Vitamin D and Parkinson’s Disease
Abstract
:1. Introduction
2. Vitamin D
3. Vitamin D and Parkinson Disease
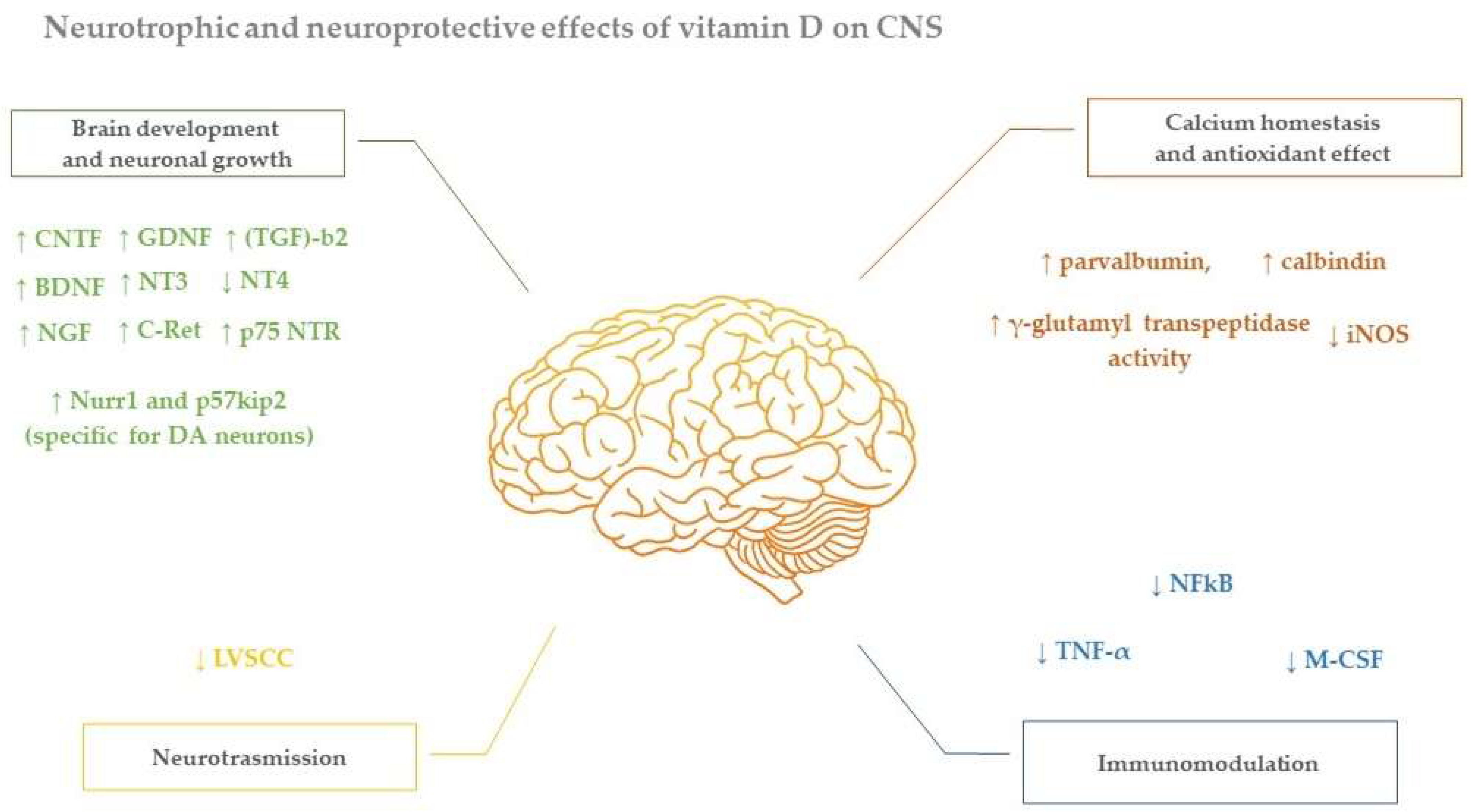
4. Neuroprotective Effect of Vitamin D in Parkinson Disease
5. Vitamin D in Relation with Parkinson Disease Symptoms and Disease Progression
6. Vitamin D Supplementation in Parkinson Disease
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Katsnelson, A.; de Strooper, B.; Zoghbi, H.Y. Neurodegeneration: From cellular concepts to clinical applications. Sci. Transl. Med. 2016, 8, 364ps18. [Google Scholar] [CrossRef] [PubMed]
- Farghali, M.; Ruga, S.; Morsanuto, V.; Uberti, F. Can Brain Health Be Supported by Vitamin D-Based Supplements? A Critical Review. Brain Sci. 2020, 10, 660. [Google Scholar] [CrossRef] [PubMed]
- Cantorna, M.T.; Snyder, L.; Lin, Y.D.; Yang, L. Vitamin D and 1,25(OH)2D regulation of T cells. Nutrients 2015, 7, 3011–3021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herrmann, M.; Farrell, C.J.L.; Pusceddu, I.; Fabregat-Cabello, N.; Cavalier, E. Assessment of Vitamin D status—A changing landscape. Clin. Chem. Lab. Med. 2017, 55, 3–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deluca, G.C.; Kimball, S.M.; Kolasinski, J.; Ramagopalan, S.V.; Ebers, G.C. Review: The role of vitamin D in nervous system health and disease. Neuropathol. Appl. Neurobiol. 2013, 39, 458–484. [Google Scholar] [CrossRef] [PubMed]
- Rosen, C.J. Vitamin D Insufficiency. N. Engl. J. Med. 2011, 364, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Cashman, K.D.; van den Heuvel, E.G.; Schoemaker, R.J.; Prévéraud, D.P.; Macdonald, H.M.; Arcot, J. 25-Hydroxyvitamin D as a Biomarker of Vitamin D Status and Its Modeling to Inform Strategies for Prevention of Vitamin D Deficiency within the Population. Adv Nutr. 2017, 8, 947–957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eyles, D.W.; Smith, S.; Kinobe, R.; Hewison, M.; McGrath, J.J. Distribution of the Vitamin D receptor and 1α-hydroxylase in human brain. J. Chem. Neuroanat. 2005, 29, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Naveilhan, P.; Neveu, I.; Wion, D.; Brachet, P. 1,25-Dihydroxyvitamin D3, an inducer of glial cell line-derived neurotrophic factor. Neuroreport 1996, 7, 2171–2175. [Google Scholar] [CrossRef]
- Khanal, R.; Nemere, I. The ERp57/GRp58/1,25D3-MARRS Receptor: Multiple Functional Roles in Diverse Cell Systems. Curr. Med. Chem. 2007, 14, 1087–1093. [Google Scholar] [CrossRef]
