The Prospective Associations of Lipid Metabolism-Related Dietary Patterns with the Risk of Diabetes in Chinese Adults
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Dietary Assessment
2.3. Measurements of the BMI, Waist Circumference, and Plasma Lipids
2.4. Outcome Ascertainment
2.5. Covariate Assessment
2.6. Statistical Analyses
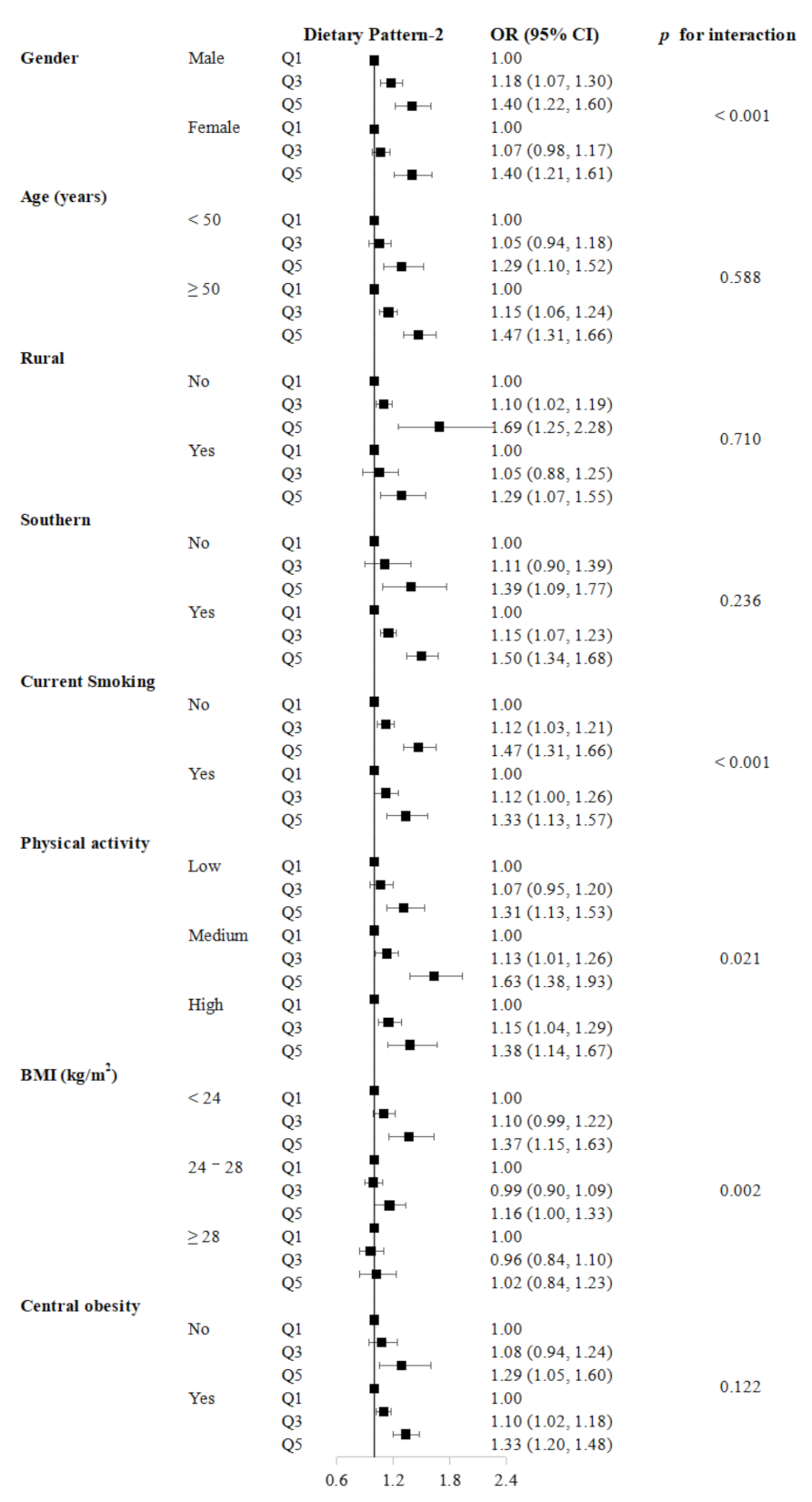
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Diabetes in the Western Pacific. Available online: https://www.who.int/westernpacific/health-topics/diabetes (accessed on 15 August 2018).
