The Effects of Prolonged Water-Only Fasting and Refeeding on Markers of Cardiometabolic Risk
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Study Population and Enrollment
3.2. Weight, BMI, and Abdominal Circumference
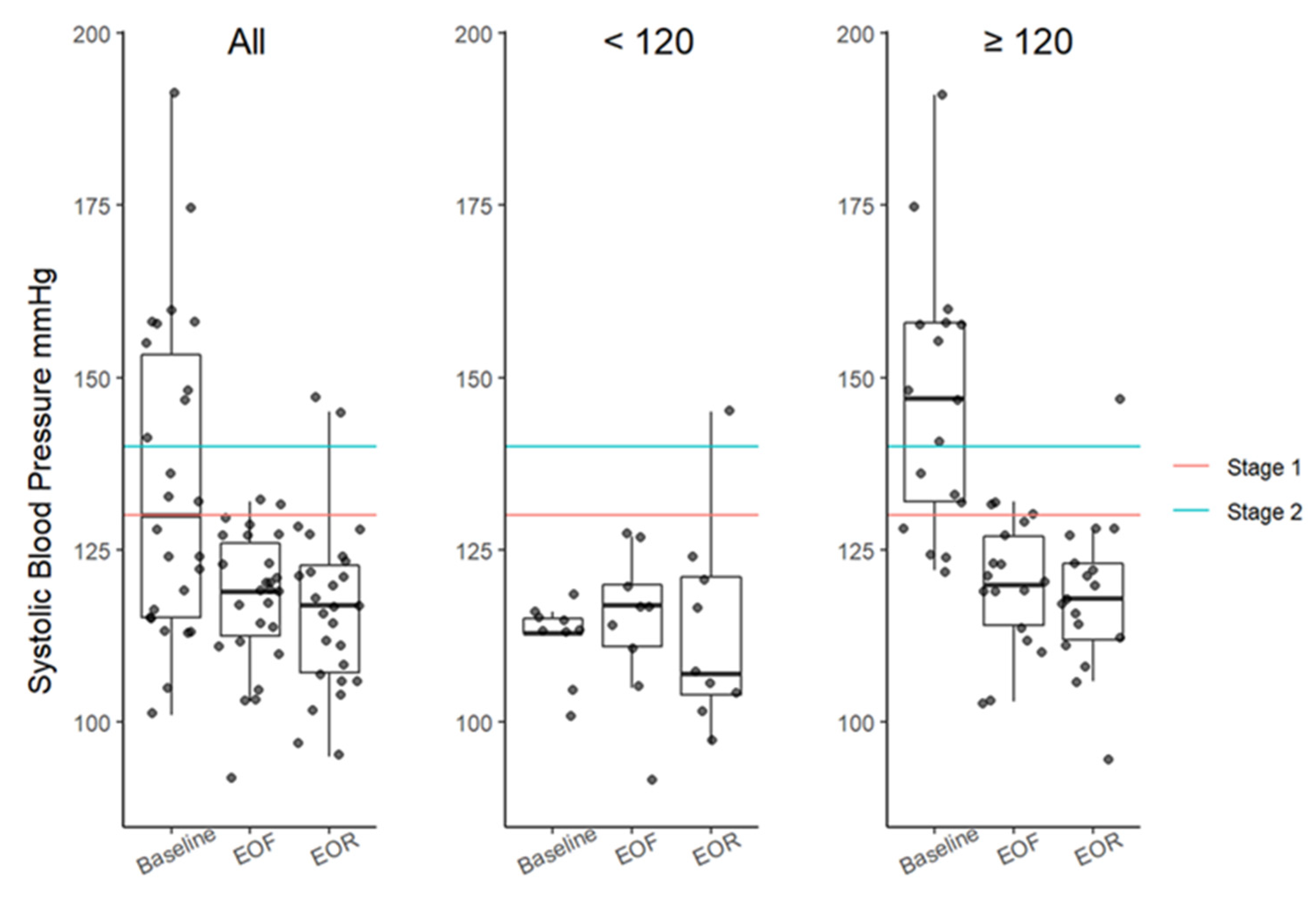
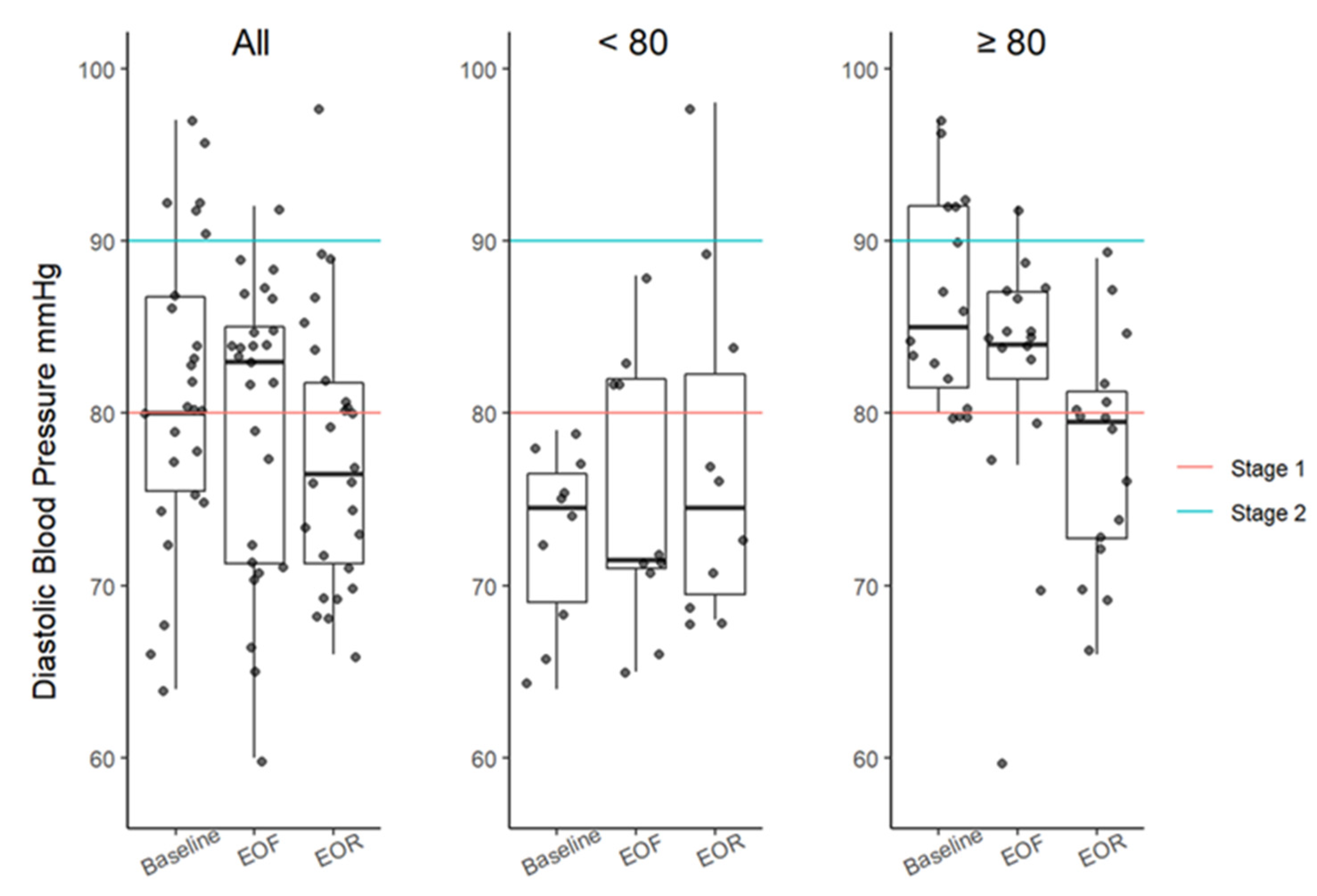
3.3. Resting Blood Pressure
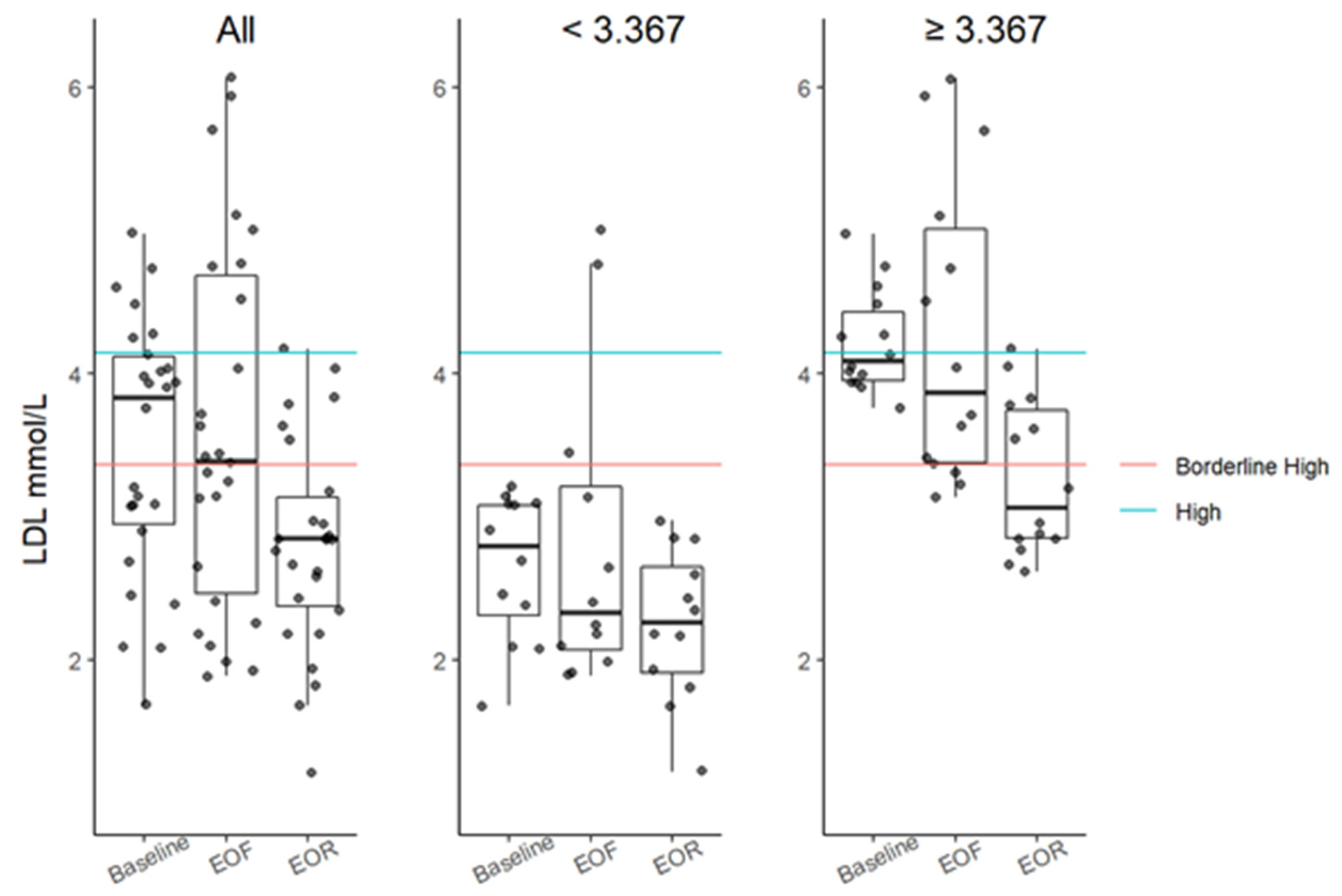
3.4. Serum Lipids
3.5. High-Sensitivity C-Reactive Protein
3.6. Homeostatic Model Assessment for Insulin Resistance (HOMA-IR)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Garcia-Garcia, F.J.; Monistrol-Mula, A.; Cardellach, F.; Garrabou, G. Nutrition, Bioenergetics, and Metabolic Syndrome. Nutrients 2020, 12, 2785. [Google Scholar] [CrossRef]
- Kirk, E.P.; Klein, S. Pathogenesis and pathophysiology of the cardiometabolic syndrome. J. Clin. Hypertens. 2009, 11, 761–765. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.; Jin, C.; Guan, Q. Causal Effects of Overall and Abdominal Obesity on Insulin Resistance and the Risk of Type 2 Diabetes Mellitus: A Two-Sample Mendelian Randomization Study. Front. Genet. 2020, 11, 603. [Google Scholar] [CrossRef]
- Czech, M.P. Mechanisms of insulin resistance related to white, beige, and brown adipocytes. Mol. Metab. 2020, 34, 27–42. [Google Scholar] [CrossRef]