- Bivona, G.; Agnello, L.; Bellia, C.; Iacolino, G.; Scazzone, C.; Lo Sasso, B.; Ciaccio, M. Non-Skeletal Activities of Vitamin D: From Physiology to Brain Pathology. Medicina 2019, 55, 341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramagopalan, S.V.; Heger, A.; Berlanga, A.J.; Maugeri, N.J.; Lincoln, M.R.; Burrel, A.; Handunnetthi, L.; Handel, A.E.; Disanto, G.; Orton, S.M.; et al. A ChIP-seq defined genome-wide map of vitamin D receptor binding: Associations with disease and evolution. Genome Res. 2010, 20, 1352–1360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- LeBlanc, T.; Nemere, L. Actin and Keratin are Binding Partners of the 1,25D3-MARRS Receptor/PDIA3/ERp57. Immunol. Endocr. Metab. Agents Med. Chem. 2014, 14, 55–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pertile, R.A.N.; Cui, X.; Hammond, L.; Eyles, D.W. Vitamin D regulation of GDNF/Ret signaling in dopaminergic neurons. FASEB J. 2018, 32, 819–828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vinh quôc Luong, K.; Thi Hoàng Nguyên, L. Vitamin D and Parkinson’s disease. J. Neurosci. Res. 2012, 90, 2227–2236. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Pelekanos, M.; Burne, T.H.J.; McGrath, J.J.; Eyles, D.W. Maternal vitamin D deficiency alters the expression of genes involved in dopamine specification in the developing rat mesencephalon. Neurosci. Lett. 2010, 486, 220–223. [Google Scholar] [CrossRef] [PubMed]
- Garcion, E.; Wion-Barbot, N.; Montero-Menei, C.N.; Berger, F.; Wion, D. New clues about vitamin D functions in the nervous system. Trends Endocrinol. Metab. 2002, 13, 100–105. [Google Scholar] [CrossRef]
- Eyles, D.; Brown, J.; Mackay-Sim, A.; McGrath, J.; Feron, F. Vitamin D3 and brain development. Neuroscience 2003, 118, 641–653. [Google Scholar] [CrossRef]
- Eyles, D.W.; Feron, F.; Cui, X.; Kesby, J.P.; Harms, L.H.; Ko, P.; McGrath, J.J.; Burne, T.H.J. Developmental vitamin D deficiency causes abnormal brain development. Psychoneuroendocrinology 2009, 34 (Suppl. S1), S247–S257. [Google Scholar] [CrossRef]
- Cui, X.; McGrath, J.J.; Burne, T.H.J.; Mackay-Sim, A.; Eyles, D.W. Maternal vitamin D depletion alters neurogenesis in the developing rat brain. Int. J. Dev. Neurosci. 2007, 25, 227–232. [Google Scholar] [CrossRef]
- Jones, G.; Strugnell, S.A.; DeLuca, H.F. Current understanding of the molecular actions of vitamin D. Physiol. Rev. 1998, 78, 1193–1231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balabanova, S.; Richter, H.P.; Antoniadis, G.; Homok, J.; Kremmer, N.; Hanle, J.; Teller, W.M. 25-hydroxyvitamin D, 24, 25-dihydroxyvitamin D and 1,25-dihydroxyvitamin D in human cerebrospinal fluid. Klin. Wochenschr. 1984, 62, 1086–1090. [Google Scholar] [CrossRef] [PubMed]
- Lang, F.; Ma, K.; Leibrock, C.B. 1,25(OH)2D3 in Brain Function and Neuropsychiatric Disease. Neuro-Signals 2019, 27, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Landel, V.; Stephan, D.; Cui, X.; Eyles, D.; Feron, F. Differential expression of vitamin D-associated enzymes and receptors in brain cell subtypes. J. Steroid Biochem. Mol. Biol. 2018, 177, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Balestrino, R.; Schapira, A.H.V. Parkinson disease. Eur. J. Neurol. 2020, 27, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Kalia, L.V.; Lang, A.E. Parkinson’s disease. Lancet 2015, 386, 896–912. [Google Scholar] [CrossRef]
