Alpha-Ketoglutarate Promotes Goblet Cell Differentiation and Alters Urea Cycle Metabolites in DSS-Induced Colitis Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Design
2.2. Tissue Collecting and Processing
2.3. Histological Examination of Goblet Cells
2.4. RNA Extraction and qPCR
2.5. Immunoblotting Analysis
2.6. Metabolite Extraction and Derivatization
2.7. Metabolomics by GC-MS
2.8. Statistical Analysis
3. Results
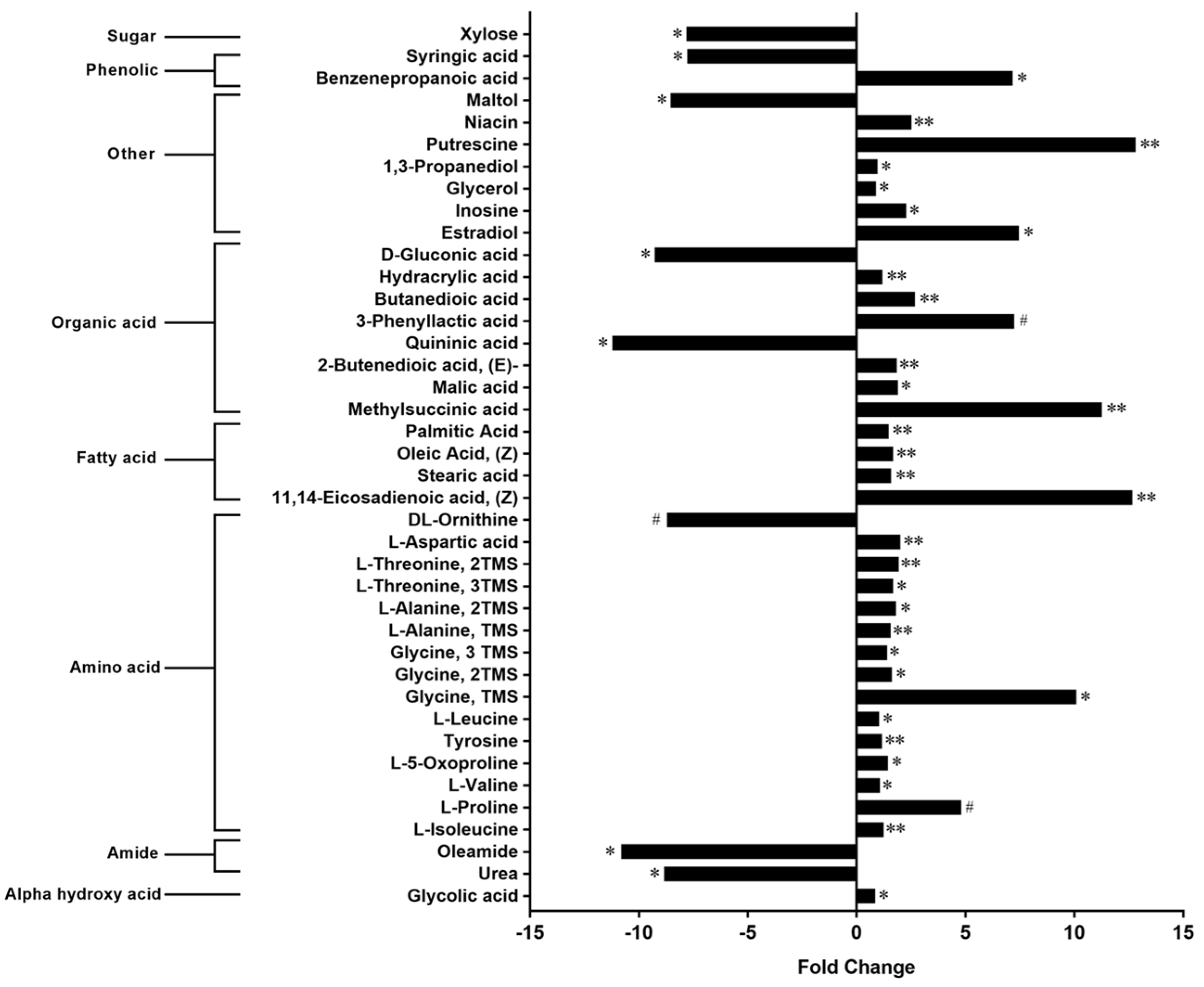
3.1. Metabolome
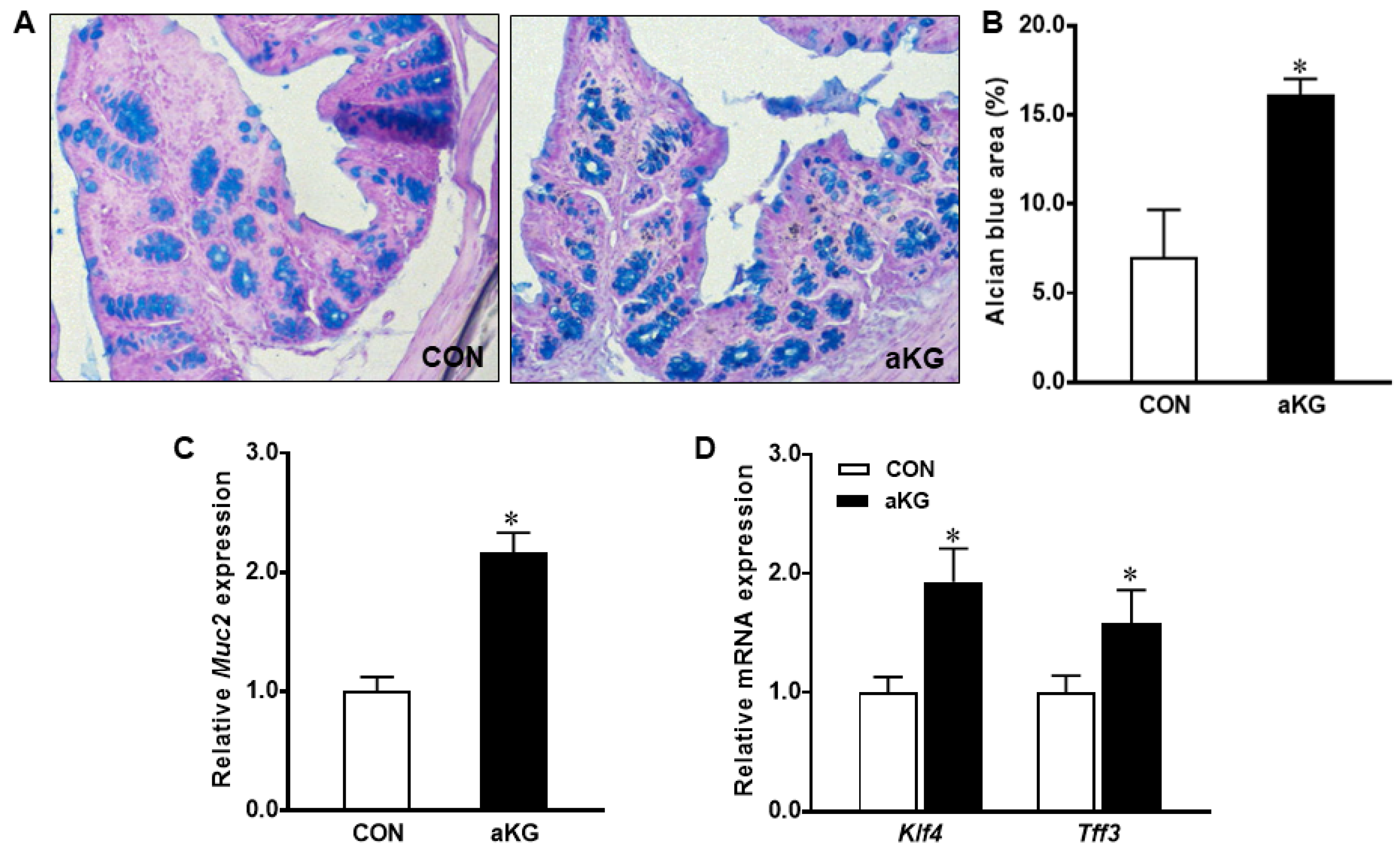
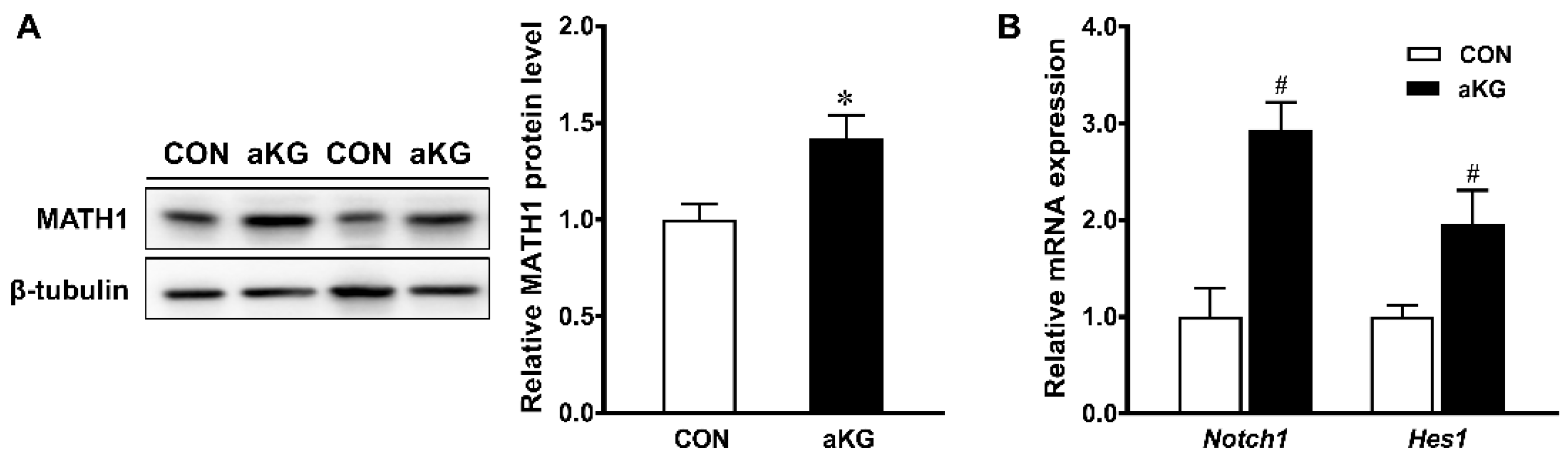
3.2. Goblet Cell Differentiation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jess, T.; Rungoe, C.; Peyrin–Biroulet, L. Risk of colorectal cancer in patients with ulcerative colitis: A meta-analysis of population-based cohort studies. Clin. Gastroenterol. Hepatol. 2012, 10, 639–645. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Goding Sauer, A.; Fedewa, S.A.; Butterly, L.F.; Anderson, J.C.; Cercek, A.; Smith, R.A.; Jemal, A. Colorectal cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 145–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khaloian, S.; Rath, E.; Hammoudi, N.; Gleisinger, E.; Blutke, A.; Giesbertz, P.; Berger, E.; Metwaly, A.; Waldschmitt, N.; Allez, M.; et al. Mitochondrial impairment drives intestinal stem cell transition into dysfunctional Paneth cells predicting Crohn’s disease recurrence. Gut 2020, 69, 1939–1951. [Google Scholar] [CrossRef] [PubMed]
- Coskun, M. Intestinal epithelium in inflammatory bowel disease. Front. Med. 2014, 1, 24. [Google Scholar] [CrossRef] [Green Version]
- Gulhane, M.; Murray, L.; Lourie, R.; Tong, H.; Sheng, Y.H.; Wang, R.; Kang, A.; Schreiber, V.; Wong, K.Y.; Magor, G.; et al. High Fat Diets Induce Colonic Epithelial Cell Stress and Inflammation that is Reversed by IL-22. Sci. Rep. 2016, 6, 28990. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Wei, X.; Sun, Y.; Du, J.; Li, X.; Xun, Z.; Li, Y.C. High-fat diet promotes experimental colitis by inducing oxidative stress in the colon. Am. J. Physiol. Gastrointest. Liver Physiol. 2019, 317, G453–G462. [Google Scholar] [CrossRef]
- Ye, Y.; Manne, S.; Treem, W.R.; Bennett, D. Prevalence of inflammatory bowel disease in pediatric and adult populations: Recent estimates from large national databases in the United States, 2007–2016. Inflamm. Bowel Dis. 2020, 26, 619–625. [Google Scholar] [CrossRef]
