; Grosse, Y.; Ghissassi, F.E.; Benbrahim-Tallaa, L.; Guha, N.; Mattock, H.; Straif, K. Carcinogenicity of consumption of red and processed meat. Lancet Oncol. 2015, 16, 1599–1600. [Google Scholar] [CrossRef] [Green Version]
- Schmit, S.L.; Edlund, C.K.; Schumacher, F.R.; Gong, J.; Harrison, T.A.; Huyghe, J.R.; Qu, C.; Melas, M.; Van Den Berg, D.J.; Wang, H.; et al. Novel Common Genetic Susceptibility Loci for Colorectal Cancer. JNCI J. Natl. Cancer Inst. 2019, 111, 146–157. [Google Scholar] [CrossRef] [PubMed]
- Huyghe, J.R.; Bien, S.A.; Harrison, T.A.; Kang, H.M.; Chen, S.; Schmit, S.L.; Conti, D.V.; Qu, C.; Jeon, J.; Edlund, C.K.; et al. Discovery of common and rare genetic risk variants for colorectal cancer. Nat. Genet. 2019, 51, 76–87. [Google Scholar] [CrossRef]
- Thomas, M.; Sakoda, L.C.; Hoffmeister, M.; Rosenthal, E.A.; Lee, J.K.; van Duijnhoven, F.J.B.; Platz, E.A.; Wu, A.H.; Dampier, C.H.; de la Chapelle, A.; et al. Genome-wide Modeling of Polygenic Risk Score in Colorectal Cancer Risk. Am. J. Hum. Genet. 2020, 107, 432–444. [Google Scholar] [CrossRef]
- Huyghe, J.R.; Harrison, T.A.; Bien, S.A.; Hampel, H.; Figueiredo, J.C.; Schmit, S.L.; Conti, D.V.; Chen, S.; Qu, C.; Lin, Y.; et al. Genetic architectures of proximal and distal colorectal cancer are partly distinct. Gut 2021, 70, 1325–1334. [Google Scholar] [CrossRef] [PubMed]
- Hutter, C.M.; Chang-Claude, J.; Slattery, M.L.; Pflugeisen, B.M.; Lin, Y.; Duggan, D.; Nan, H.; Lemire, M.; Rangrej, J.; Figueiredo, J.C.; et al. Characterization of Gene–Environment Interactions for Colorectal Cancer Susceptibility Loci. Cancer Res. 2012, 72, 2036–2044. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kantor, E.D.; Hutter, C.M.; Minnier, J.; Berndt, S.I.; Brenner, H.; Caan, B.J.; Campbell, P.T.; Carlson, C.S.; Casey, G.; Chan, A.T.; et al. Gene–Environment Interaction Involving Recently Identified Colorectal Cancer Susceptibility Loci. Cancer Epidemiol. Biomark. Prev. 2014, 23, 1824–1833. [Google Scholar] [CrossRef] [Green Version]