- Anton, S.D.; Moehl, K.; Donahoo, W.T.; Marosi, K.; Lee, S.A.; Mainous, A.G., 3rd; Leeuwenburgh, C.; Mattson, M.P. Flipping the Metabolic Switch: Understanding and Applying the Health Benefits of Fasting. Obesity 2018, 26, 254–268. [Google Scholar] [CrossRef]
- Varady, K.A.; Bhutani, S.; Klempel, M.C.; Kroeger, C.M.; Trepanowski, J.F.; Haus, J.M.; Hoddy, K.K.; Calvo, Y. Alternate day fasting for weight loss in normal weight and overweight subjects: A randomized controlled trial. Nutr. J. 2013, 12, 146. [Google Scholar] [CrossRef] [Green Version]
- Newman, J.C.; Verdin, E. Ketone bodies as signaling metabolites. Trends Endocrinol. Metab. 2014, 25, 42–52. [Google Scholar] [CrossRef] [Green Version]
- Miller, V.J.; Villamena, F.A.; Volek, J.S. Nutritional Ketosis and Mitohormesis: Potential Implications for Mitochondrial Function and Human Health. J. Nutr. Metab. 2018, 2018, 5157645. [Google Scholar] [CrossRef] [Green Version]
- Cahill, G.F., Jr. Starvation in man. Clin. Endocrinol. Metab. 1976, 5, 397–415. [Google Scholar] [CrossRef]
- Finnell, J.S.; Saul, B.C.; Goldhamer, A.C.; Myers, T.R. Is fasting safe? A chart review of adverse events during medically supervised, water-only fasting. BMC Complement. Altern. Med. 2018, 18, 67. [Google Scholar] [CrossRef]