- Wang, X.; Kong, X.; Qin, Y.; Zhu, X.; Liu, W.; Han, J. Milk phospholipids ameliorate mouse colitis associated with colonic goblet cell depletion via the Notch pathway. Food Funct. 2019, 10, 4608–4619. [Google Scholar] [CrossRef]
- Yeganeh, P.R.; Leahy, J.; Spahis, S.; Patey, N.; Desjardins, Y.; Roy, D.; Delvin, E.; Garofalo, C.; Leduc-Gaudet, J.-P.; St-Pierre, D.; et al. Apple peel polyphenols reduce mitochondrial dysfunction in mice with DSS-induced ulcerative colitis. J. Nutr. Biochem. 2018, 57, 56–66. [Google Scholar] [CrossRef]
- Tian, Q.; Iniguez, A.B.; Sun, Q.; Wang, H.; Du, M.; Zhu, M. Dietary alpha-ketoglutarate promotes epithelial metabolic transition and protects against DSS-induced colitis. Mol. Nutr. Food Res. 2021, 65, 2000936. [Google Scholar] [CrossRef]
- Sun, X.; Yang, Q.; Rogers, C.J.; Du, M.; Zhu, M.-J. AMPK improves gut epithelial differentiation and barrier function via regulating Cdx2 expression. Cell Death Differ. 2017, 24, 819–831. [Google Scholar] [CrossRef] [PubMed]
- Stringari, C.; Edwards, R.A.; Pate, K.T.; Waterman, M.L.; Donovan, P.J.; Gratton, E. Metabolic trajectory of cellular differentiation in small intestine by Phasor Fluorescence Lifetime Microscopy of NADH. Sci. Rep. 2012, 2, 568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jackson, D.N.; Panopoulos, M.; Neumann, W.L.; Turner, K.; Cantarel, B.L.; Thompson-Snipes, L.; Dassopoulos, T.; Feagins, L.A.; Souza, R.F.; Mills, J.C.; et al. Mitochondrial dysfunction during loss of prohibitin 1 triggers Paneth cell defects and ileitis. Gut 2020, 69, 1928–1938. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Z.; Qu, W.; Wang, K.; Chen, S.; Zhang, L.; Wu, D.; Chen, Z. Bisphenol A inhibits mucin 2 secretion in intestinal goblet cells through mitochondrial dysfunction and oxidative stress. Biomed. Pharmacother. 2019, 111, 901–908. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Tsuchiya, K.; Okamoto, R.; Iwasaki, M.; Kano, Y.; Sakamoto, N.; Nakamura, T.; Watanabe, M. Suppression of hath1 gene expression directly regulated by hes1 via notch signaling is associated with goblet cell depletion in ulcerative colitis. Inflamm. Bowel Dis. 2011, 17, 2251–2260. [Google Scholar] [CrossRef]
- Imaeda, H.; Andoh, A.; Aomatsu, T.; Uchiyama, K.; Bamba, S.; Tsujikawa, T.; Naito, Y.; Fujiyama, Y. Interleukin-33 suppresses Notch ligand expression and prevents goblet cell depletion in dextran sulfate sodium-induced colitis. Int. J. Mol. Med. 2011, 28, 573–578. [Google Scholar]