- Yang, T.; Li, X.; Farrington, S.M.; Dunlop, M.G.; Campbell, H.; Timofeeva, M.; Theodoratou, E. A Systematic Analysis of Interactions between Environmental Risk Factors and Genetic Variation in Susceptibility to Colorectal Cancer. Cancer Epidemiol. Biomarkers Prev. 2020, 29, 1145–1153. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Cohen, P.; Chen, S. How Big is a Big Odds Ratio? Interpreting the Magnitudes of Odds Ratios in Epidemiological Studies. Commun. Stat.-Simul. Comput. 2010, 39, 860–864. [Google Scholar] [CrossRef]
- Norton, E.C.; Dowd, B.E.; Maciejewski, M.L. Odds Ratios—Current Best Practice and Use. JAMA 2018, 320, 84. [Google Scholar] [CrossRef]
- Chen, X.; Jansen, L.; Guo, F.; Hoffmeister, M.; Chang-Claude, J.; Brenner, H. Smoking, Genetic Predisposition, and Colorectal Cancer Risk. Clin. Transl. Gastroenterol. 2021, 12, e00317. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Guo, F.; Hoffmeister, M.; Chang-Claude, J.; Brenner, H. Non-steroidal anti-inflammatory drugs, polygenic risk score and colorectal cancer risk. Aliment. Pharmacol. Ther. 2021, 54, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Brenner, H.; Chang-Claude, J.; Seiler, C.M.; Rickert, A.; Hoffmeister, M. Protection from colorectal cancer after colonoscopy: A population-based, case-control study. Ann. Intern. Med. 2011, 154, 22–30. [Google Scholar] [CrossRef]
- Brenner, H.; Chang–Claude, J.; Jansen, L.; Knebel, P.; Stock, C.; Hoffmeister, M. Reduced Risk of Colorectal Cancer Up to 10 Years After Screening, Surveillance, or Diagnostic Colonoscopy. Gastroenterology 2014, 146, 709–717. [Google Scholar] [CrossRef] [Green Version]
- Carr, P.R.; Jansen, L.; Bienert, S.; Roth, W.; Herpel, E.; Kloor, M.; Bläker, H.; Chang-Claude, J.; Brenner, H.; Hoffmeister, M. Associations of red and processed meat intake with major molecular pathological features of colorectal cancer. Eur. J. Epidemiol. 2017, 32, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Carr, P.R.; Weigl, K.; Jansen, L.; Walter, V.; Erben, V.; Chang-Claude, J.; Brenner, H.; Hoffmeister, M. Healthy Lifestyle Factors Associated With Lower Risk of Colorectal Cancer Irrespective of Genetic Risk. Gastroenterology 2018, 155, 1805–1815.e5. [Google Scholar] [CrossRef]
- Carr, P.R.; Weigl, K.; Edelmann, D.; Jansen, L.; Chang-Claude, J.; Brenner, H.; Hoffmeister, M. Estimation of Absolute Risk of Colorectal Cancer Based on Healthy Lifestyle, Genetic Risk, and Colonoscopy Status in a Population-Based Study. Gastroenterology 2020, 159, 129–138.e9. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z. Variable selection with stepwise and best subset approaches. Ann. Transl. Med. 2016, 4, 136. [Google Scholar] [CrossRef] [Green Version]