- Runcie, J.; Hilditch, T.E. Energy provision, tissue utilization, and weight loss in prolonged starvation. Br. Med. J. 1974, 2, 352–356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldhamer, A.; Lisle, D.; Parpia, B.; Anderson, S.V.; Campbell, T.C. Medically supervised water-only fasting in the treatment of hypertension. J. Manip. Physiol. Ther. 2001, 24, 335–339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldhamer, A.C.; Lisle, D.J.; Sultana, P.; Anderson, S.V.; Parpia, B.; Hughes, B.; Campbell, T.C. Medically supervised water-only fasting in the treatment of borderline hypertension. J. Altern. Complement. Med. 2002, 8, 643–650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weigle, D.S.; Duell, P.B.; Connor, W.E.; Steiner, R.A.; Soules, M.R.; Kuijper, J.L. Effect of fasting, refeeding, and dietary fat restriction on plasma leptin levels. J. Clin. Endocrinol. Metab. 1997, 82, 561–565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jackson, I.M.; McKiddie, M.T.; Buchanan, K.D. Effect of fasting on glucose and insulin metabolism of obese patients. Lancet 1969, 1, 285–287. [Google Scholar] [CrossRef]
- Jackson, I.M.; McKiddie, M.T.; Buchanan, K.D. Influence of blood-lipid levels and effect of prolonged fasting on carbohydrate metabolism in obesity. Lancet 1971, 2, 450–452. [Google Scholar] [CrossRef]
- Greenfield, M.; Kolterman, O.; Olefsky, J.M.; Reaven, G.M. The effect of ten days of fasting on various aspects of carbohydrate metabolism in obese diabetic subjects with significant fasting hyperglycemia. Metabolism 1978, 27 (Suppl. 2), 1839–1852. [Google Scholar] [CrossRef]
- Anderson, J.W.; Herman, R.H. Effect of fasting, caloric restriction, and refeeding on glucose tolerance of normal men. Am. J. Clin. Nutr. 1972, 25, 41–52. [Google Scholar] [CrossRef]
- Gayoso-Diz, P.; Otero-Gonzalez, A.; Rodriguez-Alvarez, M.X.; Gude, F.; Garcia, F.; De Francisco, A.; Quintela, A.G. Insulin resistance (HOMA-IR) cut-off values and the metabolic syndrome in a general adult population: Effect of gender and age: EPIRCE cross-sectional study. BMC Endocr. Disord. 2013, 13, 47. [Google Scholar] [CrossRef] [Green Version]
- Kassambara, A. Rstatix: Pipe-Friendly Framework for Basic Statistical Tests. R Package Version 0.7.0. 2021. Available online: https://CRAN.R-project.org/package=rstatix (accessed on 22 February 2022).