- Look AHEAD Research Group. Association of the magnitude of weight loss and changes in physical fitness with long-term cardiovascular disease outcomes in overweight or obese people with type 2 diabetes: A post-hoc analysis of the Look AHEAD randomised clinical trial. Lancet Diabetes Endocrinol. 2016, 4, 913–921. [Google Scholar] [CrossRef] [Green Version]
- Micha, R.; Wallace, S.K.; Mozaffarian, D. Red and processed meat consumption and risk of incident coronary heart disease, stroke, and diabetes mellitus: A systematic review and meta-analysis. Circulation 2010, 121, 2271–2283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, E.A.; Pan, A.; Malik, V.; Sun, Q. White rice consumption and risk of type 2 diabetes: Meta-analysis and systematic review. BMJ 2012, 344, e1454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Y.; Tian, C.; Jia, C. Association of fish and n-3 fatty acid intake with the risk of type 2 diabetes: A meta-analysis of prospective studies. Br. J. Nutr. 2012, 108, 408–417. [Google Scholar] [CrossRef] [Green Version]
- Jannasch, F.; Kröger, J.; Schulze, M.B. Dietary Patterns and Type 2 Diabetes: A Systematic Literature Review and Meta-Analysis of Prospective Studies. J. Nutr. 2017, 147, 1174–1182. [Google Scholar] [CrossRef] [Green Version]
- Weikert, C.; Schulze, M.B. Evaluating dietary patterns: The role of reduced rank regression. Curr. Opin. Clin. Nutr. Metab. Care 2016, 19, 341–346. [Google Scholar] [CrossRef]
- Ocké, M.C. Evaluation of methodologies for assessing the overall diet: Dietary quality scores and dietary pattern analysis. Proc. Nutr. Soc. 2013, 72, 191–199. [Google Scholar] [CrossRef] [Green Version]
- Hoffmann, K.; Schulze, M.B.; Schienkiewitz, A.; Nöthlings, U.; Boeing, H. Application of a New Statistical Method to Derive Dietary Patterns in Nutritional Epidemiology. Am. J. Epidemiol. 2004, 159, 935–944. [Google Scholar] [CrossRef]
- von Eckardstein, A.; Sibler, R.A. Possible contributions of lipoproteins and cholesterol to the pathogenesis of diabetes mellitus type 2. Curr. Opin. Lipidol. 2011, 22, 26–32. [Google Scholar] [CrossRef]
- Maggio, C.A.; Pi-Sunyer, F.X. Obesity and type 2 diabetes. Endocrinol. Metab. Clin. N. Am. 2003, 32, 805–822. [Google Scholar] [CrossRef]
- Chen, Z.; Lee, L.; Chen, J.; Collins, R.; Wu, F.; Guo, Y.; Peto, R. Cohort Profile: The Kadoorie Study of Chronic Disease in China (KSCDC). Int. J. Epidemiol. 2005, 34, 1243–1249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Z.; Chen, J.; Collins, R.; Guo, Y.; Peto, R.; Wu, F.; Li, L. China Kadoorie Biobank of 0.5 million people: Survey methods, baseline characteristics and long-term follow-up. Int. J. Epidemiol. 2011, 40, 1652–1666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Millwood, I.Y.; Li, L.; Smith, M.; Guo, Y.; Yang, L.; Bian, Z.; Chen, Z. Alcohol consumption in 0.5 million people from 10 diverse regions of China: Prevalence, patterns and socio-demographic and health-related correlates. Int. J. Epidemiol. 2017, 46, 2103. [Google Scholar] [CrossRef] [Green Version]
