Adverse Effects of Infant Formula Made with Corn-Syrup Solids on the Development of Eating Behaviors in Hispanic Children
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Infant Feeding Modality Determination
2.3. Eating Behavior Assessment
2.4. Participant Characteristics
2.5. Statistical Analysis
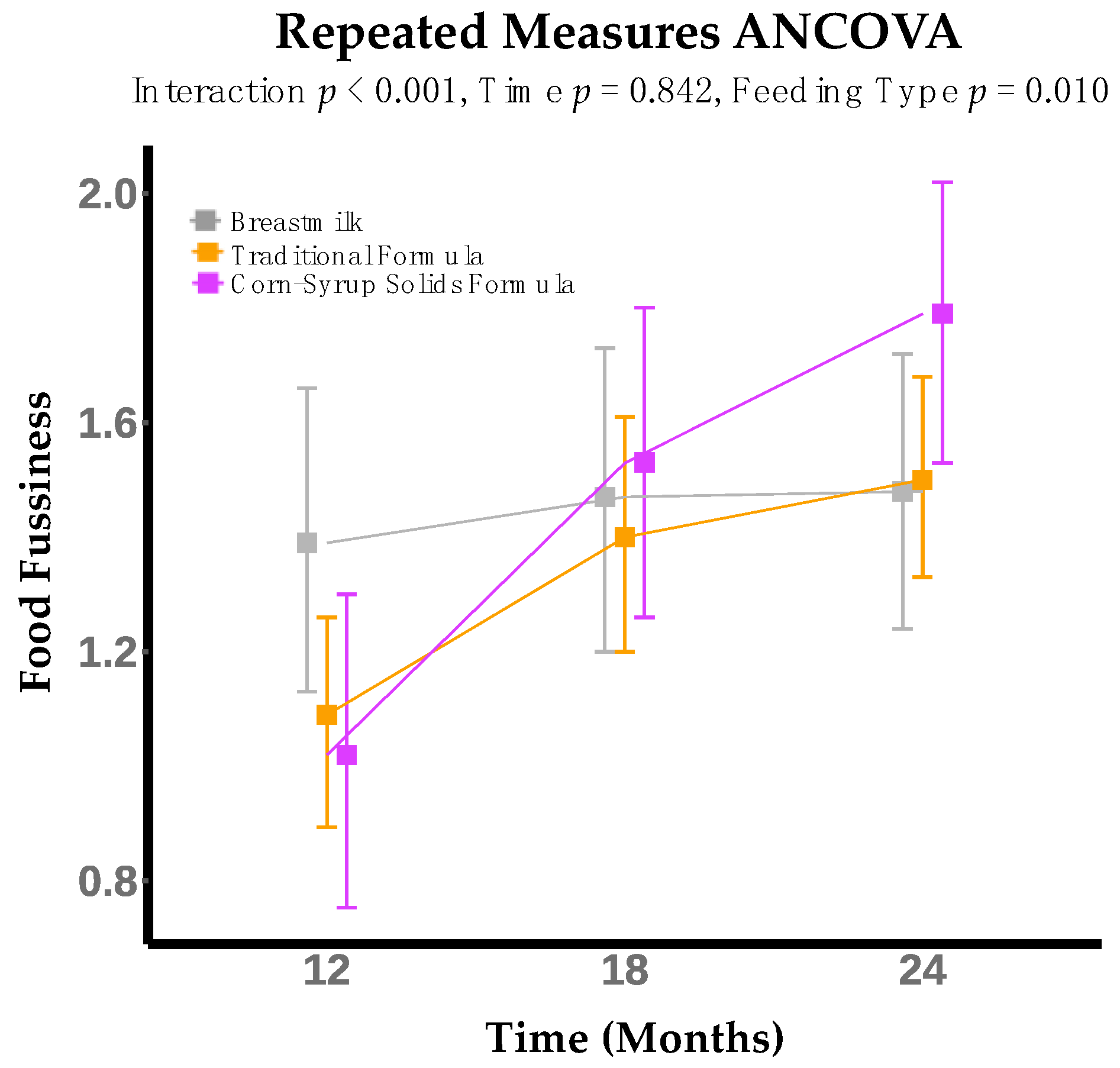
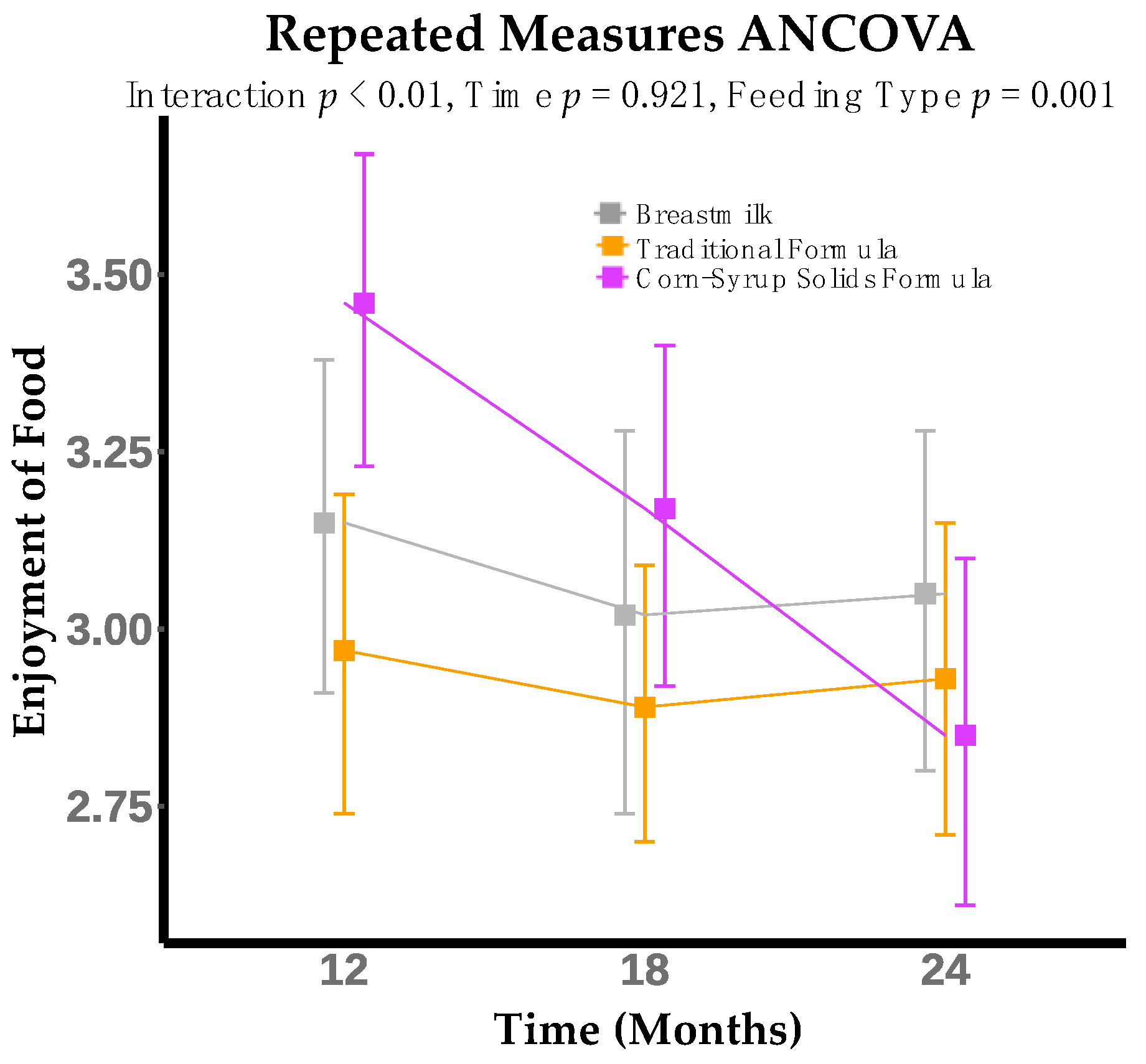
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, Q.; Zhang, Z.; Gregg, E.W.; Flanders, W.D.; Merritt, R.; Hu, F.B. Added Sugar Intake and Cardiovascular Diseases Mortality Among US Adults. JAMA Intern. Med. 2014, 174, 516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fidler Mis, N.; Braegger, C.; Bronsky, J.; Campoy, C.; Domellöf, M.; Embleton, N.D.; Hojsak, I.; Hulst, J.; Indrio, F.; Lapillonne, A.; et al. Sugar in Infants, Children and Adolescents: A Position Paper of the European Society for Paediatric Gastroenterology, Hepatology and Nutrition Committee on Nutrition. J. Pediatr. Gastroenterol. Nutr. 2017, 65, 681–696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rippe, J.M.; Angelopoulos, T.J. Relationship between Added Sugars Consumption and Chronic Disease Risk Factors: Current Understanding. Nutrients 2016, 8, 697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kong, K.L.; Burgess, B.; Morris, K.S.; Re, T.; Hull, H.R.; Sullivan, D.K.; Paluch, R.A. Association between Added Sugars from Infant Formulas and Rapid Weight Gain in US Infants and Toddlers. J. Nutr. 2021, 151, 1572–1580. [Google Scholar] [CrossRef] [PubMed]
- Murray, R.D. Savoring Sweet: Sugars in Infant and Toddler Feeding. Ann. Nutr. Metab. 2017, 70, 38–46. [Google Scholar] [CrossRef] [Green Version]
