Effects of β-Hydroxy β-Methylbutyrate Supplementation on Working Memory and Hippocampal Long-Term Potentiation in Rodents
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
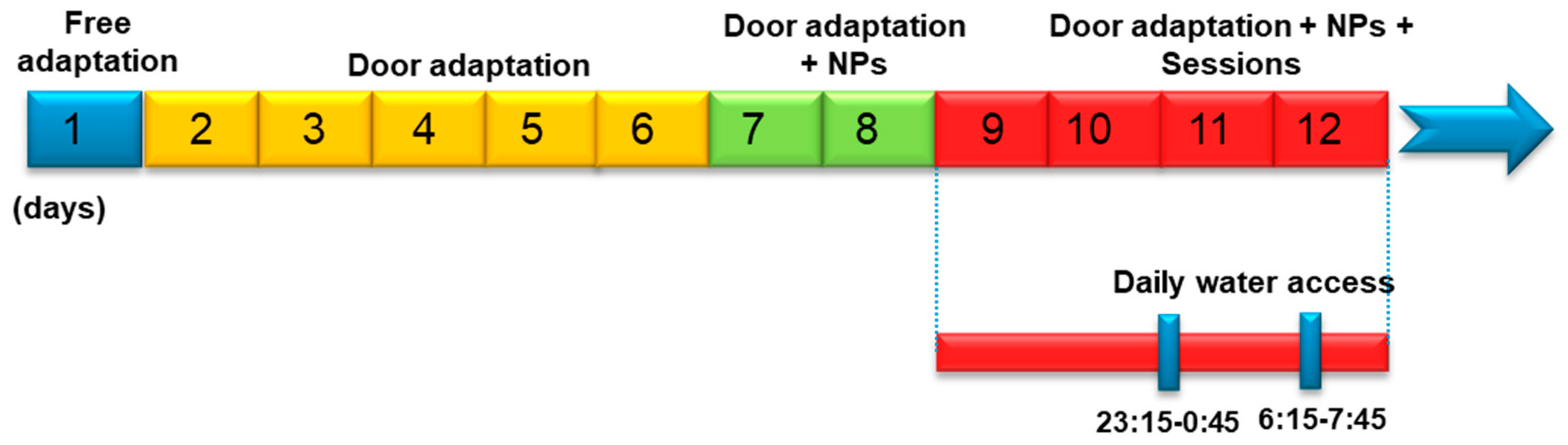
2.2. Experimental Designs
2.2.1. Long-Term Potentiation Study in Rats
2.2.2. Delayed Matching-to-Position Task in Mice
3. Results
3.1. HMB Effects on Hippocampal LTP Evoked in Alert Behaving Rats
3.2. HMB Effects on Delayed Matching to Position Task in Mice
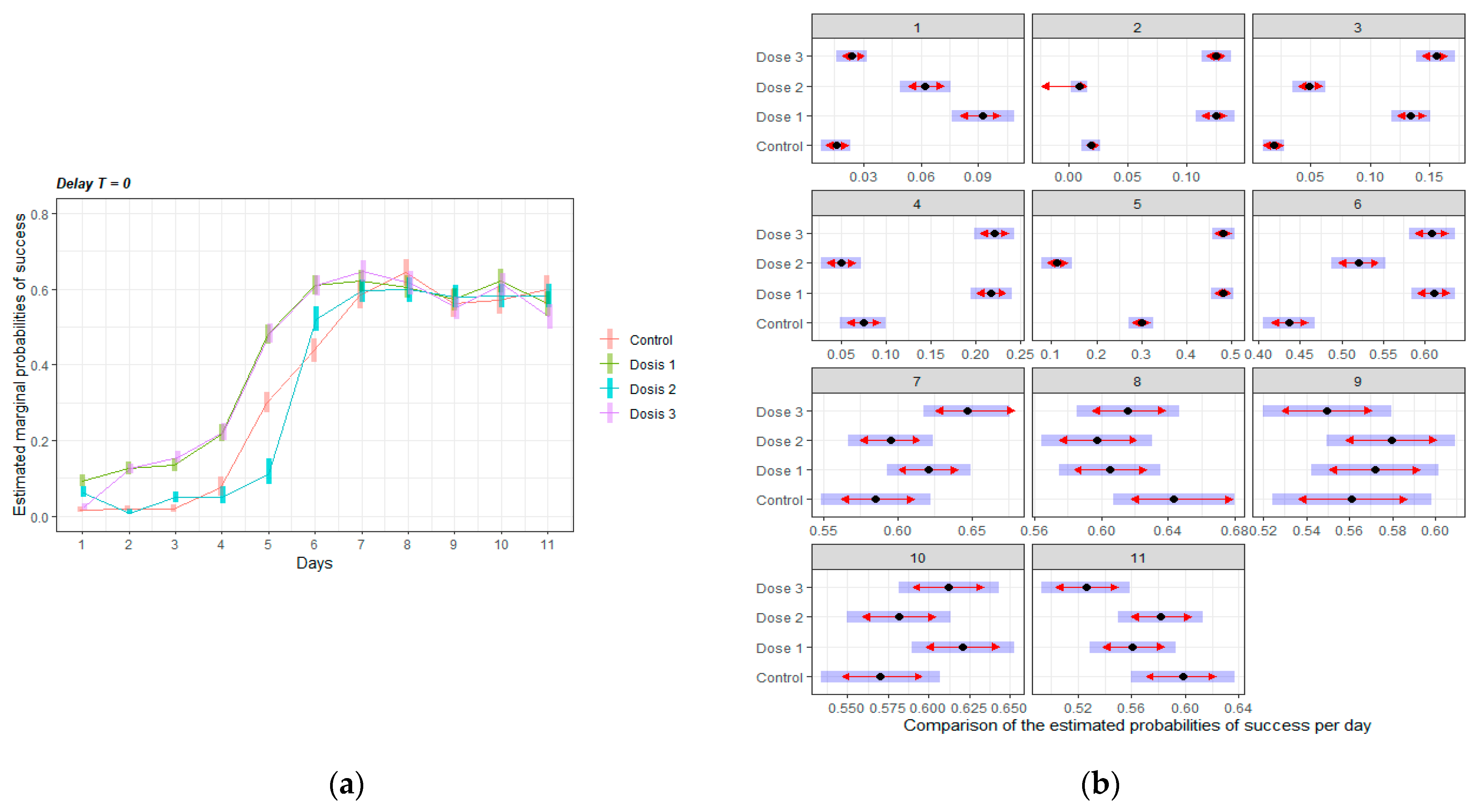
3.2.1. Acquisition of the Delayed Spatial Matching-to-Position Task
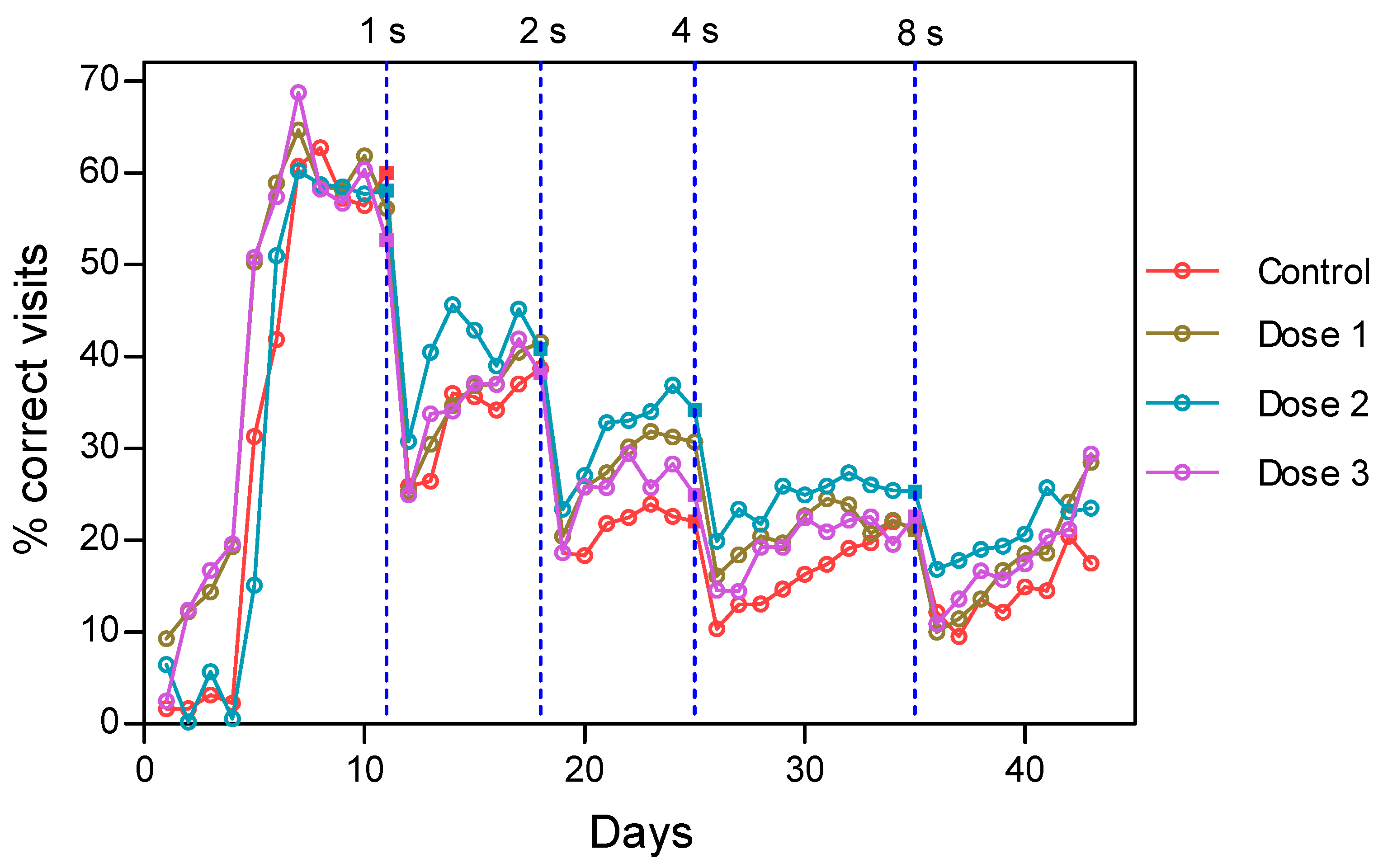
3.2.2. Intervals Test of the Delayed Spatial Matching-to-Position Task
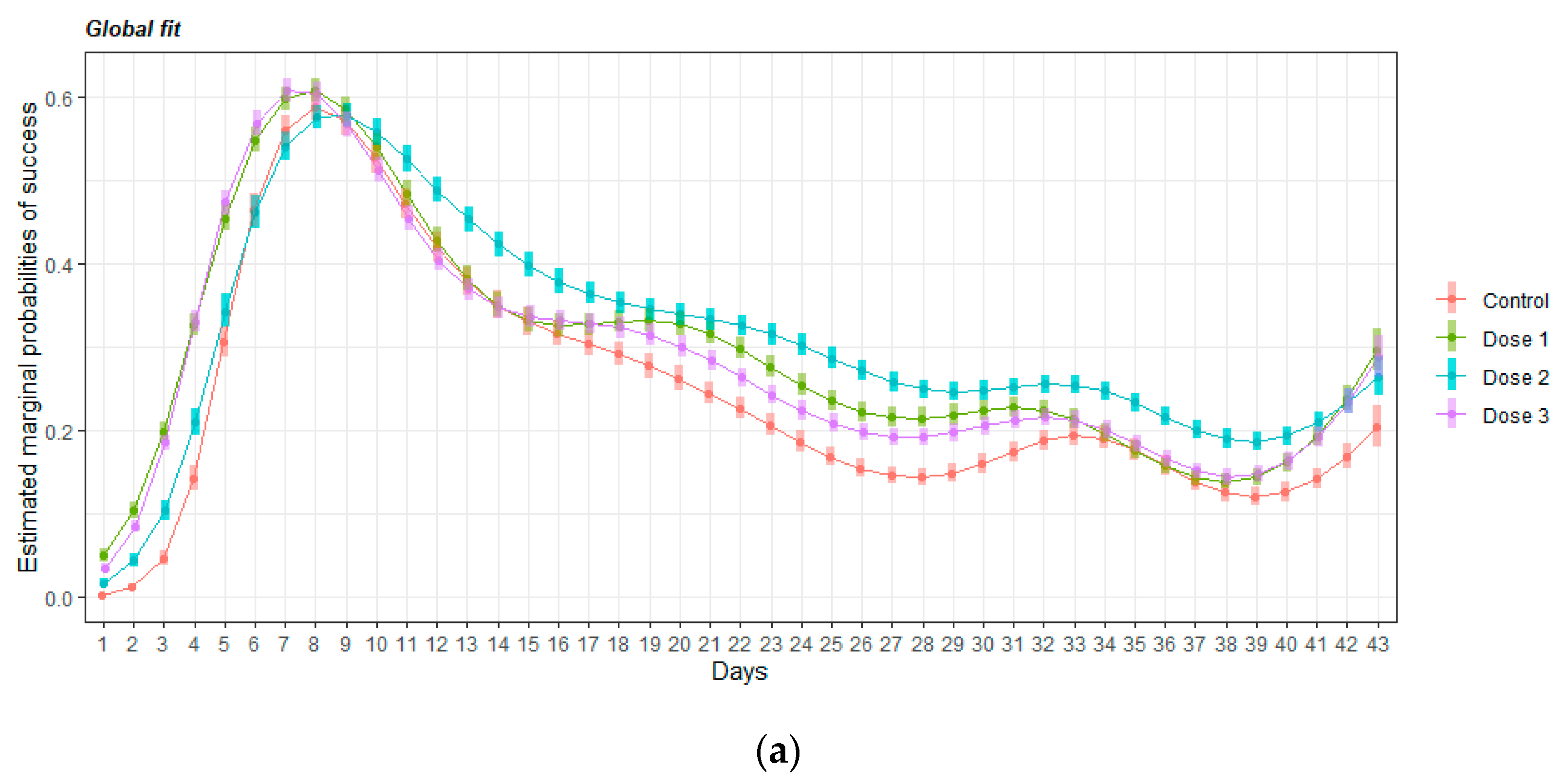
3.2.3. Retraining Effects in the Delayed Spatial Matching-to-Position Task
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Harada, C.N.; Natelson Love, M.C.; Triebel, K.L. Normal cognitive aging. Clin. Geriatr. Med. 2013, 29, 737–752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murman, D.L. The Impact of Age on Cognition. Semin. Hear. 2015, 36, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Luszcz, M.A.; Bryan, J. Toward understanding age-related memory loss in late adulthood. Gerontology 1999, 45, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Barnes, D.E.; Yaffe, K. The projected effect of risk factor reduction on Alzheimer’s disease prevalence. Lancet Neurol. 2011, 10, 819–828. [Google Scholar] [CrossRef] [Green Version]
- Klimova, B.; Valis, M.; Kuca, K. Cognitive decline in normal aging and its prevention: A review on non-pharmacological lifestyle strategies. Clin. Interv. Aging 2017, 12, 903–910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Solfrizzi, V.; Panza, F.; Frisardi, V.; Seripa, D.; Logroscino, G.; Imbimbo, B.P.; Pilotto, A. Diet and Alzheimer’s disease risk factors or prevention: The current evidence. Expert Rev. Neurother. 2011, 11, 677–708. [Google Scholar] [CrossRef] [PubMed]
