Abstract
Mussels have a particular nutritional value, representing a highly valued food source and thus sought after worldwide. Their meat is a real culinary delicacy, rich in proteins, lipids, carbohydrates, trace elements, enzymes, and vitamins. The seasonal variation of mussels’ biochemical composition has been studied to determine the best harvesting period to capitalize on various biologically active fractions. In this work biochemical determinations have been performed on fresh flesh samples of Mytilus galloprovincialis specimens from the Black Sea coast to study seasonal variations in mussels’ biochemical compounds. An analysis of significant lipid classes and the fatty acid composition of lipid extracts obtained from mussel flesh has also been performed. Since mussels retain pollutants from the marine environment, in parallel, the concentration of heavy metals in the meat of mussels collected for the analysis of the chemical composition was investigated. The impact and risk of heavy metal poisoning due to food consumption of mussels contaminated due to pollution of the marine harvesting area was evaluated by the bio-concentration factor of metals and estimated daily intakes of heavy metals through mussel consumption.
1. Introduction
Mytilus galloprovincialis is one of the most widespread invertebrates in the Black Sea, whose rich content in valuable nutrients has brought it to researchers’ attention. The remarkable nutritional quality of mussel flesh is due to the proportion of vital components necessary for human nutrition: protein, fat, carbohydrates, macro- and micronutrients, enzymes, vitamins, etc. Mussels are considered authentic delicacies, along with shrimp, lobster, crab, cuttlefish, octopus, and other seafood [1,2]. The first to use the medicinal properties of mussels were the Chinese. Even today, mollusk meat is considered a medicine used especially in oncological, cardiovascular, ocular, and gynecological diseases [3,4,5,6].
Mussel meat is known for its nutritional value due to the high quality of proteins, the presence of essential free amino acids, and water-soluble and fat-soluble vitamins. The total content of free amino acids reaches an average of 0.7% in fresh meat, and of these, 40% is represented by the fraction of essential amino acids [7,8]. Fresh mussel meat contains the same high-quality protein as red meat but has much less total fat, saturated fat, and almost 25% fewer calories. Thus, replacing red meat with mussel meat provides a low-calorie diet that benefits weight management. Like fish, seafood is highly sought after for the dietary nature of meat (low energy value) and particularly high content of minerals, especially zinc, selenium, etc. [9,10,11]. The abundance of essential amino acids, vitamins, and minerals makes mussel meat a valuable functional food for strengthening the immune system. As a result, nutritionists recommend the inclusion of seafood in general in diets to enhance immunity, especially in the current epidemiological context coupled with the increase in global pollution and even many drugs that affect the immune system [12,13,14].
Mussels have superiority in the marine environment in terms of biosynthesis of hydro and fat-soluble vitamins; remarkable is the presence of vitamins D group, which can reach up to 1% of the dry matter, and vitamin B12, which comes 6–9.7 μg/g dry matter, much more than in pork containing 1.3 μg/g [4,9]. However, variability in the biochemical composition of mussels may result in variability of nutritional value and flavor. The mussel is an important dietetic source of polyunsaturated ω-3 fatty acids, as eicosapentaenoic acid and docosahexaenoic acid: both are beneficial for health (for example, in the treatment and prevention of cardiac ischemia) [15,16]. Farmed marine mussels from the Mytilidae family, especially genera Mytilus, are essential for the human diet in providing high levels of proteins, omega-3 polyunsaturated fatty acids, fat-soluble vitamins, and carbohydrates. In recent years, the functional properties of mussel lipids have been investigated, and few dietary supplements based on lipid extracts of mussels have been presented at the market [17,18]. Essential fatty acids have beneficial effects on the heart and blood vessels. These good fats lower blood cholesterol levels, have anti-inflammatory and mild antihypertensive effects, help improve heart function and play an essential role in brain development [19,20,21].
Mussels are found in a wide variety of habitats from areas affected by the tide to completely submerged areas with a range-wide range of temperatures and salinity. They feed on phytoplankton and organic matter by constantly filtering the water sea and are therefore consistently grown in rich areas in plankton. Water quality is a significant factor in the growth of mussels. Mussels can retain and concentrate in their body pollutants from the marine environment and food elements. The most dangerous pollutants that get into the meat of mussels are heavy metals, pesticides, and pathogenic bacteria [22,23,24,25].
Mussels’ biochemical composition is influenced by geographical area, depth, nourishment, gender, age, reproductive status, climate factors, and environmental pollution level. Therefore, mussels harvested from contaminated water sources have an increased risk of infection and chemical poisoning. In addition, consumption of mussels contaminated with heavy metals, such as mercury, cadmium, or lead, can increase the risk of neurological damage and congenital disabilities [26,27,28,29]. Aquatic organisms exposed to marine contaminants such as heavy metals accumulate these elements. Sometimes, the amount of metals relative to the body mass of these organisms increases with the evolution of the food chain. In the case of fishing activities in the contaminated aquatic area and, subsequently, by consuming the catches, the toxic elements are transferred to the human body, where they can cause different organ-specific syndromes (hematological, neurological disturbances, with attention-deficit even at low concentrations of pollutants, cardiovascular diseases, renal and gastrointestinal impairments [30,31,32,33]. The severity of symptoms depends on the metal accumulated and the amount collected. The rate of bioaccumulation is influenced by certain factors such as temperature and condition physiological function of the body (sex, age, size). Heavy metals can create random bonds with cellular biomolecules such as enzymes or proteins to form complexes that may compromise their structure and function when ingested in excessive amounts.
In the present study, a series of determinations were made on mussels harvested from the Romanian Black Sea coast: analysis of seasonal variation of biochemical composition in order to identify the optimal harvesting time for nutrient recovery and analysis of heavy metals concentration in order to determine the safety of consumption.
2. Materials and Methods
2.1. Analysis of Seasonal Biochemical Composition Variation
The biochemical determinations have been performed on fresh flesh samples of Mytilus galloprovincialis specimens collected during 2020 in the harbor area (A1) near the Port of Tomis Constanta during all seasons: winter (January), spring (April), summer (July) and autumn (October) of the Romanian seaside (Figure 1) in order to evaluate the seasonal variations of the nutrients in the meat composition. In addition, for comparison purposes, analysis has also been performed on specimens collected from the harbor area (A1), the beach area (A2) near Costinesti Beach and the industrial area (A3) near Navodari of the Romanian seaside in autumn in order to evaluate the compositional differences determined by the habitat. Incidentally, the weight distribution on the components—flesh, juice, shells—also been examined.

Figure 1.
Mytilus galloprovincialis from Romanian seaside.
Proteins have been measured by the Kjeldahl method [32], whereas the Christie method [33] has been used for fats and the orcinol-sulphuric acid method for assaying total carbohydrates [34,35]. Mineral residue has been measured by means of dry tissue calcination at 550 °C. The overall biochemical composition has been expressed in %, in relation to dry weight.
2.2. Analysis of the Total Lipid Extract Isolated and Purified from Mussel
For isolation and purification of total lipid extract, adult specimens of Mytilus galloprovincialis were used, collected from the Romanian Black Sea coast. First, the flesh was separated from the shells following rinsing under running water. Then, the fat fraction was isolated and purified from the mussel meat by a simple technique [35].