- Sun, L.; Clarke, R.; Bennett, D.; Guo, Y.; Walters, R.G.; Hill, M.; Chen, Z. Causal associations of blood lipids with risk of ischemic stroke and intracerebral hemorrhage in Chinese adults. Nat. Med. 2019, 25, 569–574. [Google Scholar] [CrossRef]
- Du, H.; Bennett, D.; Li, L.; Whitlock, G.; Guo, Y.; Collins, R.; Chen, J.; Bian, Z.; Hong, L.S.; Feng, S.; et al. Physical activity and sedentary leisure time and their associations with BMI, waist circumference, and percentage body fat in 0.5 million adults: The China Kadoorie Biobank study. Am. J. Clin. Nutr. 2013, 97, 487–496. [Google Scholar] [PubMed] [Green Version]
- Zheng, B.; Yu, C.Q. RRR: Stata module to perform Reduced Rank Regression. In Statistical Software Components S 458882; Boston College Department of Economics: Boston, MA, USA, 2020. [Google Scholar]
- Lamichhane, A.P.; Liese, A.D.; Urbina, E.M.; Crandell, J.L.; Jaacks, L.M.; Dabelea, D.; Mayer-Davis, E.J. Associations of dietary intake patterns identified using reduced rank regression with markers of arterial stiffness among youth with type 1 diabetes. Eur. J. Clin. Nutr. 2014, 68, 1327–1333. [Google Scholar] [CrossRef]
- Seah, J.Y.; Ong, C.N.; Koh, W.P.; Yuan, J.M.; van Dam, R.M. A Dietary Pattern Derived from Reduced Rank Regression and Fatty Acid Biomarkers Is Associated with Lower Risk of Type 2 Diabetes and Coronary Artery Disease in Chinese Adults. J. Nutr. 2019, 149, 2001–2010. [Google Scholar] [CrossRef]
- Pastorino, S.; Richards, M.; Pierce, M.; Ambrosini, G.L. A high-fat, high-glycaemic index, low-fibre dietary pattern is prospectively associated with type 2 diabetes in a British birth cohort. Br. J. Nutr. 2016, 115, 1632–1642. [Google Scholar] [CrossRef] [Green Version]
- Shu, L.; Zheng, P.F.; Zhang, X.Y.; Si, C.J.; Yu, X.L.; Gao, W.; Liao, D. Association between Dietary Patterns and the Indicators of Obesity among Chinese: A Cross-Sectional Study. Nutrients 2015, 7, 7995–8009. [Google Scholar] [CrossRef]
- Zhubi-Bakija, F.; Bajraktari, G.; Bytyçi, I.; Mikhailidis, D.P.; Henein, M.Y.; Latkovskis, G.; Zirlik, A. The impact of type of dietary protein, animal versus vegetable, in modifying cardiometabolic risk factors: A position paper from the International Lipid Expert Panel (ILEP). Clin. Nutr. 2021, 40, 255–276. [Google Scholar] [CrossRef]
