Anthropometric Measures and Risk of Rheumatoid Arthritis in the French E3N Cohort Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. The E3N Study
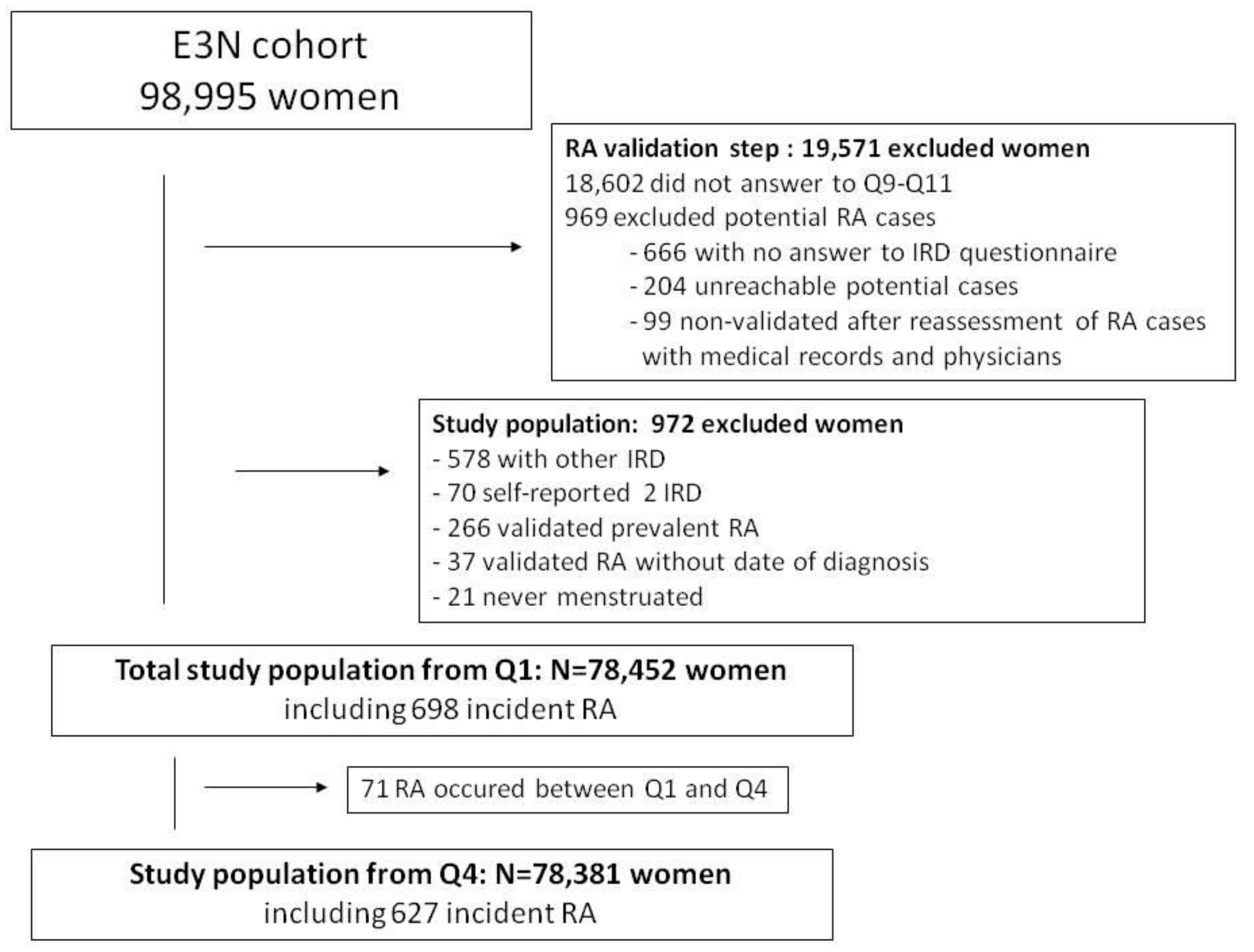
2.2. Validation of RA Cases and Study Population
2.3. Assessment of Anthropometric Measurements
2.4. Covariates
2.5. Statistical Analyses
3. Results
3.1. Study Population
3.2. RA Characteristics
3.3. Associations between Anthropometric Measures and Risk of RA
3.3.1. Obesity and Abdominal Obesity
3.3.2. Body Shapes and Their Trajectories
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Klareskog, L.; Stolt, P.; Lundberg, K.; Källberg, H.; Bengtsson, C.; Grunewald, J.; Rönnelid, J.; Harris, H.E.; Ulfgren, A.K.; Rantapää-Dahlqvist, S.; et al. A new model for an etiology of rheumatoid arthritis: Smoking may trigger HLA–DR (shared epitope)–restricted immune reactions to autoantigens modified by citrullination. Arthritis Rheum. 2006, 54, 38–46. [Google Scholar] [CrossRef]
- Linn-Rasker, S.P.; van der Helm-van Mil, A.H.M.; van Gaalen, F.A.; Kloppenburg, M.; de Vries, R.R.P.; le Cessie, S.; Breedveld, F.C.; Toes, R.E.M.; Huizinga, T.W.J. Smoking is a risk factor for anti-CCP antibodies only in rheumatoid arthritis patients who carry HLA-DRB1 shared epitope alleles. Ann. Rheum. Dis. 2006, 65, 366–371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harpsøe, M.C.; Basit, S.; Andersson, M.; Nielsen, N.M.; Frisch, M.; Wohlfahrt, J.; Nohr, E.A.; Linneberg, A.; Jess, T. Body mass index and risk of autoimmune diseases: A study within the Danish National Birth Cohort. Int. J. Epidemiol. 2014, 43, 843–855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Symmons, D.; Bankhead, C.R.; Harrison, B.J.; Brennan, P.; Silman, A.J.; Bsc, E.M.B.; Scott, D.G.I. Blood transfusion, smoking, and obesity as risk factors for the development of rheumatoid arthritis. Results from a primary care-based incident case-control study in Norfolk, England. Arthritis Care Res. 1997, 40, 1955–1961. [Google Scholar] [CrossRef]
- Pedersen, M.; Jacobsen, S.; Klarlund, M.; Pedersen, B.V.; Wiik, A.; Wohlfahrt, J.; Frisch, M. Environmental risk factors differ between rheumatoid arthritis with and without auto-antibodies against cyclic citrullinated peptides. Arthritis Res. Ther. 2006, 8, R133. [Google Scholar] [CrossRef] [Green Version]