- Dugger, B.N.; Dickson, D.W. Pathology of neurodegenerative diseases. Cold Spring Harb. Perspect. Biol. 2017, 9, a028035. [Google Scholar] [CrossRef] [PubMed]
- Samii, A.; Nutt, J.G.; Ransom, B.R. Parkinson’s disease. Lancet 2004, 363, 1783–1793. [Google Scholar] [CrossRef] [Green Version]
- Ascherio, A.; Schwarzschild, M.A. The epidemiology of Parkinson’s disease: Risk factors and prevention. Lancet Neurol. 2016, 15, 1257–1272. [Google Scholar] [CrossRef]
- D’Amelio, M.; Ragonese, P.; Sconzo, G.; Aridon, P.; Savettieri, G. Parkinson’s disease and cancer: Insights for pathogenesis from epidemiology. Ann. N. Y. Acad. Sci. 2009, 1155, 324–334. [Google Scholar] [CrossRef] [PubMed]
- D’Amelio, M.; Ragonese, P.; Morgante, L.; Epifanio, A.; Callari, G.; Salemi, G.; Savettieri, G. Tumor diagnosis preceding Parkinson’s disease: A case-control study. Mov. Disord. Off. J. Mov. Disord. Soc. 2004, 19, 807–811. [Google Scholar] [CrossRef] [PubMed]
- Berridge, M.J. Vitamin D: A custodian of cell signalling stability in health and disease. Biochem. Soc. Trans. 2015, 43, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Jankovic, J.; Tan, E.K. Parkinson’s disease: Etiopathogenesis and treatment. J. Neurol. Neurosurg. Psychiatry 2020, 91, 795–808. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Yoshioka, M.; Hashimoto, M.; Murakami, M.; Noya, M.; Takahashi, D.; Urashima, M. Randomized, double-blind, placebo-controlled trial of vitamin D supplementation in Parkinson disease. Am. J. Clin. Nutr. 2013, 97, 1004–1013. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Yoshioka, M.; Hashimoto, M.; Murakami, M.; Noya, M.; Takahashi, D.; Urashima, M. 25-hydroxyvitamin D, vitamin D receptor gene polymorphisms, and severity of Parkinson’s disease. Mov. Disord. 2012, 27, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Brewer, L.D.; Thibault, V.; Chen, K.C.; Langub, M.C.; Landfield, P.W.; Porter, N.M. Vitamin D hormone confers neuroprotection in parallel with downregulation of L-type calcium channel expression in hippocampal neurons. J. Neurosci. 2001, 21, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Riaz, S.; Malcangio, M.; Miller, M.; Tomlinson, D.R. A vitamin D3 derivative (CB1093) induces nerve growth factor and prevents neurotrophic deficits in streptozotocin-diabetic rats. Diabetologia 1999, 42, 1308–1313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Viragh, P.A.; Haglid, K.G.; Celio, M.R. Parvalbumin increases in the caudate putamen of rats with vitamin D hypervitaminosis. Proc. Natl. Acad. Sci. USA 1989, 86, 3887–3890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcion, E.; Nataf, S.; Berod, A.; Darcy, F.; Brachet, P. 1,25-Dihydroxyvitamin D3 inhibits the expression of inducible nitric oxide synthase in rat central nervous system during experimental allergic encephalomyelitis. Mol. Brain Res. 1997, 45, 255–267. [Google Scholar] [CrossRef]