The main classes of lipids were separated and purified by column chromatography on silica gel (60G). After specific treatment, the fatty acids in the lipid fractions were then analyzed by gas chromatography with a model 17 GC gas chromatograph (Shimadzu, Kyoto, Japan) provided with a flame ionization detector and a capillary column.
The process for separation of the significant lipid fractions consisted of the following steps:
- (1)
- Freshly diced and homogenized tissue (500 g) was treated with 800 mL chloro-form-methanol (2: 1 v/v) mixture using the Soxhlet method for total lipids extraction;
- (2)
- Following extraction of the lipid fraction, the extraction solvent was removed by concentration in a 4001 rotary evaporator (LABOROTA, Schwabach, Germany);
- (3)
- The total lipid extract was further purified by treatment with a mixture of chloroform: methanol: 0.9% KCl solution (10:10:9 v/v); The lower layer was next retained and concentrated by rotary evaporation, thus obtaining the purified lipid extract;
- (4)
- The purified total lipid extract was treated with 100 mL of acetone and incubated for 24 h at 4 °C, then filtered, retaining both the precipitate and the filtrate;
- (5)
- The precipitate was dissolved in chloroform, using 0.002% butylated-hydroxytoluene (BHT, Sigma-Aldrich, St. Louis, MO, USA) as an antioxidant: this fraction (fraction “a”) contains polar lipids;
- (6)
- The filtrate was evaporated in vacuum and redissolved in n-hexane, with 0.002% BHT as an antioxidant, thus yielding fraction “b”, containing neutral lipids;
- (7)
- The polar lipids in fraction “a” were separated by column chromatography on silica gel (60G Merck, Darmstadt, Germany), and residual neutral lipids were removed by elution with chloroform, yielding fraction no. 1 (containing glycolipids) by elution with acetone and fraction no. 2 (containing phospholipids) by elution with methanol;
- (8)
- Column chromatography on silica gel (60G) was used to separate neutral lipids in fraction “b”. At the same time, hydrocarbons and pigments were removed by elution with n-hexane, and the fatty acid methyl esters were removed by elution with a mixture of n-hexane: diethyl ether (70:5 v/v). Fraction no. 3 was thus obtained (containing triglycerides) by elution with chloroform, as mobile phase.
- (9)
- According to the Romanian Pharmacopoeia, the tenth edition (R Ph X) [36], the total lipid fraction isolated and purified from the mussel meat was characterized by analyzing acidic, esterification, saponification, and iodine indexes.
Analysis of fatty acids in the total lipid extract isolated and purified from the mussel flesh proceeded as follows: an internal standard (C23:0 methyl ester; Nuchek Prep Inc., Elysian, MN, USA) was added to a lipid extract sample; next, the mixture was dried under nitrogen atmosphere and then subjected to hydrolysis using a 7.9% KOH solution in methanol. After cooling, the samples were treated with a 20% boron trifluoride solution in methanol. Fatty acid methyl esters were subsequently analyzed with the Shimadzu model 17 GC gas chromatograph. The carrier gas used was helium. Assays were injected at 125 °C, following which temperature was set to increase to 170 °C at a 5 °C/min rate and maintained at this level for 4 min. Next, the temperature was again set to rise to 175 °C, at a 0.5 °C/min rate, and finally to 220 °C, at a 4 °C/min rate, when maintained for 3 min. Injector and detector temperature was maintained at 260 °C. The peak area was processed using the Shimadzu Class GC-10 software.
All samples were tested three times, and the results were expressed as means ± SD (standard deviation). A standard mix of fatty acids methyl esters (Nuchek Prep Inc., Elysian, MN, USA) was used to calibrate gas chromatography and determine response factors. All reagents used (Sigma-Aldrich) were of analytical purity.
2.3. Analysis of the Heavy Metals from Mussels
Analysis of the heavy metals has been performed on fresh flesh samples of Mytilus galloprovincialis specimens collected in all seasons during 2020 (spring, summer, autumn, and winter) from the harbor area (A1), the beach area (A2) and the industrial area (A3) of the Romanian seaside.
To analyze heavy metals (cadmium, copper, chromium, nickel, lead, zinc), mussel, water, and sediment samples were harvested from three areas of the Romanian coastline (A1, A2, and A3) to determine the optimum area for harvesting of least polluted raw material. Water samples, including surface water and bottom water, were collected from 0.5 m below the surface and 2.0 m above the bottom, and sediment samples were about 5 to 10 cm thickness of the surface marine sediment. In total, 12 samples of mussels, water, and sediment were collected from each analyzed area.
Heavy metals analysis was done using the digestion method followed by atomic absorption spectroscopy [37,38]. First, mussels collected were thoroughly rinsed, separated from the shells, grated and dried at 80 °C until constant weight, and then mortar-powdered and homogenized. Next, both powdered samples (shells and flesh, each separately) were mineralized by the wet digestion method: 1 g of each homogenized sample was introduced into the digestion vessel (DK-6 Heating Digester, Velp, Shanghai, China), together with 10 mL 65% HNO3, 5 mL 37% HCl and 2 mL 35% H2O2. Then, the mixture was heated gradually (at 150 °C, for 1 h, 200 °C, for 2 h, 250 °C, for 1 h and 300 °C, for 2 h). Next, the solutions were cooled to room temperature and transferred into a 25 mL volumetric flask, brought to volume with ultra-distilled water.
Aqueous samples (500 mL) were filtered using Whatman No. 41 (0.45 μm pore size) filter paper to estimate dissolved metal content. The filtrate and as-collected water samples (500 mL each) were preserved with 2 mL nitric acid to prevent the precipitation of metals. Both samples were tenfold concentrated on a water bath at 80 °C until the volume reached 50 mL and subjected to nitric acid digestion using the microwave-assisted technique, setting the pressure at 30 bars and power at 700 Watts [39]. After cooling, each sample filtered by filter (Whatman filter, 0.45 μm, Merck). The filtrate was diluted by deionized water to a final volume of 50 mL.
Sediment samples were dried at 80 °C until constant weight and then were mineralized by the wet digestion method, just like the mussel samples.
The resultant solutions were analyzed with a Shimadzu AA 6300 (air/acetylene flame) atomic absorption spectrophotometer in order to determine the heavy metals concentration: cadmium (λ = 228.8 nm), copper (λ = 324.7 nm), zinc (λ = 213.9 nm), chromium (λ = 359.3 nm), lead (λ = 217 nm) and nickel (λ = 232 nm). A blank digestion solution was made for comparison. A standard solution for each element under investigation was prepared and used for calibration. Triplicate determinations were performed for each metal. Results are expressed as triplicate analysis mean ± S.D (standard deviation). Data were statistically evaluated.
All reagents used for the detection of heavy metals in real samples (Merck) were of analytical purity, standard stock metal solutions containing 1000 mg of metal/mL (Titrisol, Merck). All solutions were prepared using distilled deionized water or double deionized.
The linear correlation coefficients (R2), limits of detection (LODs) and limits of quantification (LOQs) for each metal analyzed are presented in Table 1.

Table 1.
Linear correlation coefficients (R2), Limits of Detection (LOD) and Limits of Quantification (LOQ) for heavy metals.
2.4. Risk Characterisation to Consumer’s Health
To assess the impact and risk of heavy metal poisoning due to food consumption of mussels contaminated due to pollution of the marine harvesting area, the daily intake and the bio-concentration factor of metals were also calculated.