- Siegel, A.F. Robust regression using repeated medians. Biometrika 1982, 69, 242–244. [Google Scholar] [CrossRef]
- James, G.M.; Witten, D.; Hastie, T.; Tibshirani, R. An Introduction to Statistical Learning: With Applications in R; Springer Nature: Berlin, Germany, 2021. [Google Scholar]
- Leamer, E.; Leonard, H. Reporting the Fragility of Regression Estimates. Rev. Econ. Stat. 1983, 65, 306. [Google Scholar] [CrossRef]
- Allison, P.D. Change Scores as Dependent Variables in Regression Analysis. Sociol. Methodol. 1990, 20, 93. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 22 February 2022).
- Wilhelmi de Toledo, F.; Buchinger, A.; Burggrabe, H.; Holz, G.; Kuhn, C.; Lischka, E.; Lischka, N.; Lützner, H.; May, W.; Ritzmann-Widderich, M.; et al. Fasting therapy—An expert panel update of the 2002 consensus guidelines. Forsch. Komplementmed. 2013, 20, 434–443. [Google Scholar] [CrossRef] [PubMed]
- Kerndt, P.R.; Naughton, J.L.; Driscoll, C.E.; Loxterkamp, D.A. Fasting: The history, pathophysiology and complications. West J. Med. 1982, 137, 379–399. [Google Scholar]
- Bloom, W.L. Fasting as an introduction to the treatment of obesity. Metabolism 1959, 8, 214–220. [Google Scholar]
- Wilhelmi de Toledo, F.; Grundler, F.; Bergouignan, A.; Drinda, S.; Michalsen, A. Safety, health improvement and well-being during a 4 to 21-day fasting period in an observational study including 1422 subjects. PLoS ONE 2019, 14, e0209353. [Google Scholar] [CrossRef] [Green Version]
- Giesecke, K.; Magnusson, I.; Ahlberg, M.; Hagenfeldt, L.; Wahren, J. Protein and amino acid metabolism during early starvation as reflected by excretion of urea and methylhistidines. Metabolism 1989, 38, 1196–1200. [Google Scholar] [CrossRef]
- Henson, L.C.; Heber, D. Whole body protein breakdown rates and hormonal adaptation in fasted obese subjects. J. Clin. Endocrinol. Metab. 1983, 57, 316–319. [Google Scholar] [CrossRef]
- Kwon, H.; Kim, D.; Kim, J.S. Body Fat Distribution and the Risk of Incident Metabolic Syndrome: A Longitudinal Cohort Study. Sci. Rep. 2017, 7, 10955. [Google Scholar] [CrossRef] [Green Version]
- North, K.A.; Lascelles, D.; Coates, P. The mechanisms by which sodium excretion is increased during a fast but reduced on subsequent carbohydrate feeding. Clin. Sci. Mol. Med. 1974, 46, 423–432. [Google Scholar] [CrossRef]
- Kolanowski, J. On the mechanisms of fasting natriuresis and of carbohydrate-induced sodium retention. Diabetes Metab. 1977, 3, 131–143. [Google Scholar]