- Key, T.J.; Appleby, P.N.; Bradbury, K.E.; Sweeting, M.; Wood, A.; Johansson, I.; Danesh, J. Consumption of Meat, Fish, Dairy Products, and Eggs and Risk of Ischemic Heart Disease. Circulation 2019, 139, 2835–2845. [Google Scholar] [CrossRef]
- Vekic, J.; Zeljkovic, A.; Stefanovic, A.; Jelic-Ivanovic, Z.; Spasojevic-Kalimanovska, V. Obesity and dyslipidemia. Metabolism 2019, 92, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Ortiz, J.A.; Fung, T.T.; Baylin, A.; Hu, F.B.; Campos, H. Dietary patterns and risk of nonfatal acute myocardial infarction in Costa Rican adults. Eur. J. Clin. Nutr. 2006, 60, 770–777. [Google Scholar] [CrossRef] [Green Version]
- Zuniga, Y.L.; Rebello, S.A.; Oi, P.L.; Zheng, H.; Lee, J.; Tai, E.S.; Van Dam, R.M. Rice and noodle consumption is associated with insulin resistance and hyperglycaemia in an Asian population. Br. J. Nutr. 2014, 111, 1118–1128. [Google Scholar] [CrossRef] [Green Version]
- Leitner, D.R.; Frühbeck, G.; Yumuk, V.; Schindler, K.; Micic, D.; Woodward, E.; Toplak, H. Obesity and Type 2 Diabetes: Two Diseases with a Need for Combined Treatment Strategies-EASO Can Lead the Way. Obes. Facts 2017, 10, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, A.; Peeters, A.; de Courten, M.; Stoelwinder, J. The magnitude of association between overweight and obesity and the risk of diabetes: A meta-analysis of prospective cohort studies. Diabetes Res. Clin. Pract. 2010, 89, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, S.L. Diabetes and dyslipidaemia. Diabetes Obes. Metab. 2006, 8, 355–364. [Google Scholar] [CrossRef]
- Tian, S.; Xu, Q.; Jiang, R.; Han, T.; Sun, C.; Na, L. Dietary Protein Consumption and the Risk of Type 2 Diabetes: A Systematic Review and Meta-Analysis of Cohort Studies. Nutrients 2017, 9, 982. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Y.; Sang, S. Phytochemicals in whole grain wheat and their health-promoting effects. Mol. Nutr. Food Res. 2017, 61, 1600852. [Google Scholar] [CrossRef]
- Olfert, M.D.; Wattick, R.A. Vegetarian Diets and the Risk of Diabetes. Curr. Diab. Rep. 2018, 18, 101. [Google Scholar] [CrossRef] [Green Version]
- Barclay, A.W.; Petocz, P.; McMillan-Price, J.; Flood, V.M.; Prvan, T.; Mitchell, P.; Brand-Miller, J.C. Glycemic index, glycemic load, and chronic disease risk-a meta-analysis of observational studies. Am. J. Clin. Nutr. 2008, 87, 627–637. [Google Scholar] [CrossRef] [PubMed]
- Slavin, J.L.; Martini, M.C.; Jacobs, D.R., Jr.; Marquart, L. Plausible mechanisms for the protectiveness of whole grains. Am. J. Clin. Nutr. 1999, 70, 459S–463S. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.; Lv, J.; Guo, Y.; Bian, Z.; Si, J.; Yang, L.; Li, L. Associations of egg consumption with cardiovascular disease in a cohort study of 0.5 million Chinese adults. Heart 2018, 104, 1756–1763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Food or Beverage Group | Dietary Pattern-1 | Dietary Pattern-2 |
|---|---|---|
| Rice | 0.31 | −0.23 |
| Wheat | −0.79 | 0.24 |
| Other staples | −0.16 | 0.36 |
| Meat | 0.28 | 0.15 |
| Poultry | 0.22 | 0.38 |
| Fish | 0.22 | 0.43 |
| Eggs | −0.12 | −0.01 |
| Fresh vegetables | 0.02 | 0.32 |
| Fresh fruit | 0.04 | 0.34 |
| Preserved vegetables | −0.04 | 0.08 |
| Soybeans | 0.08 | 0.22 |
| Dairy products | 0.17 | 0.11 |
| Beer | −0.07 | 0.15 |
| Rice wine | 0.04 | 0.04 |
| Wine | 0.01 | 0.02 |
| Heavy spirits (≥40%) | −0.09 | −0.08 |
| Light spirits (<40%) | −0.02 | −0.02 |
| Green tea | −0.02 | 0.19 |
| Oolong tea | 0.01 | 0.19 |
| Black tea | −0.03 | −0.01 |
| Other tea | 0.07 | 0.15 |
| % Explained Variance (Correlations) | ||
| Food intakes (total) | 6.26 | 6.93 |
| Responses (total) | 4.23 | 1.44 |
| Total cholesterol | 10.56 (0.29) | 0.47 (0.07) |
| LDL cholesterol | 8.47 (0.27) | 1.42 (0.14) |
| HDL cholesterol | 4.04 (0.19) | 1.60 (−0.12) |
| Triglycerides | 0.62 (−0.07) | 0.14 (0.03) |
| BMI | 0.63 (−0.08) | 2.76 (0.17) |
| Waist circumference | 1.07 (−0.10) | 2.25 (0.15) |
| Dietary Pattern-1 | Dietary Pattern-2 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Q1 (Low) | Q2 | Q3 | Q4 | Q5 (High) | p for Trend | Q1 (Low) | Q2 | Q3 | Q4 | Q5 (High) | p for Trend | |
| n | 98,107 | 93,329 | 95,975 | 97,110 | 94,686 | - | 97,087 | 97,473 | 95,512 | 94,747 | 94,388 | - |
| Dietary pattern score | −0.87 | −0.16 | 0.16 | 0.30 | 0.57 | <0.001 | −0.36 | −0.20 | −0.04 | 0.13 | 0.47 | <0.001 |
| Age, year | 50.0 | 53.1 | 53.1 | 52.1 | 49.7 | 0.006 | 58.5 | 53.5 | 52.6 | 49.5 | 43.6 | <0.001 |
| Female, % | 13.4 | 38.2 | 67.2 | 79.7 | 79.9 | <0.001 | 75.4 | 74.8 | 61.3 | 46.5 | 26.7 | <0.001 |
| Urban area, % | 8.5 | 62.6 | 27.2 | 29.8 | 89.3 | <0.001 | 2.8 | 10.9 | 38.5 | 66.4 | 99.0 | <0.001 |
| Southern area, % | 0.7 | 18.0 | 82.7 | 98.4 | 99.6 | <0.001 | 94.8 | 63.5 | 41.0 | 39.9 | 61.1 | <0.001 |
| High school and above, % | 40.3 | 45.4 | 51.3 | 50.7 | 59.4 | <0.001 | 36.7 | 43.3 | 49.9 | 55.9 | 63.5 | <0.001 |
| Household income ≥ 20,000 CNY/year, % | 27.0 | 36.8 | 41.8 | 43.6 | 56.1 | <0.001 | 25.0 | 32.3 | 41.3 | 49.5 | 57.4 | <0.001 |
| Married, % | 87.3 | 89.4 | 90.9 | 91.9 | 92.8 | <0.001 | 85.3 | 89.8 | 90.7 | 92.3 | 94.1 | <0.001 |
| Current smoker, % | 34.4 | 29.1 | 24.7 | 22.1 | 19.7 | <0.001 | 29.7 | 25.8 | 26.7 | 25.7 | 26.2 | <0.001 |
| Weekly drinker, % | 35.5 | 25.9 | 13.2 | 7.3 | 6.6 | <0.001 | 27.8 | 13.6 | 12.4 | 10.4 | 13.9 | <0.001 |
| Physical activity, MET-h/day | 22.6 | 21.1 | 21.5 | 21.7 | 20.5 | <0.001 | 23.2 | 22.3 | 21.0 | 21.1 | 19.7 | <0.001 |
| Energy intake, kcal/day | 1441.5 | 1519.4 | 1509.1 | 1475.6 | 1561.8 | <0.001 | 1429.8 | 1465.8 | 1482.9 | 1520.9 | 1608.4 | <0.001 |
| Family history of diabetes, % | 5.4 | 5.9 | 6.4 | 6.2 | 7.1 | <0.001 | 4.6 | 5.1 | 5.9 | 6.6 | 7.3 | <0.001 |
| BMI, kg/m2 | 23.7 | 23.8 | 23.5 | 23.4 | 23.4 | <0.001 | 22.9 | 23.4 | 23.6 | 23.8 | 24.2 | <0.001 |
| Waist circumference, cm | 80.6 | 80.6 | 79.5 | 79.5 | 79.4 | <0.001 | 78.1 | 79.5 | 79.9 | 80.4 | 81.8 | <0.001 |
| Quintiles of Dietary Pattern Scores | p for Trend | |||||
|---|---|---|---|---|---|---|
| Q1 (Low) | Q2 | Q3 | Q4 | Q5 (High) | ||
| Dietary Pattern-1 | ||||||
| Cases | 1635 | 3361 | 4820 | 5300 | 3561 | |
| Incidence rate (/1000 person/year) | 1.54 | 3.38 | 4.71 | 5.06 | 3.54 | |
| Model 1 | 1.00 (Reference) | 1.17 (1.07, 1.28) | 1.24 (1.11, 1.37) | 1.24 (1.12, 1.38) | 1.15 (1.03, 1.30) | 0.024 |
| Model 2 | 1.00 (Reference) | 1.16 (1.06, 1.27) | 1.19 (1.07, 1.32) | 1.18 (1.06, 1.32) | 1.08 (0.96, 1.22) | 0.363 |
| Dietary Pattern-2 | ||||||
| Cases | 4674 | 4312 | 3282 | 3159 | 3250 | |
| Incidence rate (/1000 person/year) | 4.56 | 4.10 | 3.20 | 3.10 | 3.21 | |
| Model 1 | 1.00 (Reference) | 1.12 (1.07, 1.17) | 1.16 (1.09, 1.23) | 1.22 (1.14, 1.32) | 1.50 (1.36, 1.64) | <0.001 |
| Model 2 | 1.00 (Reference) | 1.09 (1.04, 1.15) | 1.12 (1.05, 1.19) | 1.18 (1.09, 1.27) | 1.44 (1.31, 1.59) | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Q.; Wen, Q.; Lv, J.; Shi, Z.; Guo, Y.; Pei, P.; Du, H.; Yang, L.; Chen, Y.; Zhang, X.; et al. The Prospective Associations of Lipid Metabolism-Related Dietary Patterns with the Risk of Diabetes in Chinese Adults. Nutrients 2022, 14, 980. https://doi.org/10.3390/nu14050980
Liu Q, Wen Q, Lv J, Shi Z, Guo Y, Pei P, Du H, Yang L, Chen Y, Zhang X, et al. The Prospective Associations of Lipid Metabolism-Related Dietary Patterns with the Risk of Diabetes in Chinese Adults. Nutrients. 2022; 14(5):980. https://doi.org/10.3390/nu14050980
Chicago/Turabian StyleLiu, Qi, Qiaorui Wen, Jun Lv, Zumin Shi, Yu Guo, Pei Pei, Huaidong Du, Ling Yang, Yiping Chen, Xiaofang Zhang, and et al. 2022. "The Prospective Associations of Lipid Metabolism-Related Dietary Patterns with the Risk of Diabetes in Chinese Adults" Nutrients 14, no. 5: 980. https://doi.org/10.3390/nu14050980
APA StyleLiu, Q., Wen, Q., Lv, J., Shi, Z., Guo, Y., Pei, P., Du, H., Yang, L., Chen, Y., Zhang, X., Schmidt, D., Sansome, S., Chen, J., Yu, C., Chen, Z., Li, L., & on behalf of the China Kadoorie Biobank (CKB) Collaborative Group. (2022). The Prospective Associations of Lipid Metabolism-Related Dietary Patterns with the Risk of Diabetes in Chinese Adults. Nutrients, 14(5), 980. https://doi.org/10.3390/nu14050980