2.4.1. Bioconcentration Factor
Bio-concentration Factor (BCF) can be calculated by the following equation [40,41]:
where C is the contaminant concentrations in the organisms (μg/kg), Cw is the contaminant concentration in the water (μg/L).
BCF = C/Cw
2.4.2. Estimated Daily Intake (EDI)
This parameter is obtained using the following equation [42]:
where C is the metal levels in mussels (μg/kg), Con is the daily average consumption of mussels (kg person-1/day), and Bw represents the average body weight of an adult (kg). We assumed a daily mussel consumption of 3.5 g/person, which is the average quantity consumed in the European Union [43]. Intake estimates were expressed as per unit body weight (mg/kg body wt./weekly and daily).
EDI = C × Con/Bw
2.4.3. Hazard Quotient (HQ)
This parameter is calculated for each contaminant using the following equation:
where EDI is the estimated daily intake (mg/kg/day) and RfD (mg/kg/day) is the approximation of daily tolerable exposure to which a person is expected to have any significant risk of harmful effects during a lifespan [44,45].
HQ = EDI/RfD
If the HQ value is less than 1, consumption of mussels does not pose an adverse health hazard to the exposed population in terms of the studied heavy metals. If the HQ is higher than 1, estimated daily intake exceeds the RfD revealing that there is a potential health risk associated with that contaminant.
2.5. Statistical Analyses
Statistical analysis was implemented using the open-source software R (R version 4.1.1.) [46]. The basic descriptive statistics like mean, standard deviation and 95% confidence intervals for heavy metals concentrations are reported numerically in its tables. Our input data follow a 4 × 3 balanced design where the factors involved are sample (mussel shell, mussel meat, seawater and sediment) and area (area 1, area 2 and area 3). In verifying our dataset, we perform a Shapiro-Wilk test for normality and Levene’s test to check the homoscedasticity of groups. For every sample, we investigate if there are any statistically significant differences between the means of heavy metal concentration for area levels. Thereby, to make a reliable decision we apply one-way ANOVA parametric test if both conditions are met or Welch’s ANOVA for unequal variances. Otherwise, we perform a Bootstrap version of one-way ANOVA [47]. To high-light which specific group’s means are different we use post hoc Tuckey tests for multiple comparisons. Statistical significance level was considered at alpha 5% (p < 0.05).
3. Results
3.1. Seasonal Biochemical Composition Variation of Mussel
A summary of results obtained concerning seasonal variations in mussel flesh composition is shown in Figure 2 and Table 2. In general, data observed showing maximum concentrations (protein, fat) in spring and during pre-breeding, and minimum concentration after breeding, in autumn. Instead, maximum concentration of carbohydrates occurs in autumn.
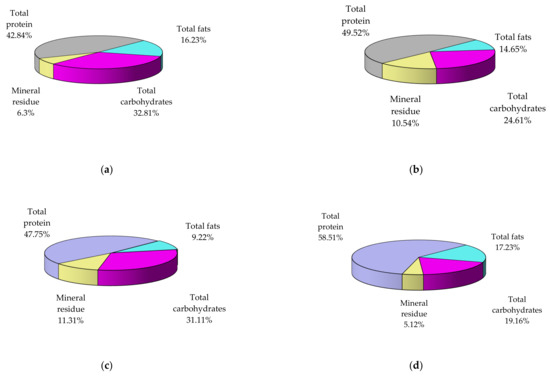
Figure 2.
The seasonal variations in biochemical composition of mussel flesh collected from A1 area, the harbor area near Port of Tomis (dry basis, %): autumn (a), winter (b), summer (c), spring (d).

Table 2.
Annual limits of biochemical composition variation in mussel flesh (% dry sample).
Three lots of mussel specimens of the same age, collected from A1, A2 and A3, respectively, showed significantly different biochemical composition values (Figure 3).
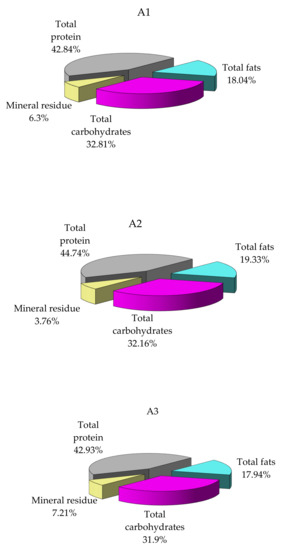
Figure 3.
Variation in concentrations of the main classes of compounds in the M. galloprovincialis flesh specimens collected from different sites (% dry sample). A1 area, the harbor area near Port of Tomis, the beach area (A2) near Costinesti Beach and the industrial area (A3) near Navodari.
Specimens collected from the area A2 display much higher protein and lipid content and approximately similar carbohydrate content, although the analysis was performed at the same time of the year (Figure 3). Differences may be attributable to specific area food conditions, which advocates for the importance of environmental factors influence. There are no significant differences in the composition of mussel samples collected from areas A1 and A3.
Weight percentage ratios of the main components resulted after cleavage of adult mussel specimens in relation to fresh weight are as follows:
- -
- flesh: 21.14–27.56%;
- -
- juice: 15.76–18.94%;
- -
- shells: 53.5–63.1%.
Mussel flesh is well-known for its nourishing value resulted from the content rich in high quality protein, presence of certain essential free amino-acids as well as of water and lipid soluble vitamins. At the same time, mussel flesh is the core of the main physiological and biochemical changes determined by physiological cycles and the influence of the marine environment. Juice is the part generally lost in processing, whereas shells are the major component part in terms of weight.
3.2. Composition of Total Lipid Extract from Mussel
Examination of distribution of the main lipid classes in total lipid (Figure 4) shows that the dominant fraction consists of neutral lipids.
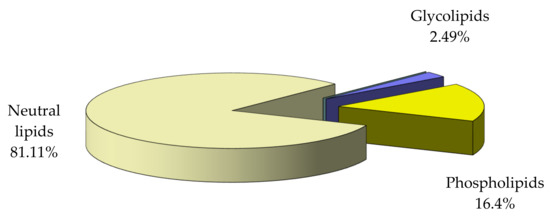
Figure 4.
Distribution of main lipid classes relative to total lipid in M. galloprovincialis flesh.
- -
- The total lipid extract represents 14.32% of dry tissue.
- -
- The total fatty acids are 69.27% of the total lipid.
Percent distribution of separate lipid fractions in the total lipid extract is shown in Figure 5.
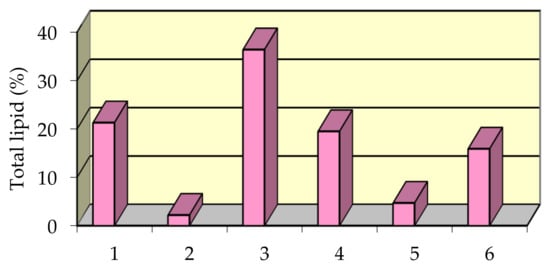
Figure 5.
Lipid fractions distribution in mussel total lipid extract: Phospholipids (1); Glycolipids (2); Triglycerides (3); Methyl esters of fatty acids (4); Hydrocarbons and pigments (5); Other lipids (sterols, fatty alcohols, fatty acids etc.) (6).