- Okamoto, R.; Tsuchiya, K.; Nemoto, Y.; Akiyama, J.; Nakamura, T.; Kanai, T.; Watanabe, M. Requirement of Notch activation during regeneration of the intestinal epithelia. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 296, G23–G35. [Google Scholar] [CrossRef] [Green Version]
- Katz, J.P.; Perreault, N.; Goldstein, B.G.; Lee, C.S.; Labosky, P.; Yang, V.W.; Kaestner, K.H. The zinc-finger transcription factor Klf4 is required for terminal differentiation of goblet cells in the colon. Development 2002, 129, 2619–2628. [Google Scholar] [CrossRef]
- Ghaleb, A.M.; Aggarwal, G.; Bialkowska, A.B.; Nandan, M.O.; Yang, V.W. Notch Inhibits Expression of the Kruppel-Like Factor 4 Tumor Suppressor in the Intestinal Epithelium. Mol. Cancer Res. 2008, 6, 1920–1927. [Google Scholar] [CrossRef] [Green Version]
- Xing, P.Y.; Pettersson, S.; Kundu, P. Microbial Metabolites and Intestinal Stem Cells Tune Intestinal Homeostasis. Proteomics 2020, 20, e1800419. [Google Scholar] [CrossRef]
- Sun, X.; Zhu, M.J. Butyrate inhibits indices of colorectal carcinogenesis via enhancing α-ketoglutarate-dependent dNA demethylation of mismatch repair genes. Mol. Nutr. Food Res. 2018, 62, e1700932. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Lee, J.M.; Lee, E.J.; Kim, D.J.; Hwang, W.B. Kynurenine promotes the goblet cell differentiation of HT-29 colon carcinoma cells by modulating Wnt, Notch and AhR signals. Oncol. Rep. 2018, 39, 1930–1938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pesi, R.; Balestri, F.; Ipata, P.L. Metabolic interaction between urea cycle and citric acid cycle shunt: A guided approach. Biochem. Mol. Biol. Educ. 2018, 46, 182–185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- LaMuraglia, G.M.; Lacaine, F.; Malt, R.A. High Ornithine Decarboxylase Activity and Polyamine Levels in Human Colorectal Neoplasia. Ann. Surg. 1986, 204, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Rao, J.N.; Rathor, N.; Zhuang, R.; Zou, T.; Liu, L.; Xiao, L.; Turner, D.J.; Wang, J.-Y. Polyamines regulate intestinal epithelial restitution through TRPC1-mediated Ca2+ signaling by differentially modulating STIM1 and STIM2. Am. J. Physiol. Cell Physiol. 2012, 303, C308–C317. [Google Scholar] [CrossRef] [Green Version]