- Wesley, A.; Bengtsson, C.; Elkan, A.-C.; Klareskog, L.; Alfredsson, L.; Wedrén, S.; Epidemiological Investigation of Rheumatoid Arthritis Study Group. Association between body mass index and anti-citrullinated protein antibody-positive and anti-citrullinated protein antibody-negative rheumatoid arthritis: Results from a population-based case-control study. Arthritis Care Res. 2013, 65, 107–112. [Google Scholar] [CrossRef] [Green Version]
- Lu, B.; Hiraki, L.T.; Sparks, J.; Malspeis, S.; Chen, C.-Y.; Awosogba, J.A.; Arkema, E.; Costenbader, K.H.; Karlson, E.W. Being overweight or obese and risk of developing rheumatoid arthritis among women: A prospective cohort study. Ann. Rheum. Dis. 2014, 73, 1914–1922. [Google Scholar] [CrossRef] [PubMed]
- Cerhan, J.R.; Saag, K.G.; Criswell, L.A.; Merlino, L.A.; Mikuls, T.R. Blood transfusion, alcohol use, and anthropometric risk factors for rheumatoid arthritis in older women. J. Rheumatol. 2002, 29, 246–254. [Google Scholar] [PubMed]
- Crowson, C.S.; Matteson, E.L.; Davis, J.M., 3rd; Gabriel, S.E. Contribution of obesity to the rise in incidence of rheumatoid arthritis. Arthritis Care Res. 2013, 65, 71–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Linauskas, A.; Overvad, K.; Symmons, D.; Johansen, M.N.; Stengaard-Pedersen, K.; de Thurah, A. Body Fat Percentage, Waist Circumference, and Obesity As Risk Factors for Rheumatoid Arthritis: A Danish Cohort Study. Arthritis Care Res. 2018, 71, 777–786. [Google Scholar] [CrossRef]
- Fagherazzi, G.; Guillas, G.; Boutron-Ruault, M.-C.; Clavel-Chapelon, F.; Mesrine, S. Body shape throughout life and the risk for breast cancer at adulthood in the French E3N cohort. Eur. J. Cancer Prev. 2013, 22, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Perquier, F.; Lasfargues, A.; Mesrine, S.; Clavel-Chapelon, F.; Fagherazzi, G. Body-size throughout life and risk of depression in postmenopausal women: Findings from the E3N cohort. Obesity 2014, 22, 1926–1934. [Google Scholar] [CrossRef]
- Fagherazzi, G.; Vilier, A.; Affret, A.; Balkau, B.; Bonnet, F.; Clavel-Chapelon, F. The association of body shape trajectories over the life course with type 2 diabetes risk in adulthood: A group-based modeling approach. Ann. Epidemiol. 2015, 25, 785–787. [Google Scholar] [CrossRef] [PubMed]