- Furman, I.; Baudet, C.; Brachet, P. Differential expression of M-CSF, LIF, and TNF-α genes in normal and malignant rat glial cells: Regulation by lipopolysaccharide and vitamin D. J. Neurosci. Res. 1996, 46, 360–366. [Google Scholar] [CrossRef]
- Garcion, E.; do Thanh, X.; Bled, F.; Dehouck, M.P.; Rigault, F.; Brachet, P.; Girault, A.; Torpier, G.; Darcy, F. 1,25-dihydroxyvitamin D3 regulates γ-glutamyl transpeptidase activity in rat brain. Neurosci. Lett. 1996, 216, 183–186. [Google Scholar] [CrossRef]
- Alfieri, D.F.; Lehmann, M.F.; Oliveira, S.R.; Flauzino, T.; Francieli Delongui, F.; Martins de Araújo, M.C.; Isaias Dichi, I.; Vinícius Daher Delfino, V.D.; Leda Mezzaroba, L.; Colado Simão, A.N.; et al. Vitamin D deficiency is associated with acute ischemic stroke, C-reactive protein, and short-term outcome. Metab. Brain Dis. 2017, 32, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Neveu, I.; Naveilhan, P.; Baudet, C.; Brachet, P.; Metsis, M. 1,25-Dihydroxyvitamin D3 regulates NT-3, NT-4 but not BDNF mRNA in astrocytes. Neuroreport 1994, 6, 124–126. [Google Scholar] [CrossRef] [PubMed]
- Musiol, I.M.; Feldman, D. 1,25-dihydroxyvitamin D3 induction of nerve growth factor in L929 mouse fibroblasts: Effect of vitamin D receptor regulation and potency of vitamin D3 analogs. Endocrinology 1997, 138, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Shirazi, H.A.; Rasouli, J.; Ciric, B.; Rostami, A.; Zhang, G.X. 1,25-Dihydroxyvitamin D3 enhances neural stem cell proliferation and oligodendrocyte differentiation. Exp. Mol. Pathol. 2015, 98, 240–245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Annweiler, C.; Montero-Odasso, M.; Hachinski, V.; Seshadri, S.; Bartha, R.; Beauchet, O. Vitamin D concentration and lateral cerebral ventricle volume in older adults. Mol. Nutr. Food Res. 2013, 57, 267–276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fleet, J.C. Rapid, membrane-initiated actions of 1,25 dihydroxyvitamin D: What are they and what do they mean? J. Nutr. 2004, 134, 3215–3218. [Google Scholar] [CrossRef]
- Veenstra, T.D.; Windebank, A.J.; Kumar, R. 1,25-dihydroxyvitamin d3 regulates the expression of N-myc, c-myc, protein kinase c, and transforming growth factor-β2 in neuroblastoma cells. Biochem. Biophys. Res. Commun. 1997, 235, 15–18. [Google Scholar] [CrossRef]
- van Etten, E.; Mathieu, C. Immunoregulation by 1,25-dihydroxyvitamin D3: Basic concepts. J. Steroid Biochem. Mol. Biol. 2005, 97, 93–101. [Google Scholar] [CrossRef]
- Ometto, F.; Stubbs, B.; Annweiler, C.; Duval, G.T.; Wooyoung Jang, W.; Kim, H.T.; McCarroll, K.; Cunningham, C.; Soysal, P.; Isik, A.T.; et al. Hypovitaminosis D and orthostatic hypotension: A systematic review and meta-analysis. J. Hypertens. 2016, 34, 1036–1043. [Google Scholar] [CrossRef]