- Flakoll, P.; Sharp, R.; Baier, S.; Levenhagen, D.; Carr, C.; Nissen, S. Effect of beta-hydroxy-beta-methylbutyrate, arginine, and lysine supplementation on strength, functionality, body composition, and protein metabolism in elderly women. Nutrition 2004, 20, 445–451. [Google Scholar] [CrossRef]
- Borack, M.S.; Volpi, E. Efficacy and Safety of Leucine Supplementation in the Elderly. J. Nutr. 2016, 146, 2625S–2629S. [Google Scholar] [CrossRef] [Green Version]
- Wu, H.; Xia, Y.; Jiang, J.; Du, H.; Guo, X.; Liu, X.; Li, C.; Huang, G.; Niu, K. Effect of beta-hydroxy-beta-methylbutyrate supplementation on muscle loss in older adults: A systematic review and meta-analysis. Arch. Gerontol. Geriatr. 2015, 61, 168–175. [Google Scholar] [CrossRef]
- Oktaviana, J.; Zanker, J.; Vogrin, S.; Duque, G. The Effect of beta-hydroxy-beta-methylbutyrate (HMB) on Sarcopenia and Functional Frailty in Older Persons: A Systematic Review. J. Nutr. Health Aging 2019, 23, 145–150. [Google Scholar] [CrossRef]
- Pimentel, G.D.; Rosa, J.C.; Lira, F.S.; Zanchi, N.E.; Ropelle, E.R.; Oyama, L.M.; do Nascimento, C.M.O.; de Mello, M.T.; Tufik, S.; Santos, R.V. β-Hydroxy-β-methylbutyrate (HMβ) supplementation stimulates skeletal muscle hypertrophy in rats via the mTOR pathway. Nutr. Metab. 2011, 8, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kimura, K.; Cheng, X.W.; Inoue, A.; Hu, L.; Koike, T.; Kuzuya, M. β-hydroxy-β-methylbutyrate facilitates pi3k/akt-dependent mammalian target of rapamycin and foxo1/3a phosphorylations and alleviates tumor necrosis factor α/interferon γ–induced murf-1 expression in c2c12 cells. Nutr. Res. 2014, 34, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Kaczka, P.; Michalczyk, M.M.; Jastrząb, R.; Gawelczyk, M.; Kubicka, K. Mechanism of action and the effect of beta-hydroxy-beta-methylbutyrate (HMB) supplementation on different types of physical performance-A systematic review. J. Hum. Kinet. 2019, 68, 211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bockaert, J.; Marin, P. mTOR in Brain Physiology and Pathologies. Physiol. Rev. 2015, 95, 1157–1187. [Google Scholar] [CrossRef]