- Runcie, J. Urinary sodium and potassium excretion in fasting obese subjects. Br. Med. J. 1971, 2, 22–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andersson, B.; Wallin, G.; Hedner, T.; Ahlberg, A.C.; Andersson, O.K. Acute effects of short-term fasting on blood pressure, circulating noradrenaline and efferent sympathetic nerve activity. Acta Med. Scand. 1988, 223, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Chinn, R.H.; Brown, J.J.; Fraser, R.; Heron, S.M.; Lever, A.F.; Murchison, L.; Robertson, J.I.S. The natriuresis of fasting: Relationship to changes in plasma renin and plasma aldosterone concentrations. Clin. Sci. 1970, 39, 437–455. [Google Scholar] [CrossRef] [Green Version]
- Gelman, A.; Sigulem, D.; Korn, D.; Ajzen, H.; Ramos, O.L. Starvation—An interesting model for the study of the renin-angiotensin-aldosterone system. Rev. Bras. Pesqui. Med. Biol. 1978, 11, 43–47. [Google Scholar]
- Feingold, K.R. Introduction to Lipids and Lipoproteins. In Endotext; Feingold, K.R., Anawalt, B., Boyce, A., Chrousos, G., de Herder, W.W., Dhatariya, K., Dungan, K., Hershman, J.M., Hofland, J., Kalra, S., et al., Eds.; South Dartmouth: Dartmouth, MA, USA, 2000. [Google Scholar]
- Goldstein, J.L.; Brown, M.S. Regulation of low-density lipoprotein receptors: Implications for pathogenesis and therapy of hypercholesterolemia and atherosclerosis. Circulation 1987, 76, 504–507. [Google Scholar] [CrossRef] [Green Version]
- Fang, Y.; Gu, Y.; Zhao, C.; Lv, Y.; Qian, J.; Zhu, L.; Yuan, N.; Zhang, S.; Wang, L.; Li, M.; et al. Impact of supervised beego, a traditional Chinese water-only fasting, on thrombosis and haemostasis. BMJ Nutr. Prev. Health 2021, 4, 1–14. [Google Scholar] [CrossRef]
- Sproston, N.R.; Ashworth, J.J. Role of C-Reactive Protein at Sites of Inflammation and Infection. Front. Immunol. 2018, 9, 754. [Google Scholar] [CrossRef]
- Li, C.; Ostermann, T.; Hardt, M.; Ludtke, R.; Broecker-Preuss, M.; Dobos, G.; Michalsen, A. Metabolic and psychological response to 7-day fasting in obese patients with and without metabolic syndrome. Forsch. Komplementmed. 2013, 20, 413–420. [Google Scholar] [CrossRef]
- Gutch, M.; Kumar, S.; Razi, S.M.; Gupta, K.K.; Gupta, A. Assessment of insulin sensitivity/resistance. Indian J. Endocrinol. Metab. 2015, 19, 160–164. [Google Scholar] [CrossRef]
- Kolterman, O.G.; Saekow, M.; Olefsky, J.M. The effects of acute and chronic starvation on insulin binding to isolated human adipocytes. J. Clin. Endocrinol. Metab. 1979, 48, 836–842. [Google Scholar] [CrossRef] [PubMed]