Table 3 indicates the values of the examined parameters, characteristic to the total lipid extract isolated and purified from mussel flesh. The high iodine value is notable, indicating high unsaturation of the fatty acids contained in the total lipid extract.

Table 3.
Values of examined parameters relative to the Mytilus galloprovincialis total lipid extract.
Percent distribution of fatty acids in the total lipid extract isolated from Mytilus galloprovincialis is shown in Table 4. Fatty acids were encoded according to number of carbon atoms, number and position of double bonds. Thus, Table 4 highlights the high concentration ω-3 fatty acids as compared to ω-6 fatty acids (ω-3/ω-6 = 6.14). At the same time, of note is the high concentration of polyunsaturated fatty acids in comparison with saturated and unsaturated fatty acids, accounting for the high unsaturation indicated by the iodine value for mussel total fat fraction. Among fatty acids, the most abundant are myristic acid (C14:0), palmitic acid (C16:0), palmitoleic acid (C16:1ω-7), oleic acid (C18:2ω-6) and eicosapentaenoic acid (C20:5ω-3).

Table 4.
Percent distribution of fatty acids in the total lipid extract isolated from Mytilus galloprovincialis.
3.3. Heavy Metals Concentration from Mussels and Seawater
Metal concentrations in mussel meat samples decrease in the order: Zn > Cu > Cr> Ni > Pb > Cd. Mean metal concentrations with their standard deviations and confidence intervals are shown in Table 5. The results showed that the content of heavy metals is higher in mussel samples collected from the industrial area than those from the harbor and beach area. The heavy metal content of meat mussels ranged between: Cd 0.19–0.63 μg/g, Cu 5.27–11.18 μg/g, Zn 8.57–14.45 μg/g, Cr 4.84–7.2 μg/g, Pb 0.68–1.84 μg/g, Ni 1.18–5.34 μg/g. The results were lower than those observed in a previous study and similar with findings from other Black Sea regions [37,42,48].

Table 5.
Heavy metals concentrations (μg/g) in mussel meat samples.
The amount of lead in the analyzed mussel meat samples exceeded the maximum allowable levels established by the Commission Regulation (EC) no. 1881/2006 [49] in the case of the samples collected from A3 (with industrial activity). Cadmium levels were within the normal range in all mussel samples. No thresholds for copper, zinc, nickel or chromium have yet been set for these marine foods.
The results showed the same distribution of heavy metal content in mussel shell samples (Zn > Cu > Cr> Ni > Pb > Cd) as in the mussel meat sample (Figure 6). The mean concentration of heavy metals in mussel shell samples with their standard deviations are given in Table 6. The amount of heavy metals in mussel shells varied within the following limits: Cd between 0.08–0.53 μg/g, Cu between 3.35–8.21 μg/g, Zn between 5.86–9.82 μg/g, Cr between 3.85–7.16 μg/g, Pb between 0.41–0.88 μg/g and Ni between 0.77–3.43 μg/g. No exceedances of the maximum permissible levels of heavy metals were noted.
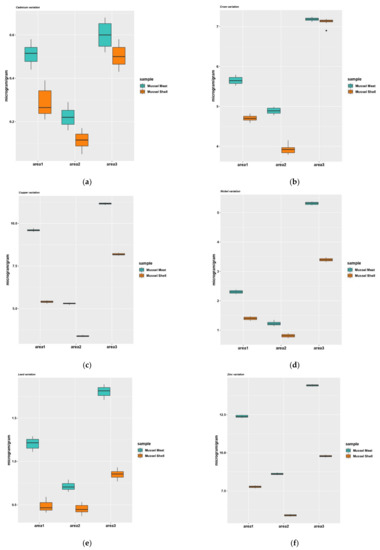
Figure 6.
The metal levels detected in mussels (Mytilus galloprovincialis) meat and shell from the Black Sea: cadmium (a), chromium (b), copper (c), nickel (d), lead (e), zinc (f). Area1, the harbor area near Port of Tomis, the beach area (area2) near Costinesti Beach and the industrial area (area3) near Navodari.

Table 6.
Heavy metals concentrations (μg/g) in mussel shell samples.
A post-hoc Tukey test for multiple comparisons was performed at the level of each heavy metal analyzed (cadmium, copper, chromium, nickel, lead, zinc) depending on the type of location (Area 1, Area 2, Area 3) and sample (mussel meat, mussel shells) and it was found that in all cases both main effects are statistically significant (Figure 6). Post-hoc tests were performed for all pairwise comparisons of heavy metal level averages taking into account the complete set of interactions. There was statistical significance among all samples analyzed (p < 0.0001), but a lower level of heavy metals was observed in the shell samples compared to meat samples collected from the same area. Experimental data indicate a high concentration of heavy metals in all samples collected from Area 3 compared to the other areas, the lowest values being recorded in the samples from Area 2. In the meat samples collected from Area 3, the values of the lead concentration exceed the maximum allowable level.
The results of seawater and sediment investigation in terms of heavy metal content indicate higher concentrations in sediments than in the seawater samples.
The content of Cd, Cu, Zn, Cr, Pb and Ni in seawater ranged between 8.65–15.26 μg/L, 5.27–39.05 μg/L, 48.15–64.23 μg/L, 56.32–75.46 μg/L, 18.40–29.24 μg/L, 27.21–46.27 μg/L, respectively. Corresponding concentrations in sediment samples ranged between 10.6–22.42 μg/g, 40.98–91.99 μg/g, 86.99–154.07 μg/g, 70.93–94.10 μg/g, 17.91–38.10 μg/g, 28.90–56.13 μg/g.
The highest concentrations of heavy metals were observed in seawater and sediment samples collected from industrial area (A3), followed by harbor area (A1) and beach area (A2) (see Table 7 and Table 8). Zinc and lead exceeded the maximum allowable levels in seawater from harbor and industrial area, while cadmium was exceeded in sediment samples picked-up from industrial area. No exceedances were recorded for the other investigated metals.

Table 7.
Heavy metals concentrations (μg/L) in sea water samples.

Table 8.
Heavy metals concentrations (μg/g) in sediment samples.
3.4. Estimated Risk to Consumers
3.4.1. Bioconcentration Factor
The analysis of the heavy metal transfer from water and sediments to mussels shows that bio-concentration factors vary by study area and by anatomical part of mussels (Figure 7).
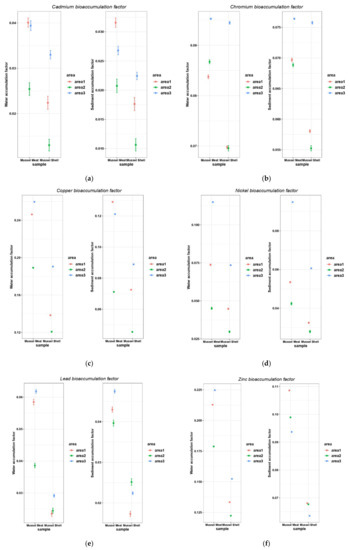
Figure 7.
The metal bioconcentration factors related to water and sediment samples in studied areas for cadmium (a), chromium (b), copper (c), nickel (d), lead (e), zinc (f). Area1, the harbor area near Port of Tomis, the beach area (area2) near Costinesti Beach and the industrial area (area3) near Navodari.