- Santos-Fandila, A.; Zafra-Gomez, A.; Barranco, A.; Navalon, A.; Rueda, R.; Ramirez, M. Quantitative determination of beta-hydroxymethylbutyrate and leucine in culture media and microdialysates from rat brain by UHPLC-tandem mass spectrometry. Anal. Bioanal. Chem. 2014, 406, 2863–2872. [Google Scholar] [CrossRef]
- Higuchi, K.; Sivaprakasam, S.; Sennoune, S.R.; Ogura, J.; Bhutia, Y.D.; Rueda, R.; Pereira, S.L.; Ganapathy, V. A Proton-Coupled Transport System for β-Hydroxy-β-Methylbutyrate (HMB) in Blood–Brain Barrier Endothelial Cell Line hCMEC/D3. Nutrients 2021, 13, 3220. [Google Scholar] [CrossRef]
- Salto, R.; Vilchez, J.D.; Giron, M.D.; Cabrera, E.; Campos, N.; Manzano, M.; Rueda, R.; Lopez-Pedrosa, J.M. beta-Hydroxy-beta-Methylbutyrate (HMB) Promotes Neurite Outgrowth in Neuro2a Cells. PLoS ONE 2015, 10, e0135614. [Google Scholar] [CrossRef] [Green Version]
- Kougias, D.G.; Nolan, S.O.; Koss, W.A.; Kim, T.; Hankosky, E.R.; Gulley, J.M.; Juraska, J.M. Beta-hydroxy-beta-methylbutyrate ameliorates aging effects in the dendritic tree of pyramidal neurons in the medial prefrontal cortex of both male and female rats. Neurobiol. Aging 2016, 40, 78–85. [Google Scholar] [CrossRef]
- Kougias, D.G.; Hankosky, E.R.; Gulley, J.M.; Juraska, J.M. Beta-hydroxy-beta-methylbutyrate (HMB) ameliorates age-related deficits in water maze performance, especially in male rats. Physiol. Behav. 2017, 170, 93–99. [Google Scholar] [CrossRef]
- Hankosky, E.R.; Sherrill, L.K.; Ruvola, L.A.; Haake, R.M.; Kim, T.; Hammerslag, L.R.; Kougias, D.G.; Juraska, J.M.; Gulley, J.M. Effects of beta-hydroxy-beta-methyl butyrate on working memory and cognitive flexibility in an animal model of aging. Nutr. Neurosci. 2017, 20, 379–387. [Google Scholar] [CrossRef]
- Costa Riela, N.A.; Alvim Guimaraes, M.M.; Oliveira de Almeida, D.; Araujo, E.M.Q. Effects of Beta-Hydroxy-Beta-Methylbutyrate Supplementation on Elderly Body Composition and Muscle Strength: A Review of Clinical Trials. Ann. Nutr. Metab. 2021, 77, 16–22. [Google Scholar] [CrossRef]
- Szczesniak, K.A.; Ostaszewski, P.; Fuller, J.C., Jr.; Ciecierska, A.; Sadkowski, T. Dietary supplementation of beta-hydroxy-beta-methylbutyrate in animals—A review. J. Anim. Physiol. Anim. Nutr. 2015, 99, 405–417. [Google Scholar] [CrossRef] [PubMed]
- Masuda, A.; Kobayashi, Y.; Itohara, S. Automated, Long-term Behavioral Assay for Cognitive Functions in Multiple Genetic Models of Alzheimer’s Disease, Using IntelliCage. JoVE J. Vis. Exp. 2018, 138, e58009. [Google Scholar] [CrossRef] [PubMed]
- Goto, K.; Kurashima, R.; Watanabe, S. Delayed matching-to-position performance in C57BL/6N mice. Behav. Processes 2010, 84, 591–597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lisman, J.; Cooper, K.; Sehgal, M.; Silva, A.J. Memory formation depends on both synapse-specific modifications of synaptic strength and cell-specific increases in excitability. Nat. Neurosci. 2018, 21, 309–314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nair, A.B.; Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 2016, 7, 27. [Google Scholar] [CrossRef] [Green Version]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates; Academic Press: San Diego, CA, USA, 1998. [Google Scholar]
- Gruart, A.; Munoz, M.D.; Delgado-Garcia, J.M. Involvement of the CA3-CA1 synapse in the acquisition of associative learning in behaving mice. J. Neurosci. 2006, 26, 1077–1087. [Google Scholar] [CrossRef]
- Madronal, N.; Delgado-Garcia, J.M.; Gruart, A. Differential effects of long-term potentiation evoked at the CA3 CA1 synapse before, during, and after the acquisition of classical eyeblink conditioning in behaving mice. J. Neurosci. 2007, 27, 12139–12146. [Google Scholar] [CrossRef]
- Valenzuela-Harrington, M.; Gruart, A.; Delgado-Garcia, J.M. Contribution of NMDA receptor NR2B subunit to synaptic plasticity during associative learning in behaving rats. Eur. J. Neurosci. 2007, 25, 830–836. [Google Scholar] [CrossRef]
- Gruart, A.; Sánchez-Campusano, R.; Fernández-Guizán, A.; Delgado-García, J.M. A Differential and Timed Contribution of Identified Hippocampal Synapses to Associative Learning in Mice. Cereb Cortex. 2015, 25, 2542–2555. [Google Scholar] [CrossRef] [Green Version]
- Sanchez-Campusano, R.; Gruart, A.; Delgado-Garcia, J.M. Timing and causality in the generation of learned eyelid responses. Front. Integr. Neurosci. 2011, 5, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- TSE-Systems. Available online: https://www.tse-systems.com/product-details/intellicage/ (accessed on 3 November 2021).
- Voikar, V.; Krackow, S.; Lipp, H.P.; Rau, A.; Colacicco, G.; Wolfer, D.P. Automated dissection of permanent effects of hippocampal or prefrontal lesions on performance at spatial, working memory and circadian timing tasks of C57BL/6 mice in IntelliCage. Behav. Brain Res. 2018, 352, 8–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heinla, I.; Ahlgren, J.; Vasar, E.; Voikar, V. Behavioural characterization of C57BL/6N and BALB/c female mice in social home cage—Effect of mixed housing in complex environment. Physiol. Behav. 2018, 188, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.L.; Brown, R.E. The lonely mouse: Verification of a separation-induced model of depression in female mice. Behav. Brain Res. 2010, 207, 196–207. [Google Scholar] [CrossRef]
- Krackow, S.; Vannoni, E.; Codita, A.; Mohammed, A.H.; Cirulli, F.; Branchi, I.; Alleva, E.; Reichelt, A.; Willuweit, A.; Voikar, V.; et al. Consistent behavioral phenotype differences between inbred mouse strains in the IntelliCage. Genes Brain Behav. 2010, 9, 722–731. [Google Scholar] [CrossRef]
- Dudchenko, P.A. An overview of the tasks used to test working memory in rodents. Neurosci. Biobehav. Rev. 2004, 28, 699–709. [Google Scholar] [CrossRef]
- Woolley, M.L.; Ballard, T.M. Age-related impairments in operant DMTP performance in the PS2APP mouse, a transgenic mouse model of Alzheimer’s disease. Behav. Brain Res. 2005, 161, 220–228. [Google Scholar] [CrossRef]
- Wiig, K.A.; Burwell, R.D. Memory impairment on a delayed non-matching-to-position task after lesions of the perirhinal cortex in the rat. Behav. Neurosci. 1998, 112, 827–838. [Google Scholar] [CrossRef]
- Glisky, E.L. Changes in Cognitive Function in Human Aging. In Brain Aging: Models, Methods, and Mechanisms; Riddle, D.R., Ed.; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Rakesh, G.; Szabo, S.T.; Alexopoulos, G.S.; Zannas, A.S. Strategies for dementia prevention: Latest evidence and implications. Ther. Adv. Chronic Dis. 2017, 8, 121–136. [Google Scholar] [CrossRef]
- Craik, F.I.; Winocur, G.; Palmer, H.; Binns, M.A.; Edwards, M.; Bridges, K.; Glazer, P.; Chavannes, R.; Stuss, D.T. Cognitive rehabilitation in the elderly: Effects on memory. J. Int. Neuropsychol. Soc. 2007, 13, 132–142. [Google Scholar] [CrossRef]
- Munroe, M.; Pincu, Y.; Merritt, J.; Cobert, A.; Brander, R.; Jensen, T.; Rhodes, J.; Boppart, M.D. Impact of beta-hydroxy beta-methylbutyrate (HMB) on age-related functional deficits in mice. Exp. Gerontol. 2017, 87, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Buckley, M.G.; Bast, T. A new human delayed-matching-to-place test in a virtual environment reverse-translated from the rodent watermaze paradigm: Characterization of performance measures and sex differences. Hippocampus 2018, 28, 796–812. [Google Scholar] [CrossRef] [PubMed]
- Sloan, H.L.; Dobrossy, M.; Dunnett, S.B. Hippocampal lesions impair performance on a conditional delayed matching and non-matching to position task in the rat. Behav. Brain Res. 2006, 171, 240–250. [Google Scholar] [CrossRef] [PubMed]
- Letellier, M.; Levet, F.; Thoumine, O.; Goda, Y. Differential role of pre- and postsynaptic neurons in the activity-dependent control of synaptic strengths across dendrites. PLoS Biol. 2019, 17, e2006223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zwilling, C.E.; Strang, A.; Anderson, E.; Jurcsisn, J.; Johnson, E.; Das, T.; Kuchan, M.J.; Barbey, A.K. Enhanced physical and cognitive performance in active duty Airmen: Evidence from a randomized multimodal physical fitness and nutritional intervention. Sci. Rep. 2020, 10, 17826. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barranco, A.; Garcia, L.; Gruart, A.; Delgado-Garcia, J.M.; Rueda, R.; Ramirez, M. Effects of β-Hydroxy β-Methylbutyrate Supplementation on Working Memory and Hippocampal Long-Term Potentiation in Rodents. Nutrients 2022, 14, 1090. https://doi.org/10.3390/nu14051090
Barranco A, Garcia L, Gruart A, Delgado-Garcia JM, Rueda R, Ramirez M. Effects of β-Hydroxy β-Methylbutyrate Supplementation on Working Memory and Hippocampal Long-Term Potentiation in Rodents. Nutrients. 2022; 14(5):1090. https://doi.org/10.3390/nu14051090
Chicago/Turabian StyleBarranco, Alejandro, Llenalia Garcia, Agnes Gruart, Jose Maria Delgado-Garcia, Ricardo Rueda, and Maria Ramirez. 2022. "Effects of β-Hydroxy β-Methylbutyrate Supplementation on Working Memory and Hippocampal Long-Term Potentiation in Rodents" Nutrients 14, no. 5: 1090. https://doi.org/10.3390/nu14051090
APA StyleBarranco, A., Garcia, L., Gruart, A., Delgado-Garcia, J. M., Rueda, R., & Ramirez, M. (2022). Effects of β-Hydroxy β-Methylbutyrate Supplementation on Working Memory and Hippocampal Long-Term Potentiation in Rodents. Nutrients, 14(5), 1090. https://doi.org/10.3390/nu14051090