- Bundeszentrale Für Gesundheitliche Aufklärung Alkohol? Kenn Dein Limit. Available online: https://www.kenn-dein-limit.info/risikoarmer-konsum.html (accessed on 7 September 2021).
- Brenner, H.; Gefeller, O.; Greenland, S. Risk and rate advancement periods as measures of exposure impact on the occurrence of chronic diseases. Epidemiology 1993, 4, 229–236. [Google Scholar] [CrossRef]
- Le Marchand, L.; Wilkens, L.R.; Hankin, J.H.; Kolonel, L.N.; Lyu, L.C. A case-control study of diet and colorectal cancer in a multiethnic population in Hawaii (United States): Lipids and foods of animal origin. Cancer Causes Control 1997, 8, 637–648. [Google Scholar] [CrossRef]
- Vieira, A.R.; Abar, L.; Chan, D.S.M.; Vingeliene, S.; Polemiti, E.; Stevens, C.; Greenwood, D.; Norat, T. Foods and beverages and colorectal cancer risk: A systematic review and meta-analysis of cohort studies, an update of the evidence of the WCRF-AICR Continuous Update Project. Ann. Oncol. 2017, 28, 1788–1802. [Google Scholar] [CrossRef] [PubMed]
- Farvid, M.S.; Sidahmed, E.; Spence, N.D.; Mante Angua, K.; Rosner, B.A.; Barnett, J.B. Consumption of red meat and processed meat and cancer incidence: A systematic review and meta-analysis of prospective studies. Eur. J. Epidemiol. 2021, 36, 937–951. [Google Scholar] [CrossRef] [PubMed]
- Hemeryck, L.Y.; Rombouts, C.; De Paepe, E.; Vanhaecke, L. DNA adduct profiling of in vitro colonic meat digests to map red vs. white meat genotoxicity. Food Chem. Toxicol. 2018, 115, 73–87. [Google Scholar] [CrossRef] [PubMed]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Red Meat and Processed Meat/IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; International Agency for Research on Cancer: Lyon, France, 2018. [Google Scholar]
- Crowe, W.; Elliott, C.T.; Green, B.D. A Review of the In Vivo Evidence Investigating the Role of Nitrite Exposure from Processed Meat Consumption in the Development of Colorectal Cancer. Nutrients 2019, 11, 2673. [Google Scholar] [CrossRef] [Green Version]