Copper and zinc presented the highest accumulation levels in mussel meat collected from the harbor (A1) and industrial areas (A3). Cadmium, lead and copper were transferred in a less proportion from the aquatic environment to mussel bodies. In all studied areas, less heavy metals accumulated in mussel shells than mussel meat.
3.4.2. Estimated Risk through Mussel Consumption
Estimation of potential risks by investigating estimated daily intakes of heavy metals through mussel consumption was the subject of several studies, inclusive located on the Black Sea coasts [42,51,52,53].
Calculated EDIs and HQs for each contaminant thorough mussel consumption in study areas are presented in Table 9. The highest values for estimated daily intakes were observed in industrial area, in which we also recorded the highest values of HQ, followed by harbor area and beach area. Even if HQs were below the critical value 1, so no possible risks are associated with mussel consumption collected from the study areas, we noted the case of lead which registered the highest calculated HQs from all heavy metals: 0.2 in A1, 0.03 in A3, 0.012 in A2, respectively.

Table 9.
Estimated daily intake rates and hazard quotient of metals through mussel consumption in A1 (harbor), A2 (beach) and A3 (industrial).
Our results are in accordance with findings of other studies conducted in order to assess potential human risks associated with mussel consumption in the region of Black Sea [54,55,56]. It should be noted also that calculated hazard quotients for investigated heavy metals within the current research were higher than values found in other investigated food categories in Romania (honey, food supplements) [57,58].
4. Discussion
There are people with a long tradition of seafood consumption worldwide (Caribbean, Asia, Mediterranean area, Scandinavian peninsula). Specialists unanimously accept that constant consumption of fish and seafood brings multiple health benefits. In general, people with a long tradition of consuming seafood have had easy access to marine resources [59]. Unfortunately, there are also exceptions, such as the Romanian people, possible due to influences of others peoples, whose food base is supported mainly by proteins from the meat of terrestrial animals [60,61].
Mussels are rich in high biological value proteins lipids rich in essential fatty acids, vitamins, and minerals. Therefore, mussels are among the most popular and cultivated seafood. There are many countries where culture mussels are produced in large quantities and consumed by the local population, and used for export: China, Chile, Japan, the Republic of Korea, the United States of America, Spain, etc. [62].
Data obtained from the analysis of mussel biochemical composition and their main compounds classes (proteins, fats, carbohydrates, minerals) and significantly related to the seasonal composition variation are helpful to determine the best harvesting period for capitalization of the various active fractions biologically. The main categories of nutrients, depending on the season, vary within the following limits in the analyzed samples (% dry sample): total proteins (42.84–58.51), total carbohydrates (12.22–32.81), total fats (9.22–17.23), mineral residue (5.12–11.31).
Experimental data showed an increased protein (58.51%) and lipid (17.23%) content in spring specimens, according to similar studies [27,63]. Therefore, to benefit from the valuable proteins in the composition of mussel meat, it is recommended to consume the samples harvested in spring, when their content is maximum in proteins rich in essential amino acids and lipids, or winter (49.52%).
It is well-known that the proteins from mussels have a remarkable biological value due to their high content of easily digestible essential amino acids, which leads to increased assimilation of essential amino acids. Furthermore, with a high concentration of vitamins and oligo-minerals, the constant consumption of proteins from mussel meat can significantly improve health, especially by strengthening the immune system [1,4].
It is noteworthy that the concentration of carbohydrates and minerals in the spring is minimal compared to the other four seasons, which could be an advantage for people who, for various reasons, should benefit from a hypoglycemic diet and/or poor in electrolytes. In addition, in the analyzed mussel samples, it can also be seen that in autumn, they accumulate a large amount of carbohydrates (32.81%, the highest of the four seasons), but also of fats (16.23%, the second-largest value after spring), while proteins (42.84%) have the lowest values. As a result, in the case of people with significant metabolic imbalances, it might be beneficial to include only mussels harvested at certain times of the year in their diet.
Analysis of fatty acids composition of the main lipid fractions showed high concentrations of polyunsaturated fatty acids in neutral lipids (also representing the dominant fraction, 81.11%), which gives them a particular biological value. The total fatty acids are 69.27% of the total lipid in analyzed mussels.
Analysis of the total lipid extract isolated and purified from mussel flesh showed abundant polyunsaturated fatty acids content, particularly of the ω-3 group fatty acids, as compared with saturated and unsaturated fatty acids, which accounts for the high degree of unsaturation indicated by the iodine value (82.34) for the fat fraction in mussel flesh.
Among fatty acids, the most abundant in the analyzed samples are myristic acid, palmitic acid, palmitoleic acid, oleic acid, and eicosapentaenoic acid. Experimental data indicate a report ω-3/ω-6 of 6.14. The 4.96 ratio of polyunsaturated to saturated fatty acids also shows an increased content of unsaturated lipids in mussel meat.
Even if the lipid fraction in mussel meat is not found in high concentrations, 9.22–17.23% dry sample, the composition rich in polyunsaturated fatty acids (especially in ω-3 group fatty acids) makes it particularly valuable for consumers’ health [64].
The beneficial effect of long-chain ω-3 polyunsaturated fatty acids in the composition of mussel meat has been highlighted by clinical studies that have shown significant improvements in the serum lipid profile of people who regularly included mussel meat in their diet and especially at the patients with cardiovascular disease. There has even been an improvement in consumers’ health with a 20% reduction in the risk of sudden cardiac death [65,66].
Due to their filtering capacity, mussels are recognized as marine organisms with an increased potential for accumulating contaminants in the aquatic environment. Therefore, rigorous investigations are needed regarding the content of various pollutants in mussel meat intended for consumption [67].
Estimated daily intake rates of metals through consumption of mussels harvested from the Romanian Black Sea were below the daily tolerable limits recommended by the Joint FAO/WHO Expert Committee on Food Additives: 0.001 mg/kg bw for cadmium, 0.5 mg/kg bw for copper, 1 mg/kg bw for zinc, 0.14 mg/kg bw for chromium, and 0.0035 mg/kg bw for lead [44,45].
The vast majority of the investigated mussel samples did not exceed allowable values for metals ions recommended by Commission Regulation (EC) no. 1881/2006 [49], and there are no concerns about their consumption, especially when calculated hazard quotients were below the critical value 1 for all analyzed heavy metals, meaning no possible risks are associated with mussel consumption from the study areas. However, Pb concentrations in mussel meat samples taken from area 3 have slightly overrun, which could be attributed to anthropogenic changes/impacts accordingly to Catianis [68]. Over the years, previous studies of the Black Sea mussels have also reported slightly excessive Pb concentrations in that area [37,69], and the evolution trends were not very clear due to large concentration fluctuations [69]. Moreover, the accumulation of the metals in the gills, visceral mass, and remaining tissues of M. galloprovincialis was measured [70], while other studies discussed the biomagnification process of copper, lead, nickel, and chromium, which was observed at small fish (trophic level 3) in some areas [71].
There are scientific studies that highlight the possibility of capitalizing on shells, not just mussel meat [72,73]. As a result, the analysis of contaminants in the shells is also critical.