- Clavel-Chapelon, F.; E3N group van Liere, M.J.; Giubout, C.; Niravong, M.Y.; Goulard, H.; Le Corre, C.; Hoang, L.A.; Amoyel, J.; Auquier, A.; Duquesnel, E. E3N, a French cohort study on cancer risk factors. E3N Group. Etude Epidémiologique auprès de femmes de l’Education Nationale. Eur. J. Cancer Prev. 1997, 6, 473–478. [Google Scholar]
- Nguyen, Y.; Salliot, C.; Gusto, G.; Descamps, E.; Mariette, X.; Boutron-Ruault, M.C.; Seror, R. Improving accuracy of self-reported diagnoses of rheumatoid arthritis in the French prospective E3N-EPIC cohort: A validation study. BMJ Open 2019, 9, e033536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seror, R.; Henry, J.; Gusto, G.; Aubin, H.-J.; Boutron-Ruault, M.-C.; Mariette, X. Passive smoking in childhood increases the risk of developing rheumatoid arthritis. Rheumatology 2018, 58, 1154–1162. [Google Scholar] [CrossRef] [Green Version]
- Kvaskoff, M.; Bijon, A.; Mesrine, S.; Vilier, A.; Clavel-Chapelon, F.; Boutron-Ruault, M.-C. Anthropometric features and cutaneous melanoma risk: A prospective cohort study in French women. Cancer Epidemiol. 2014, 38, 357–363. [Google Scholar] [CrossRef]
- Waist circumference and waist-hip ratio. In Report of a WHO Expert Consultation: Geneva, 8–11 December 2008; World Health Organization: Geneva, Switzerland, 2011.
- Sørensen, T.I.; Stunkard, A.J.; Teasdale, T.W.; Higgins, M.W. The accuracy of reports of weight: Children’s recall of their parents’ weights 15 years earlier. Int. J. Obes. 1983, 7, 115–122. [Google Scholar] [PubMed]
- Tehard, B.; van Liere, M.J.; Com, N.C.; Clavel-Chapelon, F. Anthropometric measurements and body silhouette of women: Validity and perception. J. Am. Diet. Assoc. 2002, 102, 1779–1784. [Google Scholar] [CrossRef]
- Salliot, C.; Nguyen, Y.; Gusto, G.; Gelot, A.; Gambaretti, J.; Mariette, X.; Boutron-Ruault, M.-C.; Seror, R. Female hormonal exposures and risk of rheumatoid arthritis in the French E3N-EPIC cohort study. Rheumatology 2021, 60, 4790–4800. [Google Scholar] [CrossRef]
- Nagin, D. Analyzing developmental trajectories: A semiparametric, group based approach. Psychol. Methods 1999, 4, 139–157. [Google Scholar] [CrossRef]
- Jones, B.L.; Nagin, D.S. Advances in Group-Based Trajectory Modeling and an SAS Procedure for Estimating Them. Sociol. Methods Res. 2007, 35, 542–571. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Acosta, S.; Clavel-Chapelon, F. Dealing with missing, abnormal and incoherent data in E3N cohort study. Rev. Epidemiol. Sante Publique 1999, 47, 515–523. [Google Scholar] [PubMed]