- Seiwert, N.; Heylmann, D.; Hasselwander, S.; Fahrer, J. Mechanism of colorectal carcinogenesis triggered by heme iron from red meat. Biochim. Biophys. Acta-Rev. Cancer 2020, 1873, 188334. [Google Scholar] [CrossRef]
- Song, M.; Chan, A.T.; Sun, J. Influence of the Gut Microbiome, Diet, and Environment on Risk of Colorectal Cancer. Gastroenterology 2020, 158, 322–340. [Google Scholar] [CrossRef]
- Loke, Y.L.; Chew, M.T.; Ngeow, Y.F.; Lim, W.W.D.; Peh, S.C. Colon Carcinogenesis: The Interplay Between Diet and Gut Microbiota. Front. Cell. Infect. Microbiol. 2020, 10, 169–179. [Google Scholar] [CrossRef]
- Kraft, P.; Aschard, H. Finding the missing gene–environment interactions. Eur. J. Epidemiol. 2015, 30, 353–355. [Google Scholar] [CrossRef] [Green Version]
- Bishop, Y.M.; Fienberg, S.E.; Holland, P.W. Discrete Multivariate Analysis: Theory and Practice; MIT Press: Cambridge, MA, USA, 1975. [Google Scholar]
- Peters, U.; Jiao, S.; Schumacher, F.R.; Hutter, C.M.; Aragaki, A.K.; Baron, J.A.; Berndt, S.I.; Bézieau, S.; Brenner, H.; Butterbach, K.; et al. Identification of Genetic Susceptibility Loci for Colorectal Tumors in a Genome-Wide Meta-analysis. Gastroenterology 2013, 144, 799–807.e24. [Google Scholar] [CrossRef] [Green Version]
- Schumacher, F.R.; Schmit, S.L.; Jiao, S.; Edlund, C.K.; Wang, H.; Zhang, B.; Hsu, L.; Huang, S.-C.; Fischer, C.P.; Harju, J.F.; et al. Genome-wide association study of colorectal cancer identifies six new susceptibility loci. Nat. Commun. 2015, 6, 7138. [Google Scholar] [CrossRef] [Green Version]

| Characteristics | Cases, N (%) | Controls, N (%) | p Value 6 |
|---|---|---|---|
| Total | 5109 | 4143 | |
| Sex: Male | 3077 (60.2) | 2540 (61.4) | |
| Age: Median (Q1, Q3) | 69 (62, 76) | 70 (62, 76) | |
| School education (years) | |||
| <9 | 3336 (65.3) | 2280 (55.2) | |
| 9–10 | 906 (17.7) | 877 (21.2) | <0.0001 |
| >10 | 858 (16.8) | 970 (23.5) | |
| Red and processed meat intake 1 | |||
| ≤1 time/week | 404 (7.9) | 482 (11.7) | |
| multiple times/week | 3067 (60.0) | 2514 (60.8) | |
| 1 time/day | 1411 (27.6) | 1010 (24.4) | <0.0001 |
| >1 time/day | 227 (4.4) | 128 (3.1) | |
| Smoking status | |||
| Never | 2283 (44.7) | 2088 (50.5) | |
| Former | 2039 (39.9) | 1586 (38.4) | <0.0001 |
| Current | 767 (15.0) | 449 (10.9) | |
| Alcohol consumption 2 | |||
| Above recommended threshold | 1318 (25.8) | 936 (22.6) | <0.001 |