Although pollution is quite low according to our experimental results and does not affect consumers’ health, there are still differences between the areas investigated in terms of the level of pollutants. In areas with intense human activity, permanent monitoring is required of analyzed pollutants and an extension of the tested parameters (mercury, aromatic hydrocarbons, pesticides, microplastics, etc.).
5. Conclusions
Mussels are an essential source of valuable nutrients (proteins, fats, carbohydrates), and their composition is influenced by seasonal factors and the quality of the environment in which they grow. Consequently, it is essential to analyze the influence of these factors to optimize the harvesting conditions of these marine species to benefit from specimens rich in nutrients and poor in contaminants.
In spring specimens, experimental data showed an increased protein (58.51%) and lipid (17.23%) content. Therefore, to benefit from the valuable proteins in the composition of mussel meat, it is recommended to harvest in spring when its content is maximum, or winter (49.52%). At the same time, the samples from spring present the advantage of containing a minimum level of carbohydrates and minerals, while in autumn, they accumulate a large amount of carbohydrates (the highest of the four seasons), and fats (the second-largest value after spring), with low protein concentration. As a result, in the case of people with significant metabolic imbalances, it might be beneficial to include only mussels harvested at certain times of the year in their diet.
Analysis of fatty acids composition of the main lipid fractions showed high concentrations of polyunsaturated fatty acids in neutral lipids (also representing the dominant fraction, 81.11%), which gives them a particular biological value. Among fatty acids, the most abundant in the analyzed samples are myristic acid, palmitic acid, palmitoleic acid, oleic acid, and eicosapentaenoic acid, with a 4.96 ratio of polyunsaturated/saturated fatty acids and a report ω-3/ω-6 of 6.14.
The vast majority of the investigated mussel samples did not exceed recommended allowable values for metals ions [49], so there are no concerns about their consumption, especially as the calculated hazard quotients were below the critical value 1 for all analyzed heavy metals.
Author Contributions
Conceptualization, M.M., E.O. and D.D.; methodology, M.G. and M.N.; software, M.G. and M.N.; validation, S.M.N., D.-E.D., T.O.N. and A.C.R.; formal analysis, M.G. and T.O.N.; investigation, M.M.; resources, M.M.; data curation, D.D.; writing—original draft preparation, S.M.N.; writing—review and editing, E.O.; visualization, D.-E.D.; supervision, E.O. and A.C.R.; project administration, M.M.; funding acquisition, M.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Venugopal, V.; Gopakumar, K. Shellfish: Nutritive Value, Health Benefits, and Consumer Safety. Compr. Rev. Food Sci. Food Saf. 2017, 16, 1219–1242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Food and Agriculture Organization (FAO); International Network of Food Data Systems (INFOODS). Global Food Composition Database for Fish and Shellfish; Version 10-uFiSh10; Food and Agriculture Organization (FAO): Rome, Italy, 2016. [Google Scholar]
- Cherifi, H.; Ajjabi, L.C.; Sadok, S. Nutritional value of the Tunisian mussel Mytilus galloprovincialis with a special emphasis on lipid quality. Food Chem. 2018, 268, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Grienke, U.; Silke, J.; Tasdemir, D. Bioactive compounds from marine mussels and their effects on human health. Food Chem. 2014, 142, 48–60. [Google Scholar] [CrossRef] [PubMed]
- Tenikoff, D.; Murphy, K.J.; Le, M.; Howe, P.R.; Howarth, G.S. Lyprinol (stabilised lipid extract of New Zealand green-lipped mussel): A potential preventative treatment modality for inflammatory bowel disease. J. Gastroenterol. 2005, 40, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Jung, W.K.; Kim, S.K. Isolation and characterization of an anticoagulant oligopeptide from blue mussel, Mytilus edulis. Food Chem. 2009, 117, 687–692. [Google Scholar] [CrossRef]
- Fuentes, A.; Fernández-Segovia, I.; Escriche, I.; Serra, J.A. Comparison of physico-chemical parameters and composition of mussels (Mytilus galloprovincialis Lmk.) from different Spanish origins. Food Chem. 2009, 112, 295–302. [Google Scholar] [CrossRef]
- Chi, C.F.; Zhang, J.S.; Wu, C.W.; Xu, M.Y.; Wang, B. Analysis and evaluation of nutrition composition of mussel. Adv. Mater. Res. 2012, 554–556, 1455–1458. [Google Scholar]
- Bohrer, B.M. Review: Nutrient density and nutritional value of meat products and non-meat foods high in protein. Trends Food Sci. Technol. 2017, 65, 103–112. [Google Scholar] [CrossRef]
- Mititelu, M.; Ioniţă, A.C.; Moroşan, E. Research regarding integral processing of mussels from Black Sea. Farmacia 2014, 62, 625–632. [Google Scholar]
- Ferreira, J.G.; Hawkins, A.J.S.; Bricker, S.B. Management of productivity, environmental effects and profitability of shellfish aquaculture—The Farm Aquaculture Resource Management (FARM) model. Aquaculture 2007, 264, 160–174. [Google Scholar] [CrossRef]
- Pérez-Cano, F.J. Mediterranean Diet, Microbiota and Immunity. Nutrients 2022, 14, 273. [Google Scholar] [CrossRef]
- Ioniţă, A.C.; Ghica, M.; Moroşan, E.; Nicolescu, F.; Mititelu, M. In vitro effects of some synthesized aminoacetanilide N’-substituted on human leukocytes separated from peripheral blood. Farmacia 2019, 67, 684–690. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Wu, J.H. Omega-3 fatty acids and cardiovascular disease: Effects on risk factors, molecular pathways, and clinical events. J. Am. Coll. Cardiol. 2011, 58, 2047–2067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gil, A.; Gil, F. Fish, a Mediterranean source of n-3 PUFA: Benefits do not justify limiting consumption. Br. J. Nutr. 2015, 113 (Suppl. 2), S58–S67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, G.P.; Fu, Y.Q.; Zheng, J.S.; Li, D. Anti-inflammatory activity and mechanism of a lipid extract from hard-shelled mussel (Mytilus coruscus) on chronic arthritis in rats. Mar. Drugs 2014, 12, 568–588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patterson, A.C.; Chalil, A.; Henao, J.J.A.; Streit, I.T.; Stark, K.D. Omega-3 polyunsaturated fatty acid blood biomarkers increase linearly in men and women after tightly controlled intakes of 0.25, 0.5, and 1 g/d of EPA + DHA. Nutr. Res. 2015, 35, 1040–1051. [Google Scholar] [CrossRef]
- Calder, P.C. Marine omega-3 fatty acids and inflammatory processes: Effects, mechanisms and clinical relevance. Biochim. Biophys. Acta. 2015, 1851, 469–484. [Google Scholar] [CrossRef]