- Marchand, N.E.; Sparks, J.A.; Tedeschi, S.K.; Malspeis, S.; Costenbader, K.H.; Karlson, E.W.; Lu, B. Abdominal Obesity in Comparison with General Obesity and Risk of Developing Rheumatoid Arthritis in Women. J. Rheumatol. 2021, 48, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Lahiri, M.; Luben, R.; Morgan, C.; Bunn, D.K.; Marshall, T.; Lunt, M.; Verstappen, S.; Symmons, D.; Khaw, K.-T.; Wareham, N.; et al. Using lifestyle factors to identify individuals at higher risk of inflammatory polyarthritis (results from the European Prospective Investigation of Cancer-Norfolk and the Norfolk Arthritis Register—the EPIC-2-NOAR Study). Ann. Rheum. Dis. 2013, 73, 219–226. [Google Scholar] [CrossRef]
- Tedeschi, S.K.; Cui, J.; Arkema, E.V.; Robinson, W.H.; Sokolove, J.; Lingampalli, N.; Sparks, J.A.; Karlson, E.W.; Costenbader, K.H. Elevated BMI and antibodies to citrullinated proteins interact to increase rheumatoid arthritis risk and shorten time to diagnosis: A nested case-control study of women in the Nurses’ Health Studies. Semin. Arthritis Rheum. 2017, 46, 692–698. [Google Scholar] [CrossRef]
- de Hair, M.J.; Landewé, R.B.; van de Sande, M.G.; van Schaardenburg, D.; van Baarsen, L.G.; Gerlag, D.M.; Tak, P.P. Smoking and overweight determine the likelihood of developing rheumatoid arthritis. Ann. Rheum. Dis. 2013, 72, 1654–1658. [Google Scholar] [CrossRef]
- Versini, M.; Jeandel, P.-Y.; Rosenthal, E.; Shoenfeld, Y. Obesity in autoimmune diseases: Not a passive bystander. Autoimmun. Rev. 2014, 13, 981–1000. [Google Scholar] [CrossRef]
- Abella, V.; Scotece, M.; Conde, J.; Pino, J.; Gonzalez-Gay, M.A.; Gómez-Reino, J.J.; Mera, J.J.G.-R.A.; Lago, F.; Gómez, R.; Gualillo, O. Leptin in the interplay of inflammation, metabolism and immune system disorders. Nat. Rev. Rheumatol. 2017, 13, 100–109. [Google Scholar] [CrossRef]
- Francisco, V.L.G.; Ruiz-Fernández, C.; Pino, J.; Mera, A.; Gonzalez-Gay, M.A.; Gómez, R.; Lago, F.; Mobasheri, A.; Gualillo, O. Adipokines: Linking metabolic syndrome, the immune system, and arthritic diseases. Biochem. Pharmacol. 2019, 165, 196–206. [Google Scholar] [CrossRef]