- Knekt, P.; Kilkkinen, A.; Rissanen, H.; Marniemi, J.; Sääksjärvi, K.; Heliövaara, M. Serum vitamin D and the risk of Parkinson disease. Arch. Neurol. 2010, 67, 808–811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fahmy, E.M.; Elawady, M.E.; Sharaf, S.; Heneidy, S.; Ismail, R.S. Vitamin D status in idiopathic Parkinson’s disease: An Egyptian study. Egypt. J. Neurol. Psychiatry Neurosurg. 2020, 56, 45. [Google Scholar] [CrossRef]
- Moghaddasi, M.; Mamarabadi, M.; Aghaii, M. Serum 25-hydroxyvitamin D3 concentration in Iranian patients with Parkinson’s disease. Iran. J. Neurol. 2013, 12, 56–59. [Google Scholar] [PubMed]
- Wang, L.; Evatt, M.L.; Maldonado, L.G.; Perry, W.R.; Ritchie, J.C.; Beecham, G.W.; Martin, E.R.; Haines, J.L.; Pericak-Vance, M.A.; Vance, J.M.; et al. Vitamin D from different sources is inversely associated with Parkinson disease. Mov. Disord. 2015, 30, 560–566. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Dhima, K.; Lockhart, K.C.; Locascio, J.J.; Hoesing, A.N.; Duong, K.; Trisini-Lipsanopoulos, A.; Hayes, M.T.; Sohur, U.S.; Wills, A.-M.; et al. Unrecognized vitamin D3 deficiency is common in Parkinson disease: Harvard Biomarker Study. Neurology 2013, 81, 1531–1537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evatt, M.L.; DeLong, M.R.; Khazai, N.; Rosen, A.; Triche, S.; Tangpricha, V. Prevalence of vitamin D insufficiency in patients with Parkinson disease and Alzheimer disease. Arch. Neurol. 2008, 65, 1348–1352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lv, L.; Tan, X.; Peng, X.; Bai, R.; Xiao, Q.; Zou, T.; Tan, J.; Zhang, H.; Wang, C. The relationships of vitamin D, vitamin D receptor gene polymorphisms, and vitamin D supplementation with Parkinson’s disease. Transl. Neurodegener. 2020, 9, 34. [Google Scholar] [CrossRef]
- van den Bos, F.; Speelman, A.D.; van Nimwegen, M.; van der Schouw, Y.T.; Backx, F.J.G.; Bloem, B.R.; Munneke, M.; Verhaar, H.J.J. Bone mineral density and vitamin D status in Parkinson’s disease patients. J. Neurol. 2013, 260, 754–760. [Google Scholar] [CrossRef]
- Abou-Raya, S.; Helmii, M.; Abou-Raya, A. Bone and mineral metabolism in older adults with Parkinson’s disease. Age Ageing 2009, 38, 675–680. [Google Scholar] [CrossRef] [Green Version]
- Annweiler, C.; Llewellyn, D.J.; Beauchet, O. Low serum vitamin D concentrations in Alzheimer’s disease: A systematic review and meta-analysis. J. Alzheimer’s Dis. 2013, 33, 659–674. [Google Scholar] [CrossRef] [Green Version]