| Physical activity (MET-hour/week) 3 | |||
| Q1 (≤121.6) | 1145 (22.4) | 1032 (25.0) | |
| Q2 (121.7–178.5) | 1247 (24.4) | 1030 (24.9) | |
| Q3 (178.6–244.7) | 1234 (24.2) | 1030 (24.9) | 0.0029 |
| Q4 (>244.7) | 1421 (27.8) | 1031 (24.9) | |
| BMI (kg/m2, about 10 years before enrolment) | |||
| <25 | 1537 (30.1) | 1578 (38.2) | |
| 25–<30 | 2365 (46.3) | 1875 (45.4) | <0.0001 |
| 30+ | 1137 (22.3) | 649 (15.7) | |
| History of diabetes | 971 (19.0) | 559 (13.5) | <0.0001 |
| Family history of colorectal cancer | 738 (14.4) | 450 (10.9) | <0.0001 |
| Use of NSAIDs 4 | 1453 (28.4) | 1572 (38.0) | <0.0001 |
| Use of statins 5 | 874 (17.1) | 927 (22.4) | <0.0001 |
| History of colonoscopy | 1359 (26.6) | 2493 (60.3) | <0.0001 |
| Fish < 1 time/week 1 | 1562 (30.6) | 1191 (28.8) | 0.065 |
| Whole grains < 1 time/day 1 | 3257 (63.8) | 2202 (53.3) | <0.0001 |
| Vegetables < 1 time/day 1 | 4243 (83.0) | 3108 (75.2) | <0.0001 |
| Fruits < 1 time/day 1 | 1907 (37.3) | 1223 (29.6) | <0.0001 |
| Dairy foods ≤ 1 time/day 1 | 2906 (56.9) | 2059 (49.8) | <0.0001 |
| Red and Processed Meat Intake | Cases, N (%) | Controls, N (%) | OR (95% CI) 1 | OR (95% CI) 2 |
|---|---|---|---|---|
| ≤1 time/week | 387 (7.9) | 469 (11.7) | Ref. | Ref. |
| Multiple times/week | 2925 (59.8) | 2444 (60.9) | 1.49 (1.29, 1.73) | 1.19 (1.01, 1.41) |
| 1 time/day | 1358 (27.8) | 979 (24.4) | 1.76 (1.49, 2.06 | 1.41 (1.18, 1.70) |
| >1 time/day | 222 (4.5) | 124 (3.1) | 2.27 (1.75, 2.95) | 1.73 (1.30, 2.32) |
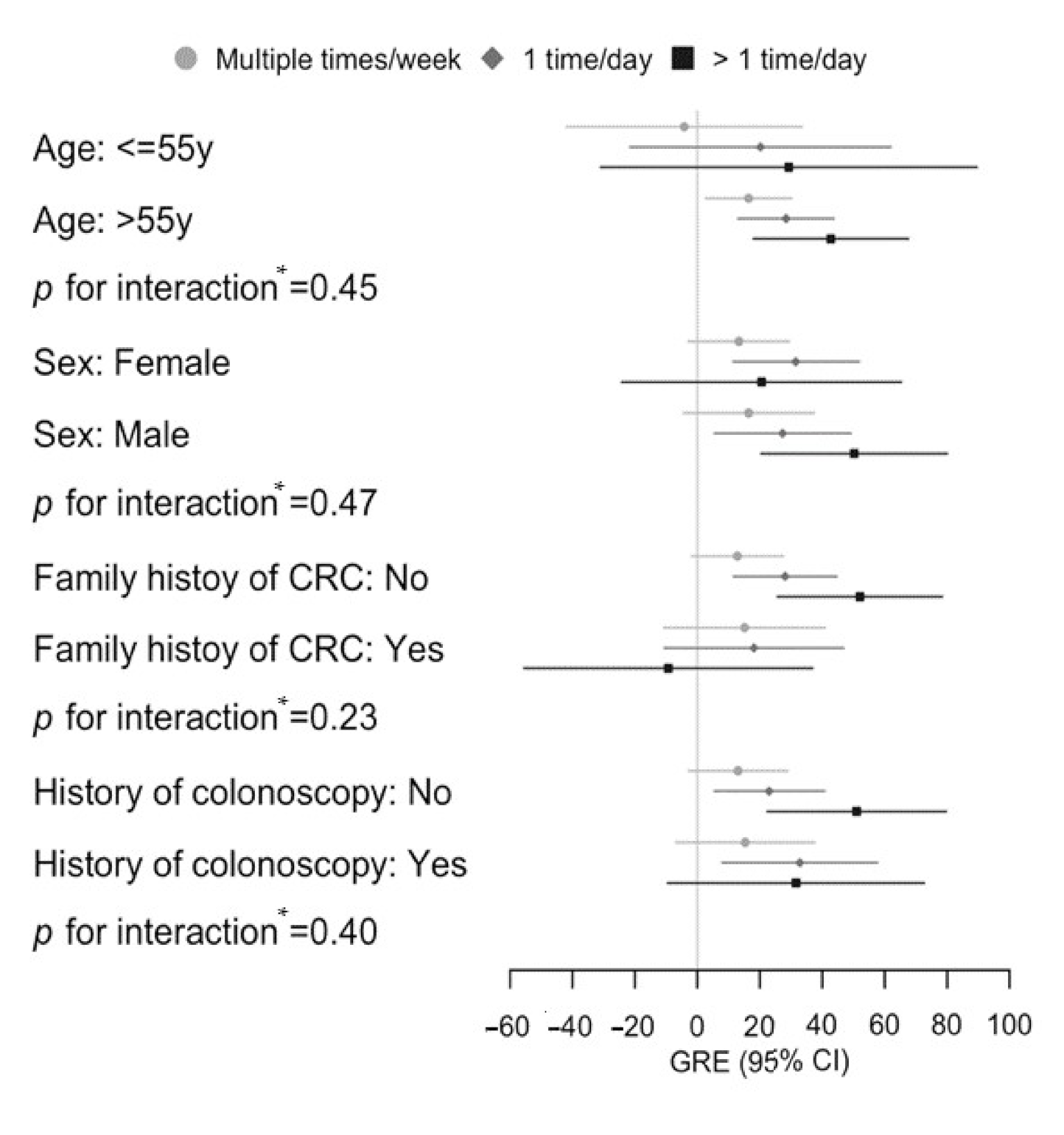
| p value for interaction 3 | 0.97 |
| PRS 1 | Red and Processed Meat Intake | |||||
|---|---|---|---|---|---|---|
| ≤1 Time/Week | Multiple Times/Week | 1 Time/Day | >1 Time/Day | Per Category Increase | ||
| Very low | Cases, N (%) | 13 (6.0) | 128 (59.3) | 65 (30.1) | 10 (4.6) | |
| Controls, N (%) | 48 (11.9) | 232 (57.7) | 105 (26.1) | 17 (4.2) | ||
| OR (95% CI) 2 | Ref. | 2.07 (1.11, 4.12) | 2.32 (1.18, 4.80) | 2.35 (0.85, 6.49) | 1.27 (0.99, 1.62) | |
| OR (95% CI) 3 | Ref. | 1.64 (0.81, 3.51) | 1.83 (0.86, 4.08) | 1.82 (0.57, 5.80) | 1.19 (0.90, 1.57) | |
| Low | Cases, N (%) | 41 (8.3) | 290 (58.7) | 136 (27.5) | 27 (5.5) | |
| Controls, N (%) | 70 (11.6) | 376 (62.0) | 143 (23.6) | 17 (2.8) | ||