- Mennella, J.A.; Bobowski, N.K. The Sweetness and Bitterness of Childhood: Insights from Basic Research on Taste Preferences. Physiol. Behav. 2015, 152, 502–507. [Google Scholar] [CrossRef] [Green Version]
- Mennella, J.A.; Bobowski, N.K.; Reed, D.R. The Development of Sweet Taste: From Biology to Hedonics. Rev. Endocr. Metab. Disord. 2016, 17, 171–178. [Google Scholar] [CrossRef] [Green Version]
- Birch, L.L.; Fisher, J.O. Development of Eating Behaviors among Children and Adolescents. Pediatrics 1998, 101, 539–549. [Google Scholar] [CrossRef]
- Hill, C.; Saxton, J.; Webber, L.; Blundell, J.; Wardle, J. The Relative Reinforcing Value of Food Predicts Weight Gain in a Longitudinal Study of 7–10-y-Old Children. Am. J. Clin. Nutr. 2009, 90, 276–281. [Google Scholar] [CrossRef] [Green Version]
- Mennella, J.A.; Forestell, C.A.; Morgan, L.K.; Beauchamp, G.K. Early Milk Feeding Influences Taste Acceptance and Liking during Infancy12345. Am. J. Clin. Nutr. 2009, 90, 780S–788S. [Google Scholar] [CrossRef] [Green Version]
- Noble, E.E.; Hsu, T.M.; Jones, R.B.; Fodor, A.A.; Goran, M.I.; Kanoski, S.E. Early-Life Sugar Consumption Affects the Rat Microbiome Independently of Obesity. J. Nutr. 2017, 147, 20–28. [Google Scholar] [CrossRef] [Green Version]
- Jones, R.B.; Berger, P.K.; Plows, J.F.; Alderete, T.L.; Millstein, J.; Fogel, J.; Iablokov, S.N.; Rodionov, D.A.; Osterman, A.L.; Bode, L.; et al. Lactose-Reduced Infant Formula with Added Corn Syrup Solids Is Associated with a Distinct Gut Microbiota in Hispanic Infants. Gut Microbes 2020, 12, 1813534. [Google Scholar] [CrossRef] [PubMed]