- Zhu, D.; Liu, G.Y.; Lv, Z.; Wen, S.R.; Bi, S.; Wang, W.Z. Inverse associations of outdoor activity and vitamin D intake with the risk of Parkinson’s disease. J. Zhejiang Univ. Sci. B 2014, 15, 923–927. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fullard, M.E.; Duda, J.E. A Review of the Relationship between Vitamin D and Parkinson Disease Symptoms. Front. Neurol. 2020, 11, 454. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, S.; Lutsey, P.L.; Alonso, A.; Huang, X.; Mosley, T.H.; Chen, H. Serum 25-hydroxyvitamin D concentrations in Mid-adulthood and Parkinson’s disease risk. Mov. Disord. 2016, 31, 972–978. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Khemka, V.K.; Ganguly, A.; Roy, D.; Ganguly, U.; Chakrabarti, S. Vitamin D and Alzheimer’s disease: Neurocognition to therapeutics. Int. J. Alzheimer’s Dis. 2015, 2015, 192747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, X.; Gooch, H.; Petty, A.; McGrath, J.J.; Eyles, D. Vitamin D and the brain: Genomic and non-genomic actions. Mol. Cell. Endocrinol. 2017, 453, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Gooch, H.; Groves, N.J.; Sah, P.; Burne, T.H.; Eyles, D.W.; McGrath, J. Vitamin D and the brain: Key questions for future research. J. Steroid Biochem. Mol. Biol. 2015, 148, 305–309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burne, T.H.J.; McGrath, J.J.; Eyles, D.W.; Mackay-Sim, A. Behavioural characterization of Vitamin D receptor knockout mice. Behav. Brain Res. 2005, 157, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Butler, M.W.; Burt, A.; Edwards, T.L.; Züchner, S.; Scott, W.K.; Martin, E.R.; Vance, J.M.; Wang, L. Vitamin D Receptor Gene as a Candidate Gene for Parkinson Disease. Ann. Hum. Genet. 2011, 75, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Calvello, R.; Cianciulli, A.; Nicolardi, G.; De Nuccio, F.; Giannotti, L.; Salvatore, R.; Porro, C.; Trotta, T.; Panaro, M.A.; Lofrumento, D.D. Vitamin D Treatment Attenuates Neuroinflammation and Dopaminergic Neurodegeneration in an Animal Model of Parkinson’s Disease, Shifting M1 to M2 Microglia Responses. J. Neuroimmune Pharmacol. 2017, 12, 327–339. [Google Scholar] [CrossRef] [PubMed]
- Cass, W.A.; Peters, L.E.; Fletcher, A.M.; Yurek, D.M. Calcitriol promotes augmented dopamine release in the lesioned striatum of 6-hydroxydopamine treated rats. Neurochem. Res. 2014, 39, 1467–1476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cass, W.A.; Peters, L.E.; Fletcher, A.M.; Yurek, D.M. Evoked dopamine overflow is augmented in the striatum of calcitriol treated rats. Neurochem. Int. 2012, 60, 186–191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.Y.; Wu, J.N.; Cherng, T.L.; Hoffer, B.J.; Chen, H.H.; Borlongan, C.V.; Wang, Y. Vitamin D3 attenuates 6-hydroxydopamine-induced neurotoxicity in rats. Brain Res. 2001, 904, 67–75. [Google Scholar] [CrossRef]
- Puchacz, E.; Stumpf, W.E.; Stachowiak, E.K.; Stachowiak, M.K. Vitamin D increases expression of the tyrosine hydroxylase gene in adrenal medullary cells. Mol. Brain Res. 1996, 36, 193–196. [Google Scholar] [CrossRef]