- Xue, Y.; Zhang, H.; Sun, X.; Zhu, M.-J. Metformin Improves Ileal Epithelial Barrier Function in Interleukin-10 Deficient Mice. PLoS ONE 2016, 11, e0168670. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Zhang, S.; Shen, Q.; Zhu, M.-J. A metabolomic explanation on beneficial effects of dietary Goji on intestine inflammation. J. Funct. Foods 2019, 53, 109–114. [Google Scholar] [CrossRef]
- S.A.S. Institute. SAS User’s Guide. Version 8; SAS Institute Inc.: Cary, NC, USA, 1999. [Google Scholar]
- Sivashanmugam, M.; Jaidev, J.; Umashankar, V.; Sulochana, K.N. Ornithine and its role in metabolic diseases: An appraisal. Biomed. Pharmacother. 2017, 86, 185–194. [Google Scholar] [CrossRef]
- Haigis, K.M.; Kendall, K.R.; Wang, Y.; Cheung, A.; Haigis, M.C.; Glickman, J.N.; Kawakita, M.; Sweet-Cordero, A.; Sebolt-Leopold, J.; Shannon, K.M.; et al. Differential effects of oncogenic K-Ras and N-Ras on proliferation, differentiation and tumor progression in the colon. Nat. Genet. 2008, 40, 600–608. [Google Scholar] [CrossRef] [Green Version]
- Maran, R.R.; Thomas, A.; Roth, M.; Sheng, Z.; Esterly, N.; Pinson, D.; Gao, X.; Zhang, Y.; Ganapathy, V.; Gonzalez, F.J.; et al. Farnesoid X Receptor Deficiency in Mice Leads to Increased Intestinal Epithelial Cell Proliferation and Tumor Development. J. Pharmacol. Exp. Ther. 2009, 328, 469–477. [Google Scholar] [CrossRef] [Green Version]
- Peterson, L.W.; Artis, D. Intestinal epithelial cells: Regulators of barrier function and immune homeostasis. Nat. Rev. Immunol. 2014, 14, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Iraporda, C.; Errea, A.; Romanin, D.E.; Cayet, D.; Pereyra, E.; Pignataro, O.; Sirard, J.-C.; Garrote, G.L.; Abraham, A.G.; Rumbo, M. Lactate and short chain fatty acids produced by microbial fermentation downregulate proinflammatory responses in intestinal epithelial cells and myeloid cells. Immunobiology 2015, 220, 1161–1169. [Google Scholar] [CrossRef] [PubMed]
- Walker, A.; Pfitzner, B.; Neschen, S.; Kahle, M.; Harir, M.; Lucio, M.; Moritz, F.; Tziotis, D.; Witting, M.; Rothballer, M.; et al. Distinct signatures of host—microbial meta-metabolome and gut microbiome in two C57BL/6 strains under high-fat diet. ISME J. 2014, 8, 2380–2396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Behr, C.; Sperber, S.; Jiang, X.; Strauss, V.; Kamp, H.; Walk, T.; Herold, M.; Beekmann, K.; Rietjens, I.M.C.M.; van Ravenzwaay, B. Microbiome-related metabolite changes in gut tissue, cecum content and feces of rats treated with antibiotics. Toxicol. Appl. Pharmacol. 2018, 355, 198–210. [Google Scholar] [CrossRef]