| OR (95% CI) 2 | Ref. | 1.34 (0.88, 2.05) | 1.66 (1.05, 2.65) | 2.78 (1.36, 5.84) | 1.33 (1.11, 1.59) | |
| OR (95% CI) 3 | Ref. | 0.96 (0.59, 1.56) | 1.12 (0.66, 1.91) | 1.70 (0.76, 3.87) | 1.16 (0.94, 1.43) | |
| Medium | Cases, N (%) | 209 (8.7) | 1433 (59.5) | 663 (27.5) | 104 (4.3) | |
| Controls, N (%) | 228 (11.3) | 1229 (61.2) | 488 (24.3) | 64 (3.2) | ||
| OR (95% CI) 2 | Ref. | 1.30 (1.06, 1.59) | 1.53 (1.22, 1.92) | 1.83 (1.27, 2.65) | 1.21 (1.11, 1.33) | |
| OR (95% CI) 3 | Ref. | 1.05 (0.83, 1.31) | 1.26 (0.98, 1.62) | 1.39 (0.93, 2.08) | 1.15 (1.04, 1.27) | |
| High | Cases, N (%) | 61 (6.6) | 555 (60.3) | 259 (28.2) | 45 (4.9) | |
| Controls, N (%) | 73 (12.2) | 365 (60.9) | 145 (24.2) | 16 (2.7) | ||
| OR (95% CI) 2 | Ref. | 1.92 (1.32, 2.78) | 2.29 (1.53, 3.46) | 3.57 (1.85, 7.18) | 1.40 (1.19, 1.65) | |
| OR (95% CI) 3 | Ref. | 1.69 (1.12, 2.56) | 2.06 (1.31, 3.24) | 2.63 (1.27, 5.65) | 1.34 (1.12, 1.61) | |
| Very high | Cases, N (%) | 63 (7.4) | 519 (60.8) | 235 (27.5) | 36 (4.2) | |
| Controls, N (%) | 50 (12.5) | 242 (60.5) | 98 (24.5) | 10 (2.5) | ||
| OR (95% CI) 2 | Ref. | 1.85 (1.22, 2.78) | 2.14 (1.36, 3.38) | 3.33 (1.53, 7.80) | 1.37 (1.14, 1.66) | |
| OR (95% CI) 3 | Ref. | 1.51 (0.94, 2.43) | 1.65 (0.98, 2.77) | 2.80 (1.16, 7.18) | 1.26 (1.02, 1.56) | |
| p value for interaction = 0.79 4 | ||||||
| PRS 1 | Red and Processed Meat Intake | |||
|---|---|---|---|---|
| ≤1 Time/Week | Multiple Times/Week | 1 Time/Day | >1 Time/Day | |
| OR (95% CI) 2 | OR (95% CI) 2 | OR (95% CI) 2 | OR (95% CI) 2 | |
| Very low | 0.30 (0.15, 0.58) | 0.49 (0.36, 0.67) | 0.58 (0.39, 0.87) | 0.50 (0.20, 1.20) |
| Low | 0.66 (0.41, 1.05) | 0.68 (0.52, 0.89) | 0.79 (0.56, 1.10) | 1.20 (0.61, 2.42) |
| Medium | Ref. | 1.05 (0.84, 1.32) | 1.28 (1.00, 1.64) | 1.42 (0.95, 2.12) |
| High | 0.83 (0.54, 1.27) | 1.38 (1.07, 1.78) | 1.71 (1.26, 2.32) | 2.34 (1.24, 4.61) |
| Very high | 1.49 (0.95, 2.36) | 1.96 (1.50, 2.56) | 2.08 (1.49, 2.90) | 3.23 (1.52, 7.41) |
| Red and Processed Meat Intake | ||||
|---|---|---|---|---|
| ≤1 Time/Week | Multiple Times/Week | 1 Time/Day | >1 Time/Day | |
| Controls, N (%) | 469 (11.7) | 2444 (60.9) | 979 (24.4) | 124 (3.1) |
| Cases (All), N (%) | 387 (7.9) | 2925 (59.8) | 1358 (27.8) | 222 (4.5) |