- Smith, M.P.; Fletcher-Turner, A.; Yurek, D.M.; Cass, W.A. Calcitriol protection against dopamine loss induced by intracerebroventricular administration of 6-hydroxydopamine. Neurochem. Res. 2006, 31, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Kalueff, A.V.; Lou, Y.R.; Laaksi, I.; Tuohimaa, P. Impaired motor performance in mice lacking neurosteroid vitamin D receptors. Brain Res. Bull. 2004, 64, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Török, R.; Török, N.; Szalardy, L.; Plangar, I.; Szolnoki, Z.; Somogyvari, F.; Vecsei, L.; Klivenyi, P. Association of vitamin D receptor gene polymorphisms and Parkinson’s disease in Hungarians. Neurosci. Lett. 2013, 551, 70–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, X.; Xue, L.; Li, Y.; Chen, B.; Xie, A. Vitamin D receptor gene polymorphism and its association with Parkinson’s disease in Chinese Han population. Neurosci. Lett. 2012, 525, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Gatto, N.M.; Sinsheimer, J.S.; Cockburn, M.; Escobedo, L.A.; Bordelon, Y.; Ritz, B. Vitamin D receptor gene polymorphisms and Parkinson’s disease in a population with high ultraviolet radiation exposure. J. Neurol. Sci. 2015, 352, 88–93. [Google Scholar] [CrossRef] [Green Version]
- Rimmelzwaan, L.M.; van Schoor, N.M.; Lips, P.; Berendse, H.W.; Eekhoff, E.M.W. Systematic Review of the Relationship between Vitamin D and Parkinson’s Disease. J. Parkinson’s Dis. 2016, 6, 29–37. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Sokal, I.; Peskind, E.R.; Quinn, J.F.; Jankovic, J.; Kenney, C.; Chung, K.A.; Millard, S.P.; Nutt, J.G.; Montine, T.J. CSF multianalyte profile distinguishes Alzheimer and Parkinson diseases. Am. J. Clin. Pathol. 2008, 129, 526–529. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.S.; Kim, Y.I.; Song, C.; Yoon, I.; Park, J.W.; Choi, Y.B.; Kim, H.T.; Lee, K.S. Association of vitamin D receptor gene polymorphism and Parkinson’s disease in Koreans. J. Korean Med. Sci. 2005, 20, 495–498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meamar, R.; Shaabani, P.; Tabibian, S.R.; Aghaye Ghazvini, M.R.; Feizi, A. The effects of uric acid, serum vitamin D3, and their interaction on parkinson’s disease severity. Parkinson’s Dis. 2015, 2015, 463483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sleeman, I.; Aspray, T.; Lawson, R.; Coleman, S.; Duncan, G.; Khoo, T.K.; Schoenmakers, I.; Rochester, L.; Burn, D.; Yarnall, A. The Role of Vitamin D in Disease Progression in Early Parkinson’s Disease. J. Parkinson’s Dis. 2017, 7, 669–675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, X.; Ou, R.; Dutta, R.; Tian, Y.; Xiong, H.; Shang, H. Association between serum Vitamin D levels and Parkinson’s disease: A systematic review and meta-analysis. Front. Neurol. 2018, 9, 909. [Google Scholar] [CrossRef] [Green Version]