- Armand, L.; Andriamihaja, M.; Gellenoncourt, S.; Bitane, V.; Lan, A.; Blachier, F. In vitro impact of amino acid-derived bacterial metabolites on colonocyte mitochondrial activity, oxidative stress response and DNA integrity. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2019, 1863, 1292–1301. [Google Scholar] [CrossRef]
- Qiao, Y.; Sun, J.; Ding, Y.; Le, G.; Shi, Y. Alterations of the gut microbiota in high-fat diet mice is strongly linked to oxidative stress. Appl. Microbiol. Biotechnol. 2013, 97, 1689–1697. [Google Scholar] [CrossRef]
- Shiomi, Y.; Nishiumi, S.; Ooi, M.; Hatano, N.; Shinohara, M.; Yoshie, T.; Kondo, Y.; Furumatsu, K.; Shiomi, H.; Kutsumi, H.; et al. GCMS-based metabolomic study in mice with colitis induced by dextran sulfate sodium. Inflamm. Bowel Dis. 2011, 17, 2261–2274. [Google Scholar] [CrossRef]
- Long, L.H.; Halliwell, B. Artefacts in cell culture: α-Ketoglutarate can scavenge hydrogen peroxide generated by ascorbate and epigallocatechin gallate in cell culture media. Biochem. Biophys. Res. Commun. 2011, 406, 20–24. [Google Scholar] [CrossRef]
- Ooi, M.; Nishiumi, S.; Yoshie, T.; Shiomi, Y.; Kohashi, M.; Fukunaga, K.; Nakamura, S.; Matsumoto, T.; Hatano, N.; Shinohara, M.; et al. GC/MS-based profiling of amino acids and TCA cycle-related molecules in ulcerative colitis. Inflamm. Res. 2011, 60, 831–840. [Google Scholar] [CrossRef]
- Alves, A.; Bassot, A.; Bulteau, A.-L.; Pirola, L.; Morio, B. Glycine Metabolism and Its Alterations in Obesity and Metabolic Diseases. Nutrients 2019, 11, 1356. [Google Scholar] [CrossRef] [Green Version]
- Wittemans, L.B.L.; Lotta, L.A.; Oliver-Williams, C.; Stewart, I.D.; Surendran, P.; Karthikeyan, S.; Day, F.R.; Koulman, A.; Imamura, F.; Zeng, L.; et al. Assessing the causal association of glycine with risk of cardio-metabolic diseases. Nat. Commun. 2019, 10, 1060. [Google Scholar] [CrossRef] [PubMed]
- Tsune, I.; Ikejima, K.; Hirose, M.; Yoshikawa, M.; Enomoto, N.; Takei, Y.; Sato, N. Dietary glycine prevents chemical-induced experimental colitis in the rat. Gastroenterology 2003, 125, 775–785. [Google Scholar] [CrossRef]
- El-Hafidi, M.; Franco, M.; Ramírez, A.R.; Sosa, J.S.; Flores, J.A.P.; Acosta, O.L.; Salgado, M.C.; Cardoso-Saldaña, G. Glycine Increases Insulin Sensitivity and Glutathione Biosynthesis and Protects against Oxidative Stress in a Model of Sucrose-Induced Insulin Resistance. Oxid. Med. Cell. Longev. 2018, 2018, 2101562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCarty, M.F.; O’Keefe, J.H.; DiNicolantonio, J.J. Dietary Glycine Is Rate-Limiting for Glutathione Synthesis and May Have Broad Potential for Health Protection. Ochsner J. 2018, 18, 81–87. [Google Scholar]