- Nguyen, Y.; Mariette, X.; Salliot, C.; Gusto, G.; Boutron-Ruault, M.-C.; Seror, R. Chronic diarrhoea and risk of rheumatoid arthritis: Findings from the French E3N-EPIC Cohort Study. Rheumatology 2020, 59, 3767–3775. [Google Scholar] [CrossRef] [PubMed]
- Rogol, A.D.; Roemmich, J.N.; Clark, P.A. Growth at puberty. J. Adolesc. Health 2002, 31, 192–200. [Google Scholar] [CrossRef]
- Pasquali, R.; Pelusi, C.; Genghini, S.; Cacciari, M.; Gambineri, A. Obesity and reproductive disorders in women. Hum. Reprod. Update 2003, 9, 359–372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
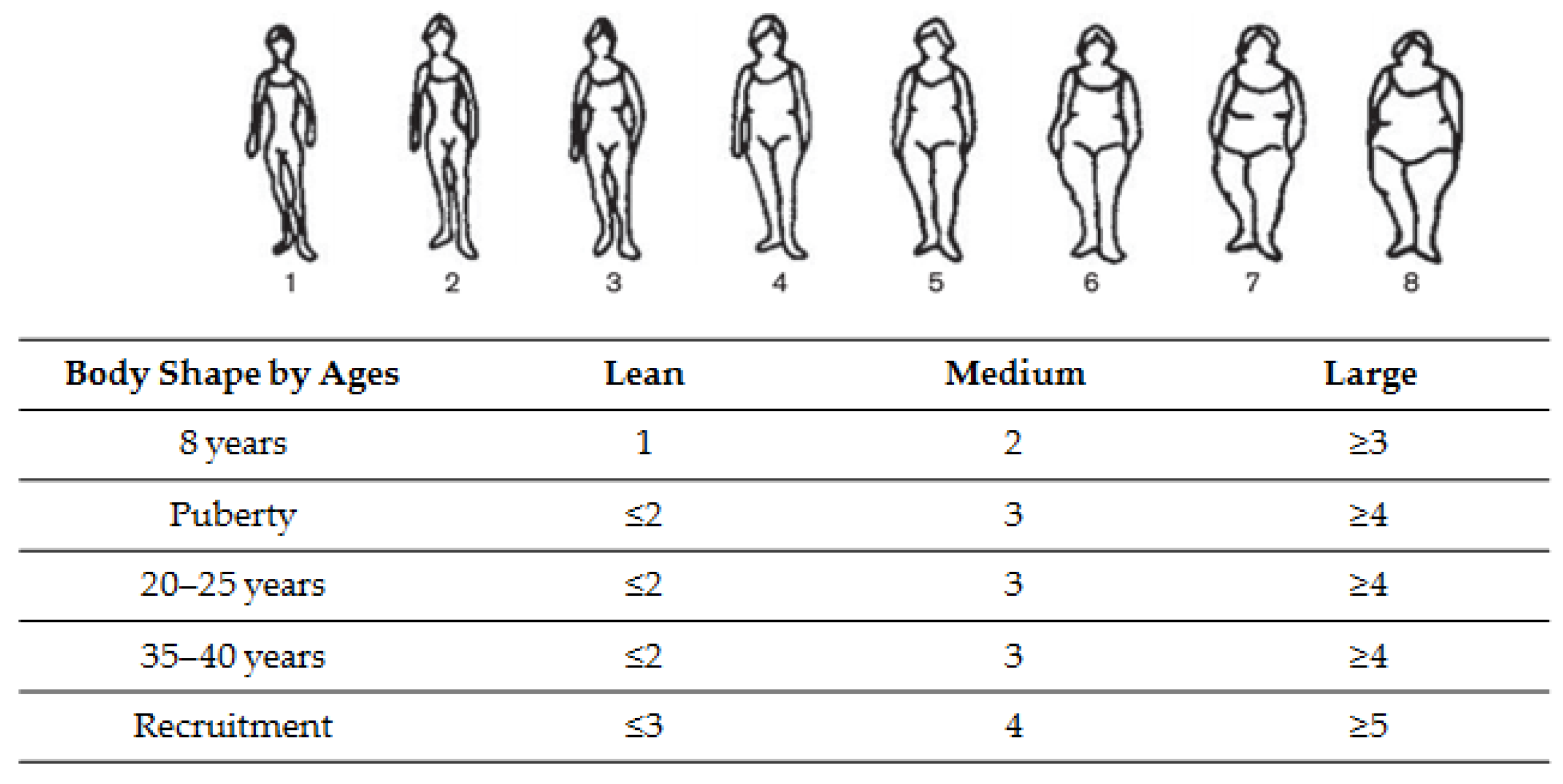

| Body Mass Index (BMI in kg/m2) * | |||||||||
| All Population (N = 78,452) | Ever-Smokers (N = 41,816) | Never-Smokers (N = 36,634) | |||||||
| RA | Non Cases | HRs (95% CI) | RA | Non Cases | HRs (95% CI) | RA | Non Cases | HRs (95% CI) | |
| BMI < 18.5 | 17 | 2874 | 0.75 (0.5–1.2) | 10 | 1575 | 0.73 (0.4–1.4) | 7 | 1299 | 0.80 (0.4–1.7) |
| BMI = 18.5–25 | 455 | 49,253 | Ref | 266 | 26,091 | Ref | 187 | 23,162 | Ref |
| BMI = 25–30 | 171 | 19,690 | 1.10 (0.9–1.3) | 97 | 10,448 | 1.10 (0.8–1.3) | 74 | 9242 | 1.10 (0.8–1.4) |
| BMI ≥ 30 | 55 | 5937 | 1.26 (0.9–1.5) | 35 | 3294 | 1.35 (0.9–1.9) | 20 | 2643 | 1.15 (0.7–1.8) |
| ptrend | 0.0559 | 0.0736 | 0.3487 | ||||||
| Abdominal Obesity (Waist Circumference >88 cm) *,† | |||||||||
| All Population (N = 78,379) | Ever-Smokers (N = 41,771) | Never-Smokers (N = 36,608) | |||||||
| RA | Non Cases | HRs (95% CI) | RA | Non Cases | HRs (95% CI) | RA | Non Cases | HRs (95% CI) | |
| No | 300 | 34,976 | Ref | 170 | 18,477 | Ref | 128 | 16,530 | Ref |
| Yes | 139 | 15,582 | 1.25 (1.0–1.5) | 85 | 8527 | 1.32 (0.9–1.8) | 54 | 7146 | 1.12 (0.8–1.6) |
| p-value | 0.0338 | 0.0697 | 0.5367 | ||||||
| All Population (N = 78,452) | Ever-Smokers (N = 41,816) | Never-Smokers (N = 36,634) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| RA | Non Cases | HRs (95% CI) | RA | Non Cases | HRs (95% CI) | RA | Non Cases | HRs (95% CI) | |
| Body shape at puberty | |||||||||
| Lean | 332 | 40,485 | Ref | 183 | 20,423 | Ref | 148 | 20,062 | Ref |
| Medium | 179 | 17,903 | 1.22 (1.0–1.5) | 103 | 9835 | 1.17 (0.9–1.5) | 75 | 8068 | 1.30 (1.0–1.7) |
| Large | 147 | 15,984 | 1.10 (0.90–1.4) | 92 | 9511 | 1.10 (0.8–1.4) | 55 | 6473 | 1.14 (0.8–1.6) |
| ptrend | 0.1760 | 0.3899 | 0.2358 | ||||||
| Body shape at baseline (perimenopausal period) | |||||||||