- Harnack, L. Nutrition Data System for Research (NDSR). In Encyclopedia of Behavioral Medicine; Gellman, M.D., Turner, J.R., Eds.; Springer: New York, NY, USA, 2013; pp. 1348–1350. ISBN 978-1-4419-1005-9. [Google Scholar]
- EnfamilŴ GentleaseŴ|Mead Johnson Nutrition. Available online: http://www.hcp.meadjohnson.com/products/mild-digestive-issues-products/enfamil-gentlease/ (accessed on 16 July 2021).
- Rossen, L.M.; Simon, A.E.; Herrick, K.A. Types of Infant Formulas Consumed in the United States. Clin. Pediatr. 2016, 55, 278–285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carnell, S.; Wardle, J. Measuring Behavioural Susceptibility to Obesity: Validation of the Child Eating Behaviour Questionnaire. Appetite 2007, 48, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Wardle, J.; Guthrie, C.A.; Sanderson, S.; Rapoport, L. Development of the Children’s Eating Behaviour Questionnaire. J. Child Psychol. Psychiatry 2001, 42, 963–970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Child Eating Behaviour Questionnaire (CEBQ). Available online: http://www.midss.ie/content/child-eating-behaviour-questionnaire-cebq (accessed on 9 August 2021).
- Peterson, R.A. BestNormalize: Normalizing Transformation Functions. 2021. Available online: https://CRAN.R-project.org/package=bestNormalize (accessed on 6 July 2021).
- Revelle, W. Psych: Procedures for Psychological, Psychometric, and Personality Research. 2021. Available online: https://CRAN.R-project.org/package=psych (accessed on 16 July 2021).
- The Comprehensive R Archive Network. Available online: https://cran.r-project.org/ (accessed on 14 February 2021).
- Taylor, C.M.; Emmett, P.M. Picky Eating in Children: Causes and Consequences. Proc. Nutr. Soc. 2019, 78, 161–169. [Google Scholar] [CrossRef] [Green Version]
- Van der Horst, K. Overcoming Picky Eating. Eating Enjoyment as a Central Aspect of Children’s Eating Behaviors. Appetite 2012, 58, 567–574. [Google Scholar] [CrossRef]
- Shepard, D.N.; Chandler-Laney, P.C. Prospective Associations of Eating Behaviors with Weight Gain in Infants. Obesity 2015, 23, 1881–1885. [Google Scholar] [CrossRef] [Green Version]
- Mennella, J.; Jagnow, C.; Beauchamp, G. Prenatal and Postnatal Flavor Learning by Human Infants. Pediatrics 2001, 107, E88. [Google Scholar] [CrossRef] [Green Version]
- Di Rienzi, S.C.; Britton, R.A. Adaptation of the Gut Microbiota to Modern Dietary Sugars and Sweeteners. Adv. Nutr. 2020, 11, 616–629. [Google Scholar] [CrossRef] [Green Version]
- Townsend, G.E.; Han, W.; Schwalm, N.D.; Raghavan, V.; Barry, N.A.; Goodman, A.L.; Groisman, E.A. Dietary Sugar Silences a Colonization Factor in a Mammalian Gut Symbiont. Proc. Natl. Acad. Sci. USA 2019, 116, 233–238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van de Wouw, M.; Schellekens, H.; Dinan, T.G.; Cryan, J.F. Microbiota-Gut-Brain Axis: Modulator of Host Metabolism and Appetite. J. Nutr. 2017, 147, 727–745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Avena, N.M.; Long, K.A.; Hoebel, B.G. Sugar-Dependent Rats Show Enhanced Responding for Sugar after Abstinence: Evidence of a Sugar Deprivation Effect. Physiol. Behav. 2005, 84, 359–362. [Google Scholar] [CrossRef] [PubMed]
- Coelho, A.I.; Berry, G.T.; Rubio-Gozalbo, M.E. Galactose Metabolism and Health. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 422–427. [Google Scholar] [CrossRef]
- Belamarich, P.F.; Bochner, R.E.; Racine, A.D. A Critical Review of the Marketing Claims of Infant Formula Products in the United States. Clin. Pediatr. 2016, 55, 437–442. [Google Scholar] [CrossRef]

| Participant Characteristics | Analysis of Variance | |||
|---|---|---|---|---|
| (Between-Group Differences by Feeding Type at 6 Months) | ||||
| BM * | TF * | CSSF * | p-Values | |
| Infant Birth Weight (mean, kg) | 3.37 | 3.42 | 3.40 | 0.840 |
| Socioeconomic Status Index | 25.40 | 26.10 | 27.00 | 0.850 |
| Infant Sex (% Female) | 58% | 46% | 65% | 0.290 |
| Mode of Delivery (%Vaginal) | 81% | 76% | 71% | 0.580 |
| Maternal Pre-Pregnancy BMI (mean, kg/m2) | 27.20 | 29.40 | 27.50 | 0.140 |
| Eating Behaviors | Time | Feeding Type | Interaction a |
|---|---|---|---|
| Food Responsiveness | 0.902 | 0.727 | 0.748 |
| Food Fussiness | 0.187 | 0.109 | 0.004 ** |
| Enjoyment of Food | 0.388 | 0.002 ** | 0.001 ** |
| Desire to Drink | 0.005 ** | 0.765 | 0.409 |
| Emotional Undereating | 0.732 | 0.860 | 0.989 |
| Emotional Overeating | 0.391 | 0.484 | 0.418 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hampson, H.E.; Jones, R.B.; Berger, P.K.; Plows, J.F.; Schmidt, K.A.; Alderete, T.L.; Goran, M.I. Adverse Effects of Infant Formula Made with Corn-Syrup Solids on the Development of Eating Behaviors in Hispanic Children. Nutrients 2022, 14, 1115. https://doi.org/10.3390/nu14051115
Hampson HE, Jones RB, Berger PK, Plows JF, Schmidt KA, Alderete TL, Goran MI. Adverse Effects of Infant Formula Made with Corn-Syrup Solids on the Development of Eating Behaviors in Hispanic Children. Nutrients. 2022; 14(5):1115. https://doi.org/10.3390/nu14051115
Chicago/Turabian StyleHampson, Hailey E., Roshonda B. Jones, Paige K. Berger, Jasmine F. Plows, Kelsey A. Schmidt, Tanya L. Alderete, and Michael I. Goran. 2022. "Adverse Effects of Infant Formula Made with Corn-Syrup Solids on the Development of Eating Behaviors in Hispanic Children" Nutrients 14, no. 5: 1115. https://doi.org/10.3390/nu14051115
APA StyleHampson, H. E., Jones, R. B., Berger, P. K., Plows, J. F., Schmidt, K. A., Alderete, T. L., & Goran, M. I. (2022). Adverse Effects of Infant Formula Made with Corn-Syrup Solids on the Development of Eating Behaviors in Hispanic Children. Nutrients, 14(5), 1115. https://doi.org/10.3390/nu14051115