- Ardite, E.; Sans, M.; Panes, J.; Romero, F.J.; Piqué, J.M.; Fernández-Checa, J.C. Replenishment of Glutathione Levels Improves Mucosal Function in Experimental Acute Colitis. Lab. Investig. 2000, 80, 735–744. [Google Scholar] [CrossRef] [Green Version]
- Faure, M.; Mettraux, C.; Moennoz, D.; Godin, J.P.; Vuichoud, J.; Rochat, F.; Breuillé, D.; Obled, C.; Corthésy-Theulaz, I. Specific amino acids increase mucin synthesis and microbiota in dextran sulfate sodium-treated rats. J. Nutr. 2006, 136, 1558–1564. [Google Scholar] [CrossRef] [Green Version]
- Bhatt, B.; Zeng, P.; Zhu, H.; Sivaprakasam, S.; Li, S.; Xiao, H.; Dong, L.; Shiao, P.; Kolhe, R.; Patel, N.; et al. Gpr109a Limits Microbiota-Induced IL-23 Production to Constrain ILC3-Mediated Colonic Inflammation. J. Immunol. 2018, 200, 2905–2914. [Google Scholar] [CrossRef] [Green Version]
- Petersen, K.F.; Dufour, S.; Cline, G.W.; Shulman, G.I. Regulation of hepatic mitochondrial oxidation by glucose-alanine cycling during starvation in humans. J. Clin. Investig. 2019, 129, 4671–4675. [Google Scholar] [CrossRef]
- Vaziri, N.D.; Yuan, J.; Norris, K. Role of Urea in Intestinal Barrier Dysfunction and Disruption of Epithelial Tight Junction in Chronic Kidney Disease. Am. J. Nephrol. 2013, 37, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Lin, H.-C.; Visek, W.J. Colon Mucosal Cell Damage by Ammonia in Rats. J. Nutr. 1991, 121, 887–893. [Google Scholar] [CrossRef]
- Chen, J.; Yang, H.; Long, L.; Zhao, Y.; Jiang, Q.; Wu, F.; Kang, B.; Liu, S.; Adebowale, T.O.; Fu, C.; et al. The effects of dietary supplementation with α-ketoglutarate on the intestinal microbiota, metabolic profiles, and ammonia levels in growing pigs. Anim. Feed Sci. Technol. 2017, 234, 321–328. [Google Scholar] [CrossRef]
- Pillai, R.B.; Tolia, V.; Rabah, R.; Simpson, P.M.; Vijesurier, R.; Lin, C.-H. Increased Colonic Ornithine Decarboxylase Activity in Inflammatory Bowel Disease in Children. Dig. Dis. Sci. 1999, 44, 1565–1570. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, A.; Kurihara, S.; Takahashi, D.; Ohashi, W.; Nakamura, Y.; Kimura, S.; Onuki, M.; Kume, A.; Sasazawa, Y.; Furusawa, Y.; et al. Symbiotic polyamine metabolism regulates epithelial proliferation and macrophage differentiation in the colon. Nat. Commun. 2021, 12, 2105. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-Y.; Johnson, L.R. Polyamines and ornithine decarboxylase during repair of duodenal mucosa after stress in rats. Gastroenterology 1991, 100, 333–343. [Google Scholar] [CrossRef]