- Quinn, W.J., 3rd; Wan, M.; Shewale, S.V.; Gelfer, R.; Rader, D.J.; Birnbaum, M.J.; Titchenell, P.M. mTORC1 stimulates phosphatidylcholine synthesis to promote triglyceride secretion. J. Clin. Investig. 2017, 127, 4207–4215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fazeli, P.K.; Zhang, Y.; O’Keefe, J.; Pesaresi, T.; Lun, M.; Lawney, B.; Steinhauser, M.L. Prolonged fasting drives a program of metabolic inflammation in human adipose tissue. Mol. Metab. 2020, 42, 101082. [Google Scholar] [CrossRef]
- Michalsen, A. Prolonged fasting as a method of mood enhancement in chronic pain syndromes: A review of clinical evidence and mechanisms. Curr. Pain Headache Rep. 2010, 14, 80–87. [Google Scholar] [CrossRef]
- Brandhorst, S.; Choi, I.Y.; Wei, M.; Cheng, C.W.; Sedrakyan, S.; Navarrete, G.; Dubeau, L.; Yap, L.P.; Park, R.; Vinciguerra, M.; et al. A Periodic Diet that Mimics Fasting Promotes Multi-System Regeneration, Enhanced Cognitive Performance, and Healthspan. Cell Metab. 2015, 22, 86–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Median (IQR) | Friedman Test | Median of Differences (95% CI) | |||||
|---|---|---|---|---|---|---|---|
| Bonferroni Corrected p Value | |||||||
| Baseline | EOF | EOR | Fr (p Value) | EOF Baseline | EOR Baseline | EOR–EOF | |
| Weight, kg | 87.2 (75.1–99.5) | 78.3 (66.2–88.2) | 79.5 (67.3–89.0) | 43 (<0.0001) | −9.0 (−9.6, −8.0) <0.0001 | −7.3 (−8.5, −6.1) <0.0001 | 1.3 (0.9, 2.0) 0.0006 |
| BMI, kg/m2 (18.5–24.9 kg/m2) | 30.7 (27.5–35.3) | 27.6 (24.6–31.5) | 28.8 (25.1–31.9) | 43 (<0.0001) | −3.2 (−3.4, −2.8) <0.0001 | −2.5 (−3.1, −2.2) <0.0001 | 0.5 (0.3, 0.7) 0.0001 |
| AC, cm (<101.6 cm for men and <88.9 cm for women) | 97.6 (93.8–108.3) | 90.7 (84.9–100.6) | 90.8 (84.8–102.3) | 46 (<0.0001) | −7.1 (−9.2, −6.4) <0.0001 | −5.7 (−7.5, −4.9) <0.0001 | 1.7 (1.1, 2.6) 0.0054 |
| SBP, mmHg (<130 mmHg) | 130 (115–153) | 119 (113–126) | 117 (107–123) | 13 (0.0017) | −14 (−26, −6) 0.0054 | −13 (−27, −9) 0.0012 | −3 (−6, 3) 1.5507 |
| DBP, mmHg (<80 mmHg) | 80 (76–87) | 83 (71–85) | 77 (71–82) | 7 (0.0368) | −3 (−6, 2) 1.4533 | −6 (−8, −1) 0.0874 | −3 (−6, 1) 0.6872 |
| TC, mmol/L (3.24–5.18 mmol/L) | 5.67 (4.71–6.18) | 5.27 (4.29–6.03) | 4.87 (4.46–5.26) | 7 (0.0280) | 0 (−0.47, 0.35) 2.4280 | −0.52 (−0.88, −0.23) 0.0031 | −0.45 (−0.92, −0.08) 0.0667 |
| HDL, mmol/L (≥1.17 mmol/L for men and ≥1.30 mmol/L for women) | 1.28 (1.04–1.52) | 1.14 (0.96–1.23) | 1.09 (0.98–1.29) | 9 (0.0094) | −0.12 (−1.26, −0.03) 0.0544 | −0.09 (−0.22, −0.04) 0.03176 | 0.01 (−0.08, 0.12) 2.0988 |
| LDL, mmol/L (<2.59 mmol/L) | 3.83 (2.95–4.12) | 3.39 (2.47–4.68) | 2.85 (2.38–3.13) | 19 (<0.0001) | −0.03 (−0.36, 0.48) 2.4666 | −0.75 (−97, −0.45) 0.0004 | −0.82 (−1.14, −0.41) 0.0010 |