- Evatt, M.L.; DeLong, M.R.; Kumari, M.; Auinger, P.; McDermott, M.P.; Tangpricha, V. High prevalence of hypovitaminosis D status in patients with early Parkinson disease. Arch. Neurol. 2011, 68, 314–319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gezen-Ak, D.; Alaylıoğlu, M.; Genç, G.; Gündüz, A.; Candaş, E.; Bilgiç, B.; Atasoy, I.L.; Apaydın, H.; Kızıltan, G.; Gürvit, H.; et al. GC and VDR SNPs and Vitamin D Levels in Parkinson’s Disease: The Relevance to Clinical Features. Neuromolecular Med. 2017, 19, 24–40. [Google Scholar] [CrossRef] [PubMed]
- Peterson, A.L.; Mancini, M.; Horak, F.B. The relationship between balance control and vitamin D in Parkinson’s disease—A pilot study. Mov. Disord. 2013, 28, 1133–1137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asaka, T.; Yahata, K.; Mani, H.; Wang, Y. Modulations of muscle modes in automatic postural responses induced by external surface translations. J. Mot. Behav. 2011, 43, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Torres-Oviedo, G.; Ting, L.H. Muscle synergies characterizing human postural responses. J. Neurophysiol. 2007, 98, 2144–2156. [Google Scholar] [CrossRef] [Green Version]
- di Monaco, M.; Vallero, F.; di Monaco, R.; Tappero, R.; Cavanna, A. Bone Mineral Density in Hip-Fracture Patients with Parkinson’s Disease: A Case-Control Study. Arch. Phys. Med. Rehabil. 2006, 87, 1459–1462. [Google Scholar] [CrossRef]
- van Weel, C.; Vermeulen, H.; van den Bosch, W. Falls, a community care perspective. Lancet 1995, 345, 1549–1551. [Google Scholar] [CrossRef]
- Peterson, A.L.; Murchison, C.; Zabetian, C.; Leverenz, J.B.; Watson, G.S.; Montine, T.; Carney, N.; Bowman, G.L.; Edwards, K.; Quinn, J.F. Memory, mood, and vitamin d in persons with parkinson’s disease. J. Parkinson’s Dis. 2013, 3, 547–555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.J.; Zhang, J.R.; Mao, C.J.; Li, K.; Wang, F.; Chen, J.; Liu, C.F. Relationship between 25-Hydroxyvitamin D, bone density, and Parkinson’s disease symptoms. Acta Neurol. Scand. 2019, 140, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Gatto, N.M.; Paul, K.C.; Sinsheimer, J.S.; Bronstein, J.M.; Bordelon, Y.; Rausch, R.; Ritz, B. Vitamin D receptor gene polymorphisms and cognitive decline in Parkinson’s disease. J. Neurol. Sci. 2016, 370, 100–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCarty, D.E.; Reddy, A.; Keigley, Q.; Kim, P.Y.; Marino, A.A. Vitamin D, race, and excessive daytime sleepiness. J. Clin. Sleep Med. 2012, 8, 693–697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.E.; Oh, E.; Park, J.; Youn, J.; Kim, J.S.; Jang, W. Serum 25-hydroxyvitamin D3 level may be associated with olfactory dysfunction in de novo Parkinson’s disease. J. Clin. Neurosci. 2018, 57, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Jang, W.; Park, J.; Kim, J.S.; Youn, J.; Oh, E.; Kwon, K.Y.; Jo, K.D.; Lee, M.K.; Kim, H.T. