Metabolomics Analysis Reveals the Effects of Compound Fuzhuan Brick Tea (CFBT) on Regulating Dyslipidemia and Metabolic Disorders in Mice Induced by High-Fat Diet
Abstract
:1. Introduction
2. Materials and Methods
2.1. CFBT Preparation and Extraction
2.2. Animal Experimental Design
2.3. Serum Collection and Biochemical Analysis
2.4. Preparation of Liver Tissue Homogenate
2.5. Histopathology Examination
2.6. Samples Preparation for Metabolomics
2.7. HPLC-MS Analysis
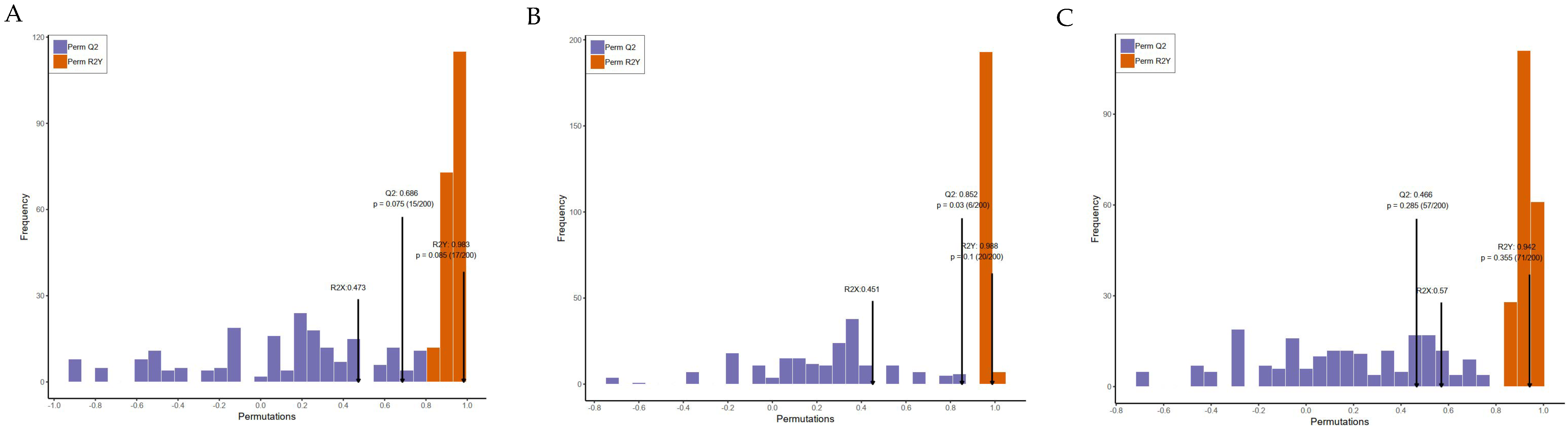
2.8. Data Processing and Statistical Analysis
3. Results
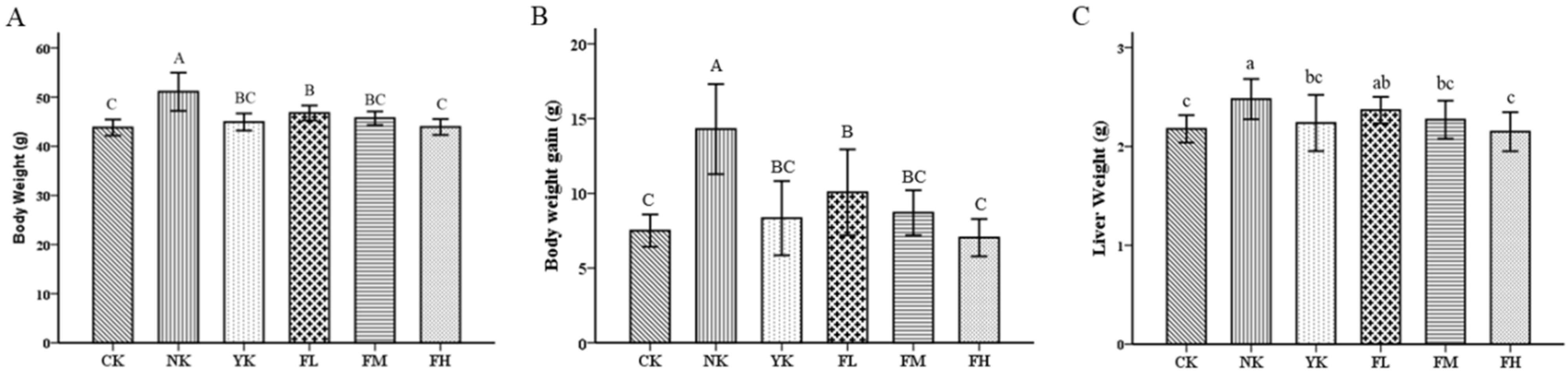
3.1. The Effects of CFBT on Body Weight and Liver Weight
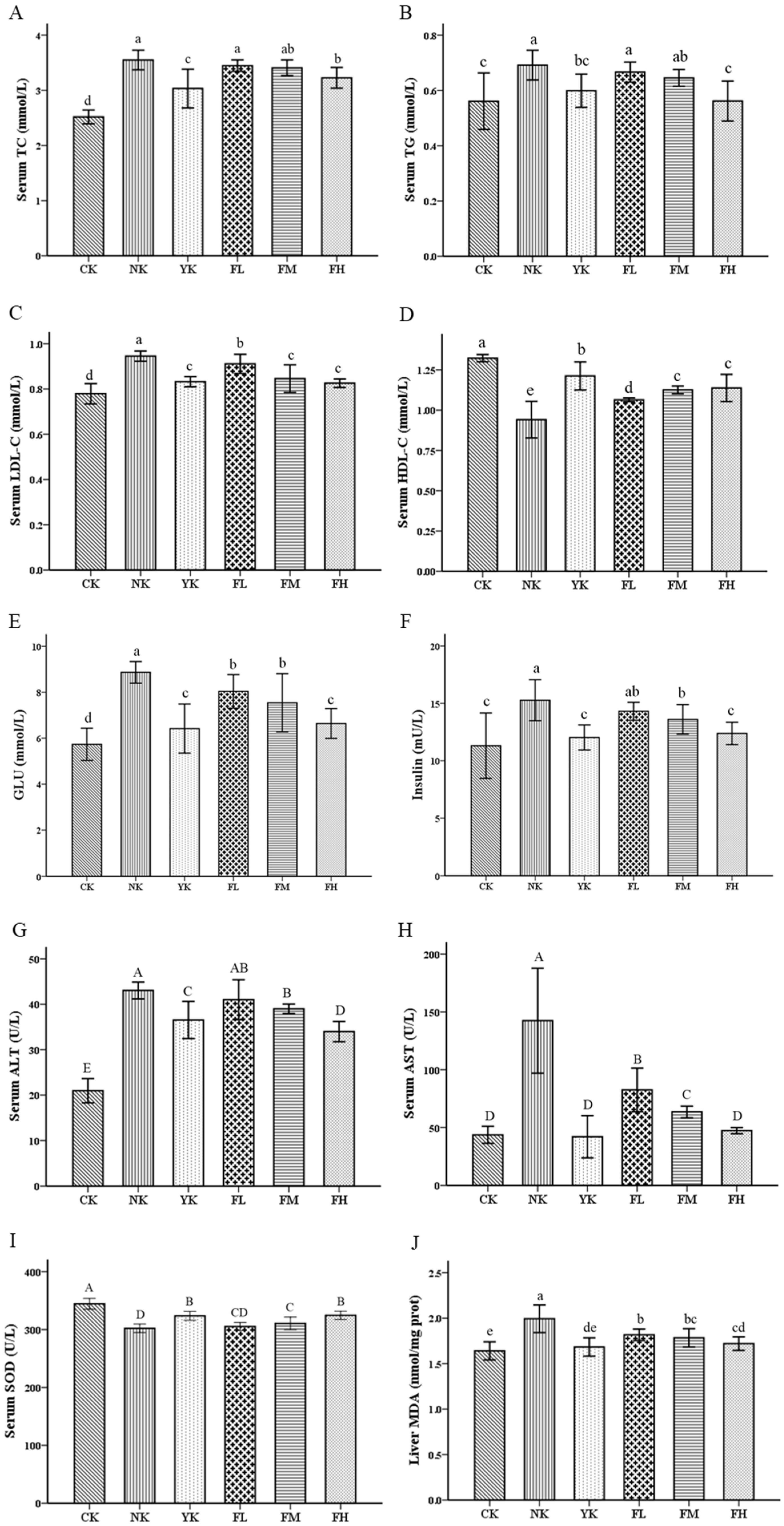
3.2. CFBT Improves Serum Biomarkers and Oxidative Stress
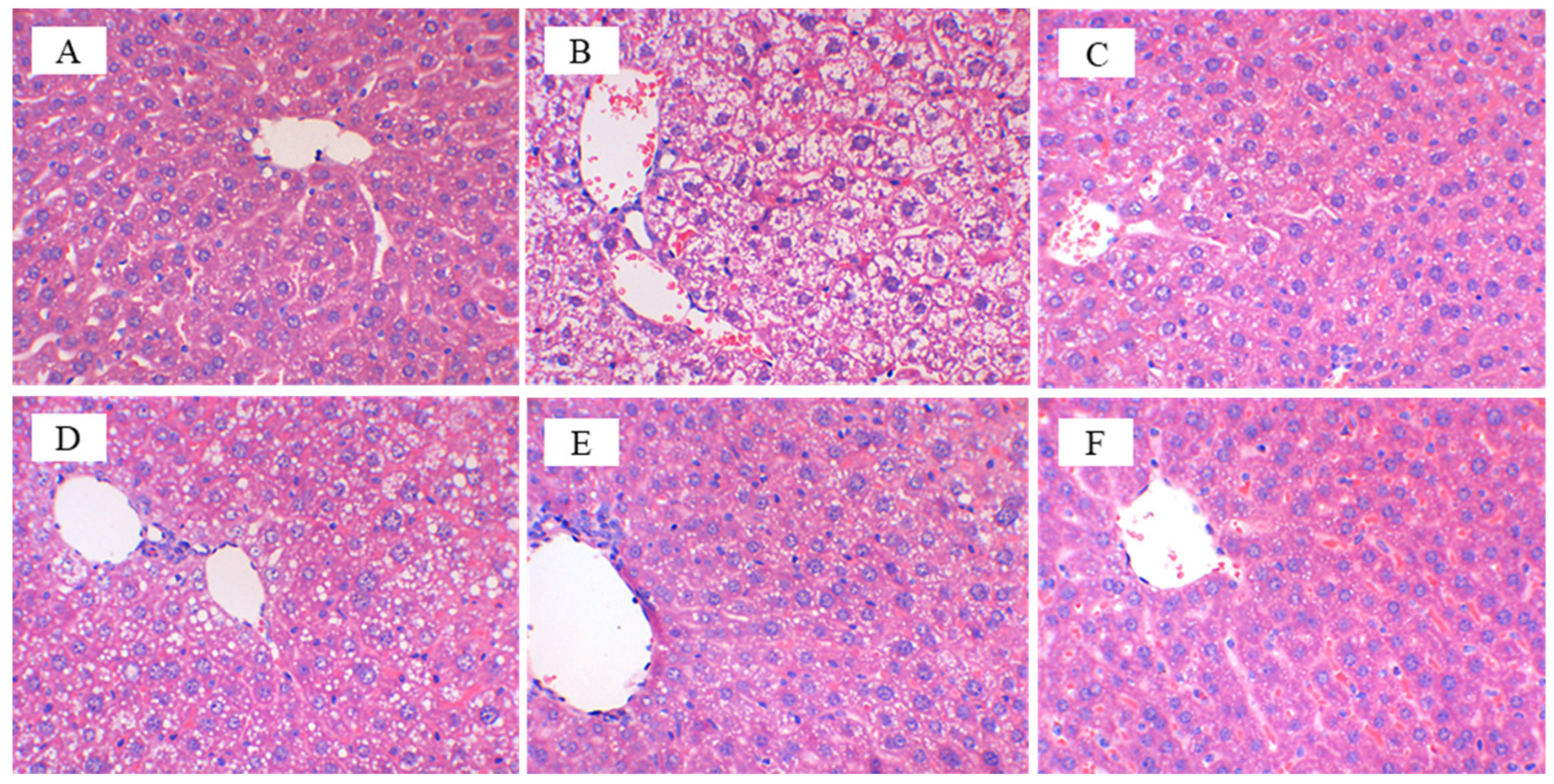
3.3. CFBT Prevents Liver Injury on HFD Mice
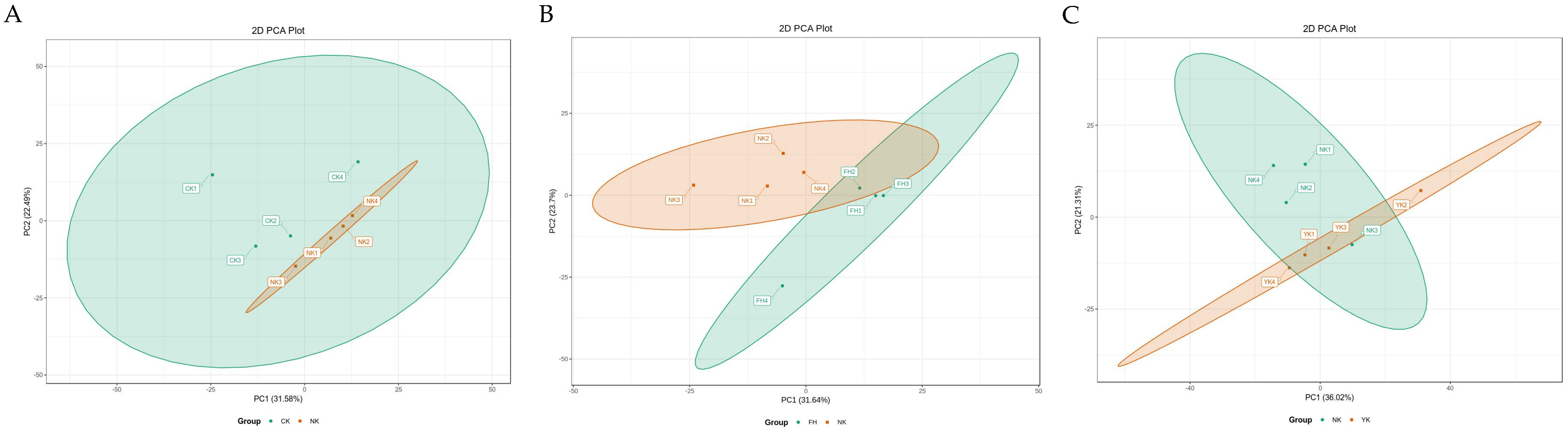
3.4. Metabolic Effects of CFBT on HFD Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Marie, N.; Tom, F.; Margaret, R.; Blake, T.; Nicholas, G.; Christopher, M.; Erin, C.M.; Stan, B.; Cristiana, A.; Semaw, F.A.; et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014, 384, 766–781. [Google Scholar]
- Lee, S.J.; Joo, Y.J.; Kim, R.Y.; Hwang, J.; Lim, S.M.; Yoon, S.J.; Kim, J.Y. Obesity May Connect Insulin Resistance to Decreased Neuronal Viability in Human Diabetic Brain. Obesity 2020, 28, 1626–1630. [Google Scholar] [CrossRef]
- Afshin, A.; Forouzanfar, M.H.; Reitsma, M.B.; Sur, P.; Estep, K.; Lee, A.; Marczak, L.; Mokdad, A.H.; Moradi-Lakeh, M.; Naghavi, M.; et al. Health Effects of Overweight and Obesity in 195 Countries over 25 Years. N. Engl. J. Med. 2017, 377, 13–27. [Google Scholar] [PubMed]
- Swinburn, B.A.; Gary, S.; Kevin, D.H.; Klim, M.; Diane, T.F.; Marjory, L.M.; Steven, L.G. The global obesity pandemic: Shaped by global drivers and local environments. Lancet 2011, 378, 804–814. [Google Scholar] [CrossRef]
- Popkin, B.M.; Adair, L.S.; Ng, S.W. Global nutrition transition and the pandemic of obesity in developing countries. Nutr. Rev. 2012, 70, 3–21. [Google Scholar] [CrossRef] [Green Version]
- Ivo, P.; Andreea, D.; Michaela, S.; Barak, Z.; Beata, W.K.; Lubomira, F.; Hrvoje, P.; Ian, B.; Reneta, P. Clinical Management of High and Very High Risk Patients with Hyperlipidaemia in Central and Eastern Europe: An Observational Study. Adv. Ther. 2019, 36, 608–620. [Google Scholar]
- Vengerovskii, A.I.; Yakimova, T.V.; Nasanova, O.N. Influence of medicinal plant extracts on the functions and antioxidant protection of erythrocytes in rats with experimental diabetes mellitus. Eksp. Klin. Farmakol. 2016, 79, 29–33. [Google Scholar]
- Danielle, T.; Sunelle, R.; Namrita, L. A review on traditionally used South African medicinal plants, their secondary metabolites and their potential development into anticancer agents. J. Ethnopharmacol. 2020, 261, 113101. [Google Scholar]
- Zheng, J.P.; Zhang, J.; Guo, Y.L.; Cui, H.R.; Lin, A.Z.; Hu, B.F.; Gao, Q.H.; Chen, Y.Z.; Liu, H.T. Improvement on metabolic syndrome in high fat diet-induced obese mice through modulation of gut microbiota by sangguayin decoction. J. Ethnopharmacol. 2020, 246, 112225. [Google Scholar] [CrossRef]
- Meng, X.H.; Li, N.; Zhu, H.T.; Wang, D.; Yang, C.R.; Zhang, Y.J. Plant Resources, Chemical Constituents, and Bioactivities of Tea Plants from the Genus Camellia Section Thea. J. Agric. Food Chem. 2019, 67, 5318–5349. [Google Scholar] [CrossRef]
- Wang, Y.J.; Kan, Z.P.; Thompson, H.J.; Ling, T.J.; Ho, C.T.; Li, D.X.; Wan, X.C. Impact of Six Typical Processing Methods on the Chemical Composition of Tea Leaves Using a Single Camellia sinensis Cultivar, Longjing 43. J. Agric. Food Chem. 2019, 67, 5423–5436. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.S.; Nagabhushanam, K.; Ho, C.T.; Pan, M.H. Green tea changes serum and liver metabolomic profiles in mice with high-fat diet-induced obesity. Mol. Nutr. Food Res. 2015, 59, 784–794. [Google Scholar] [CrossRef]
- Kuo, K.L.; Weng, M.S.; Chiang, C.T.; Tsai, Y.J.; Lin-Shiau, S.Y.; Lin, J.K. Comparative studies on the hypolipidemic and growth suppressive effects of oolong, black, pu-erh, and green tea leaves in rats. J. Agric. Food Chem. 2005, 53, 480–489. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Kim, C.; Kim, Y. Green Tea (–)-Epigallocatechin-3-Gallate Reduces Body Weight with Regulation of Multiple Genes Expression in Adipose Tissue of Diet-Induced Obese Mice. Ann. Nutr. Metab. 2009, 54, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Chantre, P.; Lairon, D. Recent findings of green tea extract AR25 (Exolise) and its activity for the treatment of obesity. Phytomedicine 2002, 9, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Eva, M.R.K.; Manuela, P.G.M.L.; Ilse, N.; Margriet, S.W.P. Effects of green tea on weight maintenance after body-weight loss. Br. J. Nutr. 2004, 91, 431–437. [Google Scholar]
- Margriet, S.W.P.; Manuela, P.G.M.L.; Eva, M.R.K. Body weight loss and weight maintenance in relation to habitual caffeine intake and green tea supplementation. Obes. Res. 2005, 13, 1195–1204. [Google Scholar]
- Mohamed, H.H.; Chahira, S.; Karima, D.; Sami, F.; Robert, D.; Maude, L.G.; André, B. Tea decoctions prevent body weight gain in rats fed high-fat diet; black tea being more efficient than green tea. J. Nutr. Int. Metab. 2016, 6, 33–40. [Google Scholar]
- Zhu, J.; Cai, R.T.; Tan, Y.X.; Wu, X.Q.; Wen, Q.; Liu, Z.H.; Ouyang, S.H.; Yin, Z.N.; Yang, H.W. Preventive consumption of green tea modifies the gut microbiota and provides persistent protection from high-fat diet-induced obesity. J. Funct. Foods 2020, 64, 103621. [Google Scholar] [CrossRef]
- Li, Q.; Lai, X.; Sun, L.; Cao, J.; Ling, C.; Zhang, W. Antiobesity and anti-inflammation effects of Hakka stir-fried tea of different storage years on high-fat diet-induced obese mice model via activating the AMPK/ACC/CPT1 pathway. Food Nutr. Res. 2020, 64, 1–13. [Google Scholar] [CrossRef]
- Bae, U.J.; John, P.; Woon, P.I.; Chae, B.M.; Oh, M.R.; Jung, S.J.; Ryu, G.S.; Chae, S.W.; Park, B.H. Epigallocatechin-3-Gallate-Rich Green Tea Extract Ameliorates Fatty Liver and Weight Gain in Mice Fed a High Fat Diet by Activating the Sirtuin 1 and AMP Activating Protein Kinase Pathway. Am. J. Chin. Med. 2018, 46, 617–632. [Google Scholar] [CrossRef] [PubMed]
- Satoshi, U.; Yoshimasa, T.; Akiko, S.; Aruto, Y.; Hiroaki, Y. Prevention of diet-induced obesity by dietary black tea polyphenols extract in vitro and in vivo. Nutrition 2010, 27, 287–292. [Google Scholar]
- Tong, T.T.; Ren, N.; Soomi, P.; Wu, J.F.; Guo, N.; Kang, H.; Kim, E.; Wu, Y.Y.; He, P.M.; Tu, Y.Y.; et al. Theaflavins Improve Insulin Sensitivity through Regulating Mitochondrial Biosynthesis in Palmitic Acid-Induced HepG2 Cells. Molecules 2018, 23, 3382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yung, L.M.; Leung, F.P.; Wong, W.T.; Tian, X.Y.; Yung, L.H.; Chen, Z.Y.; Yao, X.Q.; Huang, Y. Tea polyphenols benefit vascular function. Inflammopharmacology 2008, 16, 230–234. [Google Scholar] [CrossRef] [PubMed]
- Tung, Y.C.; Hsieh, T.H.; Yang, M.J.; Hung, W.L.; Ho, C.T.; Pan, M.H. The effects of the extract of oolong tea and its metabolites from Andraca theae in high fat diet induced obese Wistar rat. Food Sci. Hum. Welln. 2018, 7, 120–124. [Google Scholar]
- Lin, J.K.; Lin-Shiau, S.Y. Mechanisms of hypolipidemic and anti-obesity effects of tea and tea polyphenols. Mol. Nutr. Food Res. 2006, 50, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Rebecca, B.; Edward, H.; Paul, A.L.; David, C.S.; Michael, L.M.; Markandeya, J.; Andrew, J.S.; Denovan, P.B.; John, D.W.; et al. Green tea, black tea, and epigallocatechin modify body composition, improve glucose tolerance, and differentially alter metabolic gene expression in rats fed a high-fat diet. Nutr. Res. 2009, 29, 784–793. [Google Scholar] [CrossRef]
- Chung, M.Y.; Hea, J.P.; Jose, E.M.; Sung, I.K.; Richard, S.B. Green tea extract protects against nonalcoholic steatohepatitis in ob/ob mice by decreasing oxidative and nitrative stress responses induced by proinflammatory enzymes. J. Nutr. Biochem. 2012, 23, 361–367. [Google Scholar] [CrossRef] [Green Version]
- Marina, V.B.; Adriane, G.R.; Isabella, C.C.; Lycia, B.G.; Samuel, S.V.; Manuella, L. Mate tea reduces high fat diet-induced liver and metabolic disorders in mice. Biomed. Pharmacother 2019, 109, 1547–1555. [Google Scholar]
- Monique, C.; Du, T.L.; Ilse, D.P. Metabolomics describes previously unknown toxicity mechanisms of isoniazid and rifampicin. Toxicol. Lett. 2020, 322, 104–110. [Google Scholar]
- Htun, K.T.; Pan, J.; Pasanta, D.; Tungjai, M.; Udomtanakunchai, C.; Chancharunee, S.; Kaewjaeng, S.; Kim, H.J.; Kaewkhao, J.; Kothan, S.; et al. Identification of Metabolic Phenotypes in Young Adults with Obesity by 1H NMR Metabolomics of Blood Serum. Life 2021, 11, 574. [Google Scholar] [CrossRef] [PubMed]
- Preter, V.D. Metabonomics and systems biology. Methods Mol. Biol. 2015, 1277, 245–255. [Google Scholar] [PubMed]
- Li, Q.; Li, Y.D.; Luo, Y.; Xiao, L.Z.; Wang, K.B.; Huang, J.A.; Liu, Z.H. Characterization of the key aroma compounds and microorganisms during the manufacturing process of Fu brick tea. Food Sci. Technol. 2020, 127, 109355. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhong, K.; Bai, J.R.; Wu, Y.P.; Gao, H. Insight into effects of isolated Eurotium cristatum from Pingwu Fuzhuan brick tea on the fermentation process and quality characteristics of Fuzhuan brick tea. J. Sci. Food Agric. 2020, 100, 3598–3607. [Google Scholar] [CrossRef]
- Zhu, M.Z.; Li, N.; Zhou, F.; Ouyang, J.; Lu, D.M.; Xu, W.; Li, J.; Lin, H.Y.; Zhang, Z.; Xiao, J.B.; et al. Microbial bioconversion of the chemical components in dark tea. Food Chem. 2020, 312, 126043. [Google Scholar] [CrossRef]
- Xu, J.; Hu, F.; Wang, W.; Wan, X.; Bao, G. Investigation on biochemical compositional changes during the microbial fermentation process of Fu brick tea by LC–MS based metabolomics. Food Chem. 2015, 186, 176–184. [Google Scholar] [CrossRef]
- Zhou, X.L.; Chen, C.J.; Ran, L.S.; Yang, Y.; Song, J.Y.; Zhou, C.B.; Fu, D.H. Formulation optimization and safety evaluation of the compound fuzhuan tea bag. Mod. Food Sci. Technol. 2020, 36, 210–219. [Google Scholar]
- Jing, N.N.; Liu, X.X.; Jin, M.L.; Yang, X.B.; Hu, X.; Li, C.Y.; Zhao, K. Fubrick tea attenuates high-fat diet induced fat deposition and metabolic disorder by regulating gut microbiota and caffeine metabolism. Food Funct. 2020, 11, 6971–6986. [Google Scholar] [CrossRef]
- Chen, G.J.; Xie, M.H.; Wan, P.; Chen, D.; Dai, Z.Q.; Ye, H.; Hu, B.; Zeng, X.X.; Liu, Z.H. Fuzhuan Brick Tea Polysaccharides Attenuate Metabolic Syndrome in High-Fat Diet Induced Mice in Association with Modulation in the Gut Microbiota. J. Agric. Food Chem. 2018, 66, 2783–2795. [Google Scholar] [CrossRef]
- Zobel, E.H.; Hansen, T.W.; Rossing, P.; Von, S.B.J. Global Changes in Food Supply and the Obesity Epidemic. Curr. Obes. Rep. 2016, 5, 449–455. [Google Scholar] [CrossRef]
- Naqvi, S.; Imani, S.; Hosseinifard, H.; Wen, Q.L.; Shahzad, M.N.; Ijaz, I.; Deng, Y.C.; Guo, M.a.n.; Xu, Y. Associations of serum low-density lipoprotein and systolic blood pressure levels with type 2 diabetic patients with and without peripheral neuropathy: Systemic review, meta-analysis and meta-regression analysis of observational studies. BMC Endocr. Disord. 2019, 19, 125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Lahou, B.; Ausman, L.M.; Penalvo, J.L.; Huggins, G.S.; Al-Hooti, S.; Al-Zenki, S.; Zhang, F.F. Dietary Patterns Associated with the Prevalence of Cardiovascular Disease Risk Factors in Kuwaiti Adults. J. Acad. Nutr. Diet. 2020, 120, 424–436. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.J.; Xie, M.H.; Dai, Z.Q.; Wan, P.; Ye, H.; Zeng, X.X.; Sun, Y. Kudingcha and Fuzhuan Brick Tea Prevent Obesity and Modulate Gut Microbiota in High-Fat Diet Fed Mice. Mol. Nutr. Food Res. 2018, 62, 1700485. [Google Scholar] [CrossRef] [PubMed]
- Yoshihiro, K.; Makoto, W.; Aya, H.; Kyoko, H. Small-Dense Low-Density Lipoprotein Cholesterol: A Subclinical Marker for the Primary Prevention of Coronary Heart Disease. J. Atheroscler. Thromb. 2020, 27, 641–643. [Google Scholar]
- Takaeko, Y.J.; Matsui, S.; Kajikawa, M.; Maruhashi, T.; Yamaji, T.; Harada, T.; Han, Y.M.; Hashimoto, H.; Kihara, Y.; Hida, E. Relationship between high-density lipoprotein cholesterol levels and endothelial function in women: A cross-sectional study. BMJ Open 2020, 10, e038121. [Google Scholar] [CrossRef]
- Lu, C.; Hwang, L.S. Polyphenol contents of Pu-Erh teas and their abilities to inhibit cholesterol biosynthesis in Hep G2 cell line. Food Chem. 2008, 111, 67–71. [Google Scholar] [CrossRef]
- Li, Y.; Rahman, S.U.; Huang, Y.Y.; Zhang, Y.F.; Ming, P.F.; Zhu, L.; Chu, X.Y.; Li, J.C.; Feng, S.B.; Wang, X.C.; et al. Green tea polyphenols decrease weight gain, ameliorate alteration of gut microbiota, and mitigate intestinal inflammation in canines with high-fat-diet-induced obesity. J. Nutr. Biochem. 2020, 78, 108324. [Google Scholar] [CrossRef]
- Foster, M.T.; Gentile, C.L.; Cox-York, K.; Wei, Y.; Wang, D.; Estrada, A.L.; Reese, L.; Miller, T.; Pagliassotti, M.J.; Weir, T.L. Fuzhuan tea consumption imparts hepatoprotective effects and alters intestinal microbiota in high saturated fat diet-fed rats. Mol. Nutr. Food Res. 2016, 60, 1213–1220. [Google Scholar] [CrossRef]
- Zarzour, R.H.A.; Ahmad, M.; Asmawi, M.Z.; Kaur, G.; Saeed, M.A.A.; Al-Mansoub, M.A.; Saghir, S.A.M.; Usman, N.S.; Al-Dulaimi, D.W.; Yam, M.F. Phyllanthus Niruri Standardized Extract Alleviates the Progression of Non-Alcoholic Fatty Liver Disease and Decreases Atherosclerotic Risk in Sprague-Dawley Rats. Nutrients 2017, 9, 766. [Google Scholar] [CrossRef] [Green Version]
- Panelli, M.F.; Pierine, D.T.; de Souza, S.L.B.; Ferron, A.J.T.; Garcia, J.L.; Santos, K.C.D.; Corrêa, C.R. Bark of Passiflora edulis Treatment Stimulates Antioxidant Capacity, and Reduces Dyslipidemia and Body Fat in db/db Mice. Antioxidants 2018, 7, 120. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.A.; Zhang, X.L.; Yan, Y.Y.; Fan, X.H. Antioxidant Evaluation and Composition Analysis of Extracts from Fuzhuan Brick Tea and Its Comparison with Two Instant Tea Products. J. AOAC Int. 2017, 100, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.X.; Jia, Z.H.; Gao, P.; Kong, H.W.; Li, X.; Chen, J.; Yang, Q.; Yin, P.Y.; Wang, J.S.; Lu, X.; et al. Metabonomics study of atherosclerosis rats by ultra fast liquid chromatography coupled with ion trap-time of flight mass spectrometry. Talanta 2009, 79, 836–844. [Google Scholar] [CrossRef] [PubMed]
- Ma, N.; Yang, Y.J.; Liu, X.W.; Kong, X.J.; Li, X.H.; Qin, Z.; Jiao, Z.H.; Li, J.Y. UPLC-Q-TOF/MS-based metabonomic studies on the intervention effects of aspirin eugenol ester in atherosclerosis hamsters. Sci. Rep. 2017, 7, 10544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y.Y.; Miao, H.; Cheng, X.L.; Wei, F. Lipidomics: Novel insight into the biochemical mechanism of lipid metabolism and dysregulation-associated disease. Chem. Biol. Int. 2015, 240, 220–238. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, K.; Xu, Y.; Xu, Y.Y.; Li, Y.Y.; Xu, J.S.; Zhu, Y.D.; Adorini, L.; Lee, Y.K.; Kasumov, T.; Yin, L.Y.; et al. Reversal of metabolic disorders by pharmacological activation of bile acid receptors TGR5 and FXR. Mol. Metab. 2018, 9, 134–140. [Google Scholar] [CrossRef]
- Hylemon, P.B.; Takabe, K.; Dozmorov, M.; Nagahashi, M.; Zhou, H.P. Bile acids as global regulators of hepatic nutrient metabolism. Liver Res. 2017, 1, 10–16. [Google Scholar] [CrossRef]
- Rebecca, M.H.; Gianella, C.C.; Marie-Louise, R. Dietary procyanidins selectively modulate intestinal farnesoid X receptor-regulated gene expression to alter enterohepatic bile acid recirculation: Elucidation of a novel mechanism to reduce triglyceridemia. Mol. Nutr. Food Res. 2016, 60, 727–736. [Google Scholar]
- Jeckelmann, J.; Erni, B. The mannose phosphotransferase system (Man-PTS)—Mannose transporter and receptor for bacteriocins and bacteriophages. BBA Biomembr. 2020, 1862, 183412. [Google Scholar] [CrossRef]
- Sherratt, S.C.R.; Mason, R.P. Eicosapentaenoic acid inhibits oxidation of high density lipoprotein particles in a manner distinct from docosahexaenoic acid. Biochem. Biophys. Res. Commun. 2018, 496, 335–338. [Google Scholar] [CrossRef]
- Das, U.N. Essential fatty acids: Biochemistry, physiology and pathology. Biotechnol. J. 2006, 1, 420–439. [Google Scholar] [CrossRef]

| Time (min) | Flow Rate (mL/min) | Temperature (°C) | Mobile Phase A (V) | Mobile Phase B (V) |
|---|---|---|---|---|
| 0.0 | 0.4 | 40 | 95 | 5 |
| 11.0 | 0.4 | 40 | 5 | 95 |
| 12.0 | 0.4 | 40 | 5 | 95 |
| 12.1 | 0.4 | 40 | 95 | 5 |
| 14.0 | 0.4 | 40 | 95 | 5 |
| Metabolites | CK vs. NK | NK vs. FH | CK vs. FH | CK vs. YK | NK vs. YK | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Log2FC | FC | Log2FC | FC | Log2FC | FC | Log2FC | FC | Log2FC | FC | |
| Taurodeoxycholic acid sodium salt | 1.43↑ | 2.69 | −1.90↓ | 0.27 | ||||||
| Tauroursodeoxycholic Acid | −1.88↓ | 0.27 | ||||||||
| Taurochenodesoxycholic Acid | 1.00↑ | 2.01 | −1.90↓ | 0.27 | ||||||
| Hyodeoxycholic Acid | −1.07↓ | 0.48 | −1.88↓ | 0.27 | ||||||
| Glycoursodeoxycholic Acid | −1.39↓ | 0.38 | ||||||||
| Glycocholic Acid | −1.22↓ | 0.43 | −2.24↓ | 0.21 | −1.02↓ | 0.49 | ||||
| Deoxycholic Acid | 1.04↑ | 2.06 | −1.60↓ | 0.33 | −1.46↓ | 0.36 | ||||
| Chenodeoxycholic Acid | 1.06↑ | 2.09 | −1.54↓ | 0.34 | −1.43↓ | 0.37 | ||||
| Taurodeoxycholic Acid sodium salt | −1.53↓ | 0.35 | ||||||||
| D-Sorbitol | 1.04↑ | 2.06 | −1.42↓ | 0.37 | 1.77↑ | 3.40 | ||||
| D-Fructose | 1.03↑ | 2.04 | −1.27↓ | 0.41 | 1.37↑ | 2.59 | ||||
| D-Galactose | 1.18↑ | 2.26 | −1.43↓ | 0.37 | 1.71↑ | 3.28 | ||||
| D-Glucose | 1.24↑ | 2.36 | −1.53↓ | 0.35 | 1.79↑ | 3.46 | ||||
| D-Mannose | 1.31↑ | 2.48 | −1.56↓ | 0.34 | 1.67↑ | 3.17 | ||||
| D-Sucrose | −3.89↓ | 0.07 | −1.30↓ | 0.41 | 4.67↑ | 25.43 | ||||
| D-Trehalose | 4.12↑ | 17.37 | −3.78↓ | 0.07 | 5.08↑ | 33.73 | ||||
| D-Glucose 6-Phosphate | 2.73↑ | 6.65 | −1.75↓ | 0.30 | 3.64↑ | 12.49 | ||||
| L-Fucose | −1.35↓ | 0.39 | ||||||||
| L-Rhamnose | −1.08↓ | 0.47 | ||||||||
| Maltose | 5.48↑ | 44.70 | ||||||||
| D-Sedoheptuiose 7-Phosphate | 1.00↑ | 2.00 | 1.30↑ | 2.47 | ||||||
| D-Glucopyranose | −1.59↓ | 0.33 | ||||||||
| D-Fructose 6-Phosphate -Disodium Salt | 2.76↑ | 6.77 | −1.62↓ | 0.32 | 1.14↑ | 2.20 | 3.71↑ | 13.13 | ||
| D-Fructose−1,6-Biphos- phate-Trisodium Salt | 2.15↑ | 4.45 | −2.01↓ | 0.25 | 2.75↑ | 6.70 | ||||
| N-Acetylglucosamine 1-Phosphate | 1.06↑ | 2.08 | ||||||||
| 3′-Sialyllactose | 3.14↑ | 8.84 | −2.11↓ | 0.23 | 1.03↑ | 2.04 | 3.08↑ | 8.45 | ||
| Galactaric Acid | 1.17↑ | 2.25 | ||||||||
| Hydroxyphenyllactic Acid | 1.47↑ | 2.78 | 1.13↑ | 2.19 | ||||||
| MARMESIN | −1.57↓ | 0.34 | 1.16↑ | 2.23 | ||||||
| L-Erythrulose | 1.58↑ | 2.99 | −1.68↓ | 0.31 | 2.19↑ | 4.55 | ||||
| D-Mannose 6-phosphate | 2.75↑ | 6.72 | −1.67↓ | 0.31 | 1.07↑ | 2.11 | 3.73↑ | 13.31 | ||
| D-Fructose 6-phosphate | 2.71↑ | 6.56 | −1.72↓ | 0.30 | 3.59↑ | 12.06 | ||||
| Formononetin | −12.99↓ | 0.00 | 11.32↑ | 2561.81 | −1.67↓ | 0.31 | −12.99↓ | 0.00 | ||
| D-Sucrose | 3.56↑ | 11.80 | 1.11↑ | 2.16 | ||||||
| Lactobionic Acid | 2.24↑ | 4.74 | 2.32↑ | 4.99 | ||||||
| Dulcitol | 2.64↑ | 6.24 | 1.01↑ | 2.02 | ||||||
| Lactose | −1.37↓ | 0.39 | 3.35↑ | 10.20 | ||||||
| Maltotriose | 12.40↑ | 5418.78 | −5.19↓ | 0.03 | 7.21↑ | 147.99 | 14.06↑ | 17110.83 | 1.66↑ | 3.16 |
| N-Acetyl-D-Glucosamine | 1.12↑ | 2.18 | ||||||||
| Raffinose | 12.57↑ | 6062.72 | −5.88↓ | 0.02 | 6.69↑ | 103.22 | 14.18↑ | 18620.56 | 1.62↑ | 3.07 |
| D-Mannitol | −1.83↓ | 0.28 | 2.41↑ | 5.33 | ||||||
| D(-)-Threose | 1.31↑ | 2.48 | ||||||||
| Octadecatrienoic Acid | −1.41↓ | 0.38 | ||||||||
| Thromboxane B2 | −2.36↓ | 0.19 | −2.58↓ | 0.17 | ||||||
| Prostaglandin D2 | −3.44↓ | 0.09 | −3.20↓ | 0.11 | ||||||
| Eicosapentaenoic Acid | −3.27↓ | 0.10 | 1.30↑ | 2.46 | −1.98↓ | 0.25 | −4.98↓ | 0.03 | −1.71↓ | 0.31 |
| Eicosatetraenoic Acid | −4.52↓ | 0.04 | 2.83↑ | 7.09 | −1.69↓ | 0.31 | −6.83↓ | 0.01 | −2.32↓ | 0.20 |
| Eicosatrienoic Acid | −1.92↓ | 0.26 | −1.71↓ | 0.31 | ||||||
| Docosahexaenoic Acid | −4.79↓ | 0.04 | 2.71↑ | 6.54 | −2.08↑ | 0.24 | −6.82↓ | 0.01 | −2.04↓ | 0.24 |
| 12-Hydroxyeicosane tetraenoic acid | −2.29↓ | 0.20 | 1.48↑ | 2.79 | −3.09↓ | 0.12 | ||||
| Lysopc 16:1 | −1.09↓ | 0.47 | ||||||||
| Lysopg 18:1 | 1.34↑ | 2.53 | ||||||||
| Lysope 18:1 | 1.29↑ | 2.44 | ||||||||
| Lysopc 20:2 | 1.15↑ | 2.21 | ||||||||
| Lysopa 18:0 | 1.79↑ | 3.47 | 2.15↑ | 4.42 | 2.03↑ | 4.08 | ||||
| Cis-11,14-Eicosadienoic Acid (C20:2) | 1.20↑ | 2.30 | 1.28↑ | 2.44 | 1.15 | 2.21 | ||||
| Arachidic Acid(C20:0) | −1.37↓ | 0.39 | −1.76↓ | 0.30 | ||||||
| 3-Methylglutaric Acid | −1.38↓ | 0.39 | ||||||||
| Tetradecanedioic Acid | −1.30↓ | 0.41 | −1.96↓ | 0.26 | ||||||
| Hexadecanedioic Acid | −1.78↓ | 0.29 | ||||||||
| PE(18:1(9Z)/0:0) | 1.37↑ | 2.58 | 1.40↑ | 2.65 | ||||||
| Punicic Acid | −2.21↓ | 0.22 | 1.26↑ | 2.39 | −2.61↓ | 0.16 | ||||
| Stearidonic Acid | −1.56↓ | 0.34 | −1.67↓ | 0.31 | 1.71↑ | 1.71 | ||||
| p-Mentha-1,3,8-triene | −1.05↓ | 0.48 | 1.63↑ | 1.63 | ||||||
| 2,2-Dimethyl Succinic Acid | −1.31↓ | 0.40 | ||||||||
| 2-Methylbutyroylcarnitine | −1.56↓ | 0.34 | −1.63↓ | 0.32 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, X.; Ge, B.; Zhang, X.; Wang, K.; Zhou, C.; Fu, D. Metabolomics Analysis Reveals the Effects of Compound Fuzhuan Brick Tea (CFBT) on Regulating Dyslipidemia and Metabolic Disorders in Mice Induced by High-Fat Diet. Nutrients 2022, 14, 1128. https://doi.org/10.3390/nu14061128
Zhou X, Ge B, Zhang X, Wang K, Zhou C, Fu D. Metabolomics Analysis Reveals the Effects of Compound Fuzhuan Brick Tea (CFBT) on Regulating Dyslipidemia and Metabolic Disorders in Mice Induced by High-Fat Diet. Nutrients. 2022; 14(6):1128. https://doi.org/10.3390/nu14061128
Chicago/Turabian StyleZhou, Xiaolu, Binggang Ge, Xuwen Zhang, Kunbo Wang, Caibi Zhou, and Donghe Fu. 2022. "Metabolomics Analysis Reveals the Effects of Compound Fuzhuan Brick Tea (CFBT) on Regulating Dyslipidemia and Metabolic Disorders in Mice Induced by High-Fat Diet" Nutrients 14, no. 6: 1128. https://doi.org/10.3390/nu14061128
APA StyleZhou, X., Ge, B., Zhang, X., Wang, K., Zhou, C., & Fu, D. (2022). Metabolomics Analysis Reveals the Effects of Compound Fuzhuan Brick Tea (CFBT) on Regulating Dyslipidemia and Metabolic Disorders in Mice Induced by High-Fat Diet. Nutrients, 14(6), 1128. https://doi.org/10.3390/nu14061128