| LDL > 3.11 mmol/L ‡ | 4.03 (3.93–4.33) | 3.87 (3.35–5.02) | 2.91 (2.83–3.66) | 16 (0.0004) | −0.13 (−0.63, 0.71) 2.1172 | −0.92 (−1.20, −0.61) 0.0018 | −0.96 (−1.53, −0.52) 0.0044 |
| VLDL, mmol/L (<0.78 mmol/L) | 0.63 (0.49–0.78) | 0.66 (0.60–0.74) | 0.87 (0.75–1.04) | 20 (<0.0001) | 0.03 (−0.10, 0.13) 1.7424 | 0.30 (0.13, 0.39) 0.0023 | 0.26 (0.17, 0.36) 0.0002 |
| TG, mmol/L (<1.70 mmol/L) | 1.39 (1.06–1.68) | 1.44 (1.30–1.63) | 1.91 (1.65–2.28) | 21 (<0.0001) | 0.04 (−0.21, 0.27) 1.7813 | 0.62 (0.30, 0.84) 0.0011 | 0.55 (0.35, 0.77) <0.0001 |
| hsCRP, mg/L (<1.0 mg/L) | 2.67 (1.00–4.60) | 3.91 (1.84–7.09) | 1.68 (0.79–3.44) | 9 (0.0088) | 0.60 (−0.07, 1.69) 0.2526 | −0.55 (−2.65, −0.07) 0.0897 | −1.46 (−3.35, 0.87) 0.0041 |
| hsCRP > 2 mg/L ‡ ‡ | 3.64 (2.70–7.01) | 5.19 (3.68–8.06) | 2.54 (1.56–3.47) | 15 (0.0007) | 1.07 (−2.05, 2.81) 1.3757 | −2.02 (−4.71, −0.81) 0.0068 | −2.43 (−5.01, 1.53) 0.0009 |
| Glucose, mmol/L (<7.8 mmol/L) | 4.94 (4.56–5.05) | 4.13 (3.90–4.66) | 5.55 (5.05–5.97) | 38 (<0.0001) | −0.61 (−0.94, −0.44) 0.0002 | 0.83 (0.47, 1.05) 0.0003 | 1.42 (1.08, 1.83) < 0.0001 |
| Insulin, pmol/L (<102 pmol/L) | 40.2 (30.8–64.5) | 36 (23.4–44.3) | 75.3 (62.0–126.8) | 34 (<0.0001) | −7.2 (−24.0, 1.2) 0.1912 | 41.7 (32.7, 60.9) <0.0001 | 36.9 (32.4, 86.7) 0.0001 |
| HOMA-IR (<1 is optimal) | 1.4 (1.0–2.6) | 1.1 (0.6–1.4) | 3.1 (2.2–6.1) | 37 (<0.0001) | −0.4 (−1.2, −0.1) 0.0329 | 2.0 (1.5, 2.9) <0.0001 | 1.8 (1.5, 4.0) 0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scharf, E.; Zeiler, E.; Ncube, M.; Kolbe, P.; Hwang, S.-Y.; Goldhamer, A.; Myers, T.R. The Effects of Prolonged Water-Only Fasting and Refeeding on Markers of Cardiometabolic Risk. Nutrients 2022, 14, 1183. https://doi.org/10.3390/nu14061183
Scharf E, Zeiler E, Ncube M, Kolbe P, Hwang S-Y, Goldhamer A, Myers TR. The Effects of Prolonged Water-Only Fasting and Refeeding on Markers of Cardiometabolic Risk. Nutrients. 2022; 14(6):1183. https://doi.org/10.3390/nu14061183
Chicago/Turabian StyleScharf, Eugene, Evelyn Zeiler, Mackson Ncube, Patricia Kolbe, Su-Yeon Hwang, Alan Goldhamer, and Toshia R. Myers. 2022. "The Effects of Prolonged Water-Only Fasting and Refeeding on Markers of Cardiometabolic Risk" Nutrients 14, no. 6: 1183. https://doi.org/10.3390/nu14061183
APA StyleScharf, E., Zeiler, E., Ncube, M., Kolbe, P., Hwang, S.-Y., Goldhamer, A., & Myers, T. R. (2022). The Effects of Prolonged Water-Only Fasting and Refeeding on Markers of Cardiometabolic Risk. Nutrients, 14(6), 1183. https://doi.org/10.3390/nu14061183