| Lean | 359 | 44,718 | Ref | 220 | 23,804 | Ref | 138 | 20,914 | Ref |
| Medium | 200 | 20,041 | 1.15 (0.9–1.4) | 103 | 10,489 | 0.94 (0.7–1.2) | 96 | 9552 | 1.46 (1.1–1.9) |
| Large | 110 | 9838 | 1.17 (0.8–1.5) | 63 | 5330 | 1.0 (0.7–1.4) | 47 | 4508 | 1.45 (0.9–2.2) |
| ptrend | 0.1956 | 0.8268 | 0.0375 | ||||||
| All Population (N =77,552) α | Ever-Smokers (N = 41,339) | Never-Smokers (N = 36,221) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| RA | Non Cases | HRs (95% CI) | RA | Non Cases | HRs (95% CI) | RA | Non Cases | HRs (95% CI) | |
| Body shape trajectories from puberty to perimenopausal period | |||||||||
| Constantly lean | 101 | 12,415 | Ref | 59 | 6182 | Ref | 42 | 6233 | Ref |
| Medium BS at puberty and sharp decrease | 114 | 13,477 | 1.04 (0.8–1.4) | 67 | 7314 | 0.97 (0.7–1.4) | 46 | 6163 | 1.14 (0.7–1.7) |
| Large BS at puberty and decrease | 152 | 19,780 | 1.0 (0.8–1.3) | 98 | 10,868 | 1.0 (0.7–1.5) | 54 | 8912 | 0.92 (0.6–1.4) |
| Constantly medium | 119 | 12,023 | 1.10 (0.8–1.4) | 57 | 6010 | 0.87 (0.6–1.3) | 62 | 6013 | 1.44 (0.9–2.2) |
| Upper midrange | 150 | 14,826 | 1.18 (0.9–1.5) | 91 | 8103 | 1.07 (0.7–1.5) | 58 | 6723 | 1.33 (0.9–2.0) |
| Constantly large | 51 | 4344 | 1.29 (0.9–1.9) | 26 | 2464 | 0.9 (0.5–1.5) | 25 | 1880 | 2.10 (1.2–3.6) |
| ptrend | 0.1243 | 0.9595 | 0.0248 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salliot, C.; Nguyen, Y.; Mariette, X.; Boutron-Ruault, M.-C.; Seror, R. Anthropometric Measures and Risk of Rheumatoid Arthritis in the French E3N Cohort Study. Nutrients 2022, 14, 934. https://doi.org/10.3390/nu14050934
Salliot C, Nguyen Y, Mariette X, Boutron-Ruault M-C, Seror R. Anthropometric Measures and Risk of Rheumatoid Arthritis in the French E3N Cohort Study. Nutrients. 2022; 14(5):934. https://doi.org/10.3390/nu14050934
Chicago/Turabian StyleSalliot, Carine, Yann Nguyen, Xavier Mariette, Marie-Christine Boutron-Ruault, and Raphaèle Seror. 2022. "Anthropometric Measures and Risk of Rheumatoid Arthritis in the French E3N Cohort Study" Nutrients 14, no. 5: 934. https://doi.org/10.3390/nu14050934
APA StyleSalliot, C., Nguyen, Y., Mariette, X., Boutron-Ruault, M.-C., & Seror, R. (2022). Anthropometric Measures and Risk of Rheumatoid Arthritis in the French E3N Cohort Study. Nutrients, 14(5), 934. https://doi.org/10.3390/nu14050934