- Sinclair, A.J.; Murphy, K.J.; Li, D. Marine lipids: Overview “news insights and lipid composition of Lyprinol”. Allerg. Immunol. 2000, 32, 261–271. [Google Scholar]
- Raikow, D.F.; Hamilton, S.K. Bivalve diets in a Midwestern U.S. stream: A stable isotope enrichment study. Limnol. Oceanogr. 2001, 46, 514–522. [Google Scholar] [CrossRef]
- Gallardi, D. Effects of bivalve aquaculture on the environment and their possible mitigation: A review. Fish. Aquac. J. 2014, 5, 1. [Google Scholar] [CrossRef] [Green Version]
- Brunner, E.J.; Jones, P.J.; Friel, S.; Bartley, M. Fish, human health and marine ecosystem health: Policies in collision. Int. J. Epidemiol. 2009, 38, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Anacleto, P.; Maulvault, A.L.; Nunes, M.L.; Carvallo, M.L.; Rosa, R.; Marques, A. Effects of depuration on metal levels and health status of bivalve mollusks. Food Control 2015, 47, 493–501. [Google Scholar] [CrossRef]
- Domingo, J.L. Nutrients and chemical pollutants in fish and shellfish: Balancing health benefits and risks of regular fish consumption. Crit. Rev. Food Sci. Nut. 2016, 56, 979–988. [Google Scholar] [CrossRef] [PubMed]
- Hargrave, B.T.; Doucette, L.I.; Cranford, P.J.; Law, B.A.; Milligan, T.G. Influence of mussel aquaculture on sediment organic enrichment in a nutrient-rich coastal embayment. Mar. Ecol. Prog. Ser. 2008, 365, 137–149. [Google Scholar] [CrossRef]
- Orban, E.; Di Lena, G.; Nevigato, T.; Casini, I.; Marzetti, A.; Caproni, R. Seasonal changes in meat content, condition index and chemical composition of mussels (Mytilus galloprovincialis) cultured in two different Italian sites. Food Chem. 2002, 77, 57–65. [Google Scholar] [CrossRef]
- Çelik, M.Y.; Karayücel, S.; Karayücel, İ.; Öztürk, R.; Eyüboğlu, B. Meat yield, condition index, and biochemical composition of mussels (Mytilus galloprovincialis Lamarck, 1819) in Sinop, South of the Black Sea. J. Aquat. Food Prod. Technol. 2012, 21, 198–205. [Google Scholar] [CrossRef]
- Järup, L. Hazards of heavy metal contamination. Br. Med. Bull. 2003, 68, 167–182. [Google Scholar] [CrossRef] [Green Version]
- Nedelescu, M.; Baconi, D.; Neagoe, A.; Iordache, V.; Stan, M.; Ciobanu, A.M.; Vardavas, A.I.; Vinceti, M.; Tsatsakis, A.M. Environmental metal contamination and health impact assessment in two industrial regions of Romania. Sci. Total Environ. 2017, 580, 984–995. [Google Scholar] [CrossRef]
- Alissa, E.M.; Ferns, G.A. Heavy Metal Poisoning and Cardiovascular Disease. J. Toxicol. 2011, 2011, 870125. [Google Scholar] [CrossRef]
- Nedelescu, M.; Stan, M.; Ciobanu, A.-M.; Bălălău, C.; Filippini, T.; Baconi, D. Attention deficit among preschool and school-aged children living near former metal-processing plants in Romania. Environ. Res. 2022, 208, 112689. [Google Scholar] [CrossRef]
- Kjeldahl, J. New method for the determination of nitrogen in organic substances. Z. Anal. Chem. 1883, 22, 366–383. [Google Scholar] [CrossRef] [Green Version]
- Christie, W.W.; Han, X. Lipid Analysis—Isolation, Separation, Identification and Lipidomic Analysis, 4th ed.; Oily Press: Bridgwater, UK, 2010; pp. 403–405. [Google Scholar]
- Fernell, W.R.; King, H.K. The simultaneous determination of pentose and hexose in mixtures of sugars. Analyst 1953, 78, 80–83. [Google Scholar] [CrossRef]
- Yamamoto, C. A method for determining sugar and glycogen in the tissues by orcinol sulphuric acid method. J. Biochem. 1940, 32, 389–399. [Google Scholar] [CrossRef]
- XXX Romanian Pharmacopoeia, 10th ed.; Editura Medicală: Bucharest, Romania, 1993.
- Mititelu, M.; Moroşan, E.; Neacsu, S.M.; Ioniţă, E.I. Research regarding the pollution degree from romanian Black Sea coast. Farmacia 2018, 66, 1059–1063. [Google Scholar] [CrossRef]
- Mititelu, M.; Ghica, M.; Ionita, A.C.; Moroşan, E. The influence of heavy metals contamination in soil on the composition of some wild edible mushrooms. Farmacia 2019, 67, 398–404. [Google Scholar] [CrossRef]
- Makedonski, L.; Ivanova, P.C.; Stancheva, M. Determination of some heavy metal of selected Black Sea fish species. Food Control 2015, 30, 313–318. [Google Scholar]
- Wang, W.X. Bioaccumulation and biomonitoring. In Marine Ecotoxicology; Academic Press: Cambridge, MA, USA, 2016; pp. 99–119. [Google Scholar]
- Jitar, O.; Teodosiu, C.; Oros, A.; Plavan, G.; Nicoara, M. Bioaccumulation of heavy metals in marine organism from the Romanian sector of the Black Sea. New Biotechnol. 2014, 32, 369–378. [Google Scholar] [CrossRef]
- Bat, L.; Arici, E.; Oztekin, A. Human Health Risk Assessment of Heavy Metals in the Black Sea: Evaluating Mussels. Curr. World Environ. 2018, 13, 15–31. [Google Scholar] [CrossRef] [Green Version]
- European Commission. Facts and Figures on the Common Fisheries Policy; European Commission: Brussels, Belgium, 2020; Available online: https://ec.europa.eu/oceans-and-fisheries/facts-and-figures/facts-and-figures-common-fisheries-policy/consumption_en (accessed on 15 January 2022).
- World Health Organization (WHO); Food and Agriculture Organization (FAO). Guidelines for the Safe Use of Wastewater and Food Stuff; Report of the Joint WHO/FAO; World Health Organization (WHO): Geneva, Switzerland, 2013; Volume 2, Available online: https://www.who.int/water_sanitation_health/wastewater/wwuvol2intro.pdf (accessed on 10 January 2022).
- Guidelines for the Simple Evaluation of Dietary Exposure to Food Additives. 2014. Available online: http://www.codexalimentarius.org (accessed on 22 January 2022).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org/ (accessed on 10 January 2022).
- Albeanu, G.; Ghica, M.; Popentiu-Vladicescu, F. On using bootstrap scenario-generation for multiperiod stochastic programming applications. Int. J. Comput. Commun. Control 2008, 3, 156–161. [Google Scholar]
- Bat, L.; Ustun, F.; Baki, O.G. Trace Element Concentrations in the Mediterranean Mussel Mytilus galloprovincialis Lamarck, 1819 Caught from Sinop Coast of the Black Sea, Turkey. Open Mar. Biol. J. 2012, 6, 1–5. [Google Scholar] [CrossRef]
- European Union. Commission Regulation (EC) No. 1881/2006 setting maximum levels for certain contaminants in foodstuffs. Off. J. Eur. Union 2006, L173, 6–9. [Google Scholar]
- Romanian Ministry of Environment and Sustainable Development. Order No. 1888 of November 28, 2007 on the Approval of the List of Organohalogenated Substances and Heavy Metals, as Well as the Maximum Permissible Limits for Organohalogenated Substances and Heavy Metals in Water and Sedimentary Substrate; Official Gazette; Romanian Ministry of Environment and Sustainable Development: Bucharest, Romania, 2007; Volume 839.
- Rodriguez-Hernandez, A.; Zumbado, M.; Henriquez-Hernandez, L.A.; Boada, L.D.; Luzardo, O.P. Dietary Intake of Essential, Toxic, and Potentially Toxic Elements from Mussels (Mytilus spp.) in the Spanish Population: A Nutritional Assessment. Nutrients 2019, 11, 864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bogdanovic, T.; Ujevic, I.; Sedak, M.; Listes, E.; Simat, V.; Petricevic, S.; Poljak, V. As, Cd, Hg and Pb in four edible shellfish species from breeding and harvesting areas along the eastern Adriatic Coast, Croatia. Food Chem. 2014, 146, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Djedjibegovic, J.; Marjanovic, A.; Tahirovic, D.; Caklovica, K.; Turalic, A.; Lugusic, A.; Omeragić, E.; Šober, M.; Čaklovica, F. Heavy metals in commercial fish and seafood products and risk assessment in adult population in Bosnia and Herzegovina. Sci. Rep. 2020, 10, 13238. [Google Scholar] [CrossRef] [PubMed]
- Peteva, Z.; Georgieva, S.; Krock, B.; Gerasimova, A.; Stancheva, M.; Merdzhanova, A. Lipophilic Marine Biotoxins in Mussels from Bulgarian Coast and Dietary Intake of Different Population Groups. Proc. Nutr. Soc. 2020, 79, E325. [Google Scholar] [CrossRef]
- Bat, L.; Oztekin, A.; Arici, E.; Sahin, F. Mytilus Galloprovincialis and Metal Contaminants: Health Risk Assessment from Sinop Coasts. Korean J. Food Health Converg. 2021, 7, 13–21. [Google Scholar]
- Yigit, M.; Celikkol, B.; Yilmaz, S.; Bulut, M.; Ozalp, B.; Dwyer, R.L.; Maita, M.; Kizilkaya, B.; Yigit, U.; Ergun, S.; et al. Bioaccumulation of trace metals in Mediterranean mussels (Mytilus galloprovincialis) from a fish farm with copper-alloy mesh pens and potential risk assessment. Hum. Ecol. Risk Assess. Int. J. 2017, 24, 465–481. [Google Scholar] [CrossRef] [Green Version]
- Mititelu, M.; Udeanu, D.I.; Nedelescu, M.; Neacsu, S.M.; Nicoara, A.C.; Oprea, E.; Ghica, M. Quality Control of Different Types of Honey and Propolis Collected from Romanian Accredited Bee-Keepers and Consumer’s Risk Assessment. Crystals 2022, 12, 87. [Google Scholar] [CrossRef]
- Mustatea, G.; Ungureanu, E.L.; Iorga, S.C.; Ciotea, D.; Popa, M.E. Risk Assessment of Lead and Cadmium in Some Food Supplements Available on the Romanian Market. Foods 2021, 10, 581. [Google Scholar] [CrossRef]
- Cisneros-Montemayor, A.M.; Pauly, D.; Lauren, V.W.; Ota, Y. A Global Estimate of Seafood Consumption by Coastal Indigenous Peoples. PLoS ONE 2016, 11, e0166681. [Google Scholar] [CrossRef]
- Klepper, N. Taste of Romania; Hippocrene: New York, NY, USA, 1999; ISBN 9780781807661. [Google Scholar]
- Năstăsescu, V.; Mititelu, M.; Stanciu, T.I.; Drăgănescu, D.; Grigore, N.D.; Udeanu, D.I.; Stanciu, G.; Neacsu, S.M.; Dinu-Pîrvu, C.E.; Oprea, E.; et al. Food Habits and Lifestyle of Romanians in the Context of the COVID-19 Pandemic. Nutrients 2022, 14, 504. [Google Scholar] [CrossRef]
- Food and Agriculture Organization (FAO). The State of World Fisheries and Aquaculture 2020: Sustainability in Action; Food and Agriculture Organization (FAO): Rome, Italy, 2020. [Google Scholar]
- Suja, N.; Muthiah, P. Variation in gross biochemical composition in relation to the gametogenic cycle of the baby clam, Marcia opima (Gmelin), from two geographically separated areas. Indian J. Fish. 2010, 57, 53–59. [Google Scholar]
- Gammone, M.A.; Riccioni, G.; Parrinello, G.; D’Orazio, N. Omega-3 Polyunsaturated Fatty Acids: Benefits and Endpoints in Sport. Nutrients 2018, 11, 46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carboni, S.; Kaur, G.; Pryce, A.; McKee, K.; Desbois, A.P.; Dick, J.R.; Galloway, S.D.R.; Hamilton, D.L. Mussel Consumption as a “Food First” Approach to Improve Omega-3 Status. Nutrients 2019, 11, 1381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albert, C.M.; Campos, H.; Stampfer, M.J.; Ridker, P.M.; Manson, J.E.; Willett, W.C.; Ma, J. Blood levels of long-chain n–3 fatty acids and the risk of sudden death. N. Engl. J. Med. 2002, 346, 1113–1118. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization (WHO). Safe Management of Shellfish and Harvest Waters; Rees, G., Pond, K., Kay, D., Bartram, J., Domingo, J.S., Eds.; IWA Publishing: London, UK, 2010; ISBN 9781843392255. Available online: https://www.who.int/water_sanitation_health/emerging/depuration (accessed on 6 February 2022).
- Catianis, I.; Ungureanu, C.; Magagnini, L.; Ulazzi, E.; Campisi, T.; Stanica, A. Environmental impact of the Midia Port—Black Sea (Romania), on the coastal sediment quality. Open Geosci. 2016, 8, 174–194. [Google Scholar] [CrossRef] [Green Version]
- Oros, A.; Gomoiu, M.T. Comparative data on the accumulation of five heavy metals (cadmium, chromium, copper, nickel, lead) in some marine species (molluscs, fish) from the Romanian sector of the Black Sea. Rech. Mar. 2010, 39, 89–108. [Google Scholar]
- Roméo, M.; Frasila, C.; Gnassia-Barelli, M.; Damiens, G.; Micu, D.; Mustata, G. Biomonitoring of trace metals in the Black Sea (Romania) using mussels Mytilus galloprovincialis. Water Res. 2005, 39, 596–604. [Google Scholar] [CrossRef]
- Coatu, V.; Oros, A.; Damir, N.; Timofte, F.; Lazăr, L. Bioaccumulation of contaminants in the main links of the pelagic trophic chain at the Romanian Black Sea coast. Rech. Mar. 2010, 39, 89–108. [Google Scholar]
- Mititelu, M.; Moroșan, E.; Nicoară, A.C.; Secăreanu, A.A.; Musuc, A.M.; Atkinson, I.; Cusu, J.P.; Nițulescu, G.M.; Ozon, E.A.; Sarbu, I.; et al. Development of immediate release tablets containing calcium lactate synthetized from Black Sea mussel shells. Mar. Drugs 2022, 20, 45. [Google Scholar] [CrossRef]
- Mititelu, M.; Stanciu, G.; Drăgănescu, D.; Ioniță, A.C.; Neacșu, S.M.; Dinu, M.; Stefanvan Staden, R.-I.; Moroșan, E. Mussel Shells, a Valuable Calcium Resource for the Pharmaceutical Industry. Mar. Drugs 2022, 20, 25. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).