- Bardócz, S.; Grant, G.; Brown, D.S.; Pusztai, A. Putrescine as a source of instant energy in the small intestine of the rat. Gut 1998, 42, 24–28. [Google Scholar] [CrossRef]
- Santandreu, F.M.; Oliver, J.; Roca, P. Improvement of mitochondrial energy and oxidative balance during intestinal differentiation. Mitochondrion 2011, 11, 89–96. [Google Scholar] [CrossRef]
- Keshteli, A.H.; Madsen, K.L.; Mandal, R.; Boeckxstaens, G.E.; Bercik, P.; De Palma, G.; Reed, D.E.; Wishart, D.; Vanner, S.; Dieleman, L.A. Comparison of the metabolomic profiles of irritable bowel syndrome patients with ulcerative colitis patients and healthy controls: New insights into pathophysiology and potential biomarkers. Aliment. Pharmacol. Ther. 2019, 49, 723–732. [Google Scholar] [CrossRef]
- Banerjee, A.; Herring, C.A.; Chen, B.; Kim, H.; Simmons, A.J.; Southard-Smith, A.; Allaman, M.M.; White, J.R.; Macedonia, M.C.; Mckinley, E.T.; et al. Succinate Produced by Intestinal Microbes Promotes Specification of Tuft Cells to Suppress Ileal Inflammation. Gastroenterology 2020, 159, 2101–2115.e5. [Google Scholar] [CrossRef]
- JanssenDuijghuijsen, L.M.; Grefte, S.; de Boer, V.; Zeper, L.W.; Van Dartel, D.A.M.; Van Der Stelt, I.; Bekkenkamp-Grovenstein, M.; Van Norren, K.; Wichers, H.; Keijer, J. Mitochondrial ATP Depletion Disrupts Caco-2 Monolayer Integrity and Internalizes Claudin 7. Front. Physiol. 2017, 8, 794. [Google Scholar] [CrossRef] [Green Version]
- Nowarski, R.; Jackson, R.; Gagliani, N.; De Zoete, M.R.; Palm, N.W.; Bailis, W.; Low, J.S.; Harman, C.C.D.; Graham, M.; Elinav, E.; et al. Epithelial IL-18 Equilibrium Controls Barrier Function in Colitis. Cell 2015, 163, 1444–1456. [Google Scholar] [CrossRef] [Green Version]
- Van der Sluis, M.; De Koning, B.A.E.; De Bruijn, A.C.J.M.; Velcich, A.; Meijerink, J.P.P.; van Goudoever, J.B.; Büller, H.A.; Dekker, J.; VAN Seuningen, I.; Renes, I.B.; et al. Muc2-Deficient Mice Spontaneously Develop Colitis, Indicating That MUC2 Is Critical for Colonic Protection. Gastroenterology 2006, 131, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Mashimo, H.; Wu, D.-C.; Podolsky, D.K.; Fishman, M.C. Impaired Defense of Intestinal Mucosa in Mice Lacking Intestinal Trefoil Factor. Science 1996, 274, 262–265. [Google Scholar] [CrossRef] [PubMed]
- Gersemann, M.; Becker, S.; Kübler, I.; Koslowski, M.; Wang, G.; Herrlinger, K.R.; Griger, J.; Fritz, P.; Fellermann, K.; Schwab, M.; et al. Differences in goblet cell differentiation between Crohn’s disease and ulcerative colitis. Differentiation 2009, 77, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Jensen, J.; Pedersen, E.E.; Galante, P.; Hald, J.; Heller, R.S.; Ishibashi, M.; Kageyama, R.; Guillemot, F.; Serup, P.; Madsen, O.D. Control of endodermal endocrine development by Hes-1. Nat. Genet. 2000, 24, 36–44. [Google Scholar] [CrossRef]
- Bibi, S.; Du, M.; Zhu, M.-J. Dietary Red Raspberry Reduces Colorectal Inflammation and Carcinogenic Risk in Mice with Dextran Sulfate Sodium–Induced Colitis. J. Nutr. 2018, 148, 667–674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, X.; Du, M.; Navarre, D.A.; Zhu, M.-J. Purple potato extract promotes intestinal epithelial differentiation and barrier function by activating AMP-activated protein kinase. Mol. Nutr. Food Res. 2018, 62, 1700536. [Google Scholar] [CrossRef]
- Bibi, S.; de Sousa Moraes, L.F.; Lebow, N.; Zhu, M.J. Dietary green pea protects against DSS-induced colitis in mice challenged with high-fat diet. Nutrients 2017, 9, 509. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bravo Iniguez, A.; Tian, Q.; Du, M.; Zhu, M.-J. Alpha-Ketoglutarate Promotes Goblet Cell Differentiation and Alters Urea Cycle Metabolites in DSS-Induced Colitis Mice. Nutrients 2022, 14, 1148. https://doi.org/10.3390/nu14061148
Bravo Iniguez A, Tian Q, Du M, Zhu M-J. Alpha-Ketoglutarate Promotes Goblet Cell Differentiation and Alters Urea Cycle Metabolites in DSS-Induced Colitis Mice. Nutrients. 2022; 14(6):1148. https://doi.org/10.3390/nu14061148
Chicago/Turabian StyleBravo Iniguez, Alejandro, Qiyu Tian, Min Du, and Mei-Jun Zhu. 2022. "Alpha-Ketoglutarate Promotes Goblet Cell Differentiation and Alters Urea Cycle Metabolites in DSS-Induced Colitis Mice" Nutrients 14, no. 6: 1148. https://doi.org/10.3390/nu14061148
APA StyleBravo Iniguez, A., Tian, Q., Du, M., & Zhu, M.-J. (2022). Alpha-Ketoglutarate Promotes Goblet Cell Differentiation and Alters Urea Cycle Metabolites in DSS-Induced Colitis Mice. Nutrients, 14(6), 1148. https://doi.org/10.3390/nu14061148