| OR (95% CI) 1 | Ref. | 1.19 (1.01, 1.40) | 1.41 (1.18, 1.69) | 1.74 (1.31, 2.33) |
| GRE (95% CI) | Ref. | 13.3 (0.6, 26.0) | 26.2 (12.0, 40.4) | 42.3 (19.6, 64.9) |
| Cases (Colon) 2, N (%) | 256 (8.6) | 1812 (60.8) | 782 (26.3) | 128 (4.3) |
| OR (95% CI) 1 | Ref. | 1.17 (0.98, 1.41) | 1.32 (1.08, 1.61) | 1.76 (1.28, 2.43) |
| GRE (95% CI) | Ref. | 12.8 (−2.3, 28.0) | 22.7 (5.9, 39.6) | 46.3 (19.1, 73.4) |
| Cases (Proximal colon), N (%) | 146 (8.8) | 1027 (62.0) | 420 (25.4) | 63 (3.8) |
| OR (95% CI) 1 | Ref. | 1.28 (1.03, 1.60) | 1.40 (1.10, 1.78) | 1.73 (1.17, 2.56) |
| GRE (95% CI) | Ref. | 21.8 (2.1, 41.5) | 29.7 (7.8, 51.6) | 48.4 (12.9, 83.9) |
| Cases (Distal colon), N (%) | 110 (8.3) | 782 (59.3) | 362 (27.4) | 65 (4.9) |
| OR (95% CI) 1 | Ref. | 1.06 (0.83, 1.37) | 1.22 (0.93, 1.61) | 1.76 (1.17, 2.65) |
| GRE (95% CI) | Ref. | 4.2 (−13.6, 21.9) | 14.2 (−5.3, 33.7) | 40.4 (10.5, 70.4) |
| Cases (Rectum), N (%) | 131 (6.8) | 1113 (58.2) | 576 (30.1) | 94 (4.9) |
| OR (95% CI) 1 | Ref. | 1.26 (1.00, 1.60) | 1.64 (1.28, 2.12) | 1.82 (1.23, 2.67) |
| GRE (95% CI) | Ref. | 17.6 (−0.6, 35.9) | 37.8 (17.4, 58.1) | 45.7 (15.5, 75.9) |
| Cases (Stages I–III), N (%) | 334 (8.0) | 2488 (59.9) | 1144 (27.5) | 187 (4.5) |
| OR (95% CI) 1 | Ref. | 1.18 (0.99, 1.40) | 1.37 (1.14, 1.66) | 1.76 (1.31, 2.38) |
| GRE (95% CI) | Ref. | 12.6 (−0.6, 25.8) | 24.0 (9.4, 38.7) | 43.1 (19.7, 66.6) |
| Cases (Stage IV), N (%) | 49 (7.1) | 410 (59.0) | 203 (29.2) | 33 (4.7) |
| OR (95% CI) 1 | Ref. | 1.31 (0.94, 1.85) | 1.66 (1.16, 2.40) | 1.80 (1.04, 3.07) |
| GRE (95% CI) | Ref. | 20.6 (−5.6, 46.8) | 38.7 (9.7, 67.7) | 44.9 (2.5, 87.2) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Hoffmeister, M.; Brenner, H. Red and Processed Meat Intake, Polygenic Risk Score, and Colorectal Cancer Risk. Nutrients 2022, 14, 1077. https://doi.org/10.3390/nu14051077
Chen X, Hoffmeister M, Brenner H. Red and Processed Meat Intake, Polygenic Risk Score, and Colorectal Cancer Risk. Nutrients. 2022; 14(5):1077. https://doi.org/10.3390/nu14051077
Chicago/Turabian StyleChen, Xuechen, Michael Hoffmeister, and Hermann Brenner. 2022. "Red and Processed Meat Intake, Polygenic Risk Score, and Colorectal Cancer Risk" Nutrients 14, no. 5: 1077. https://doi.org/10.3390/nu14051077






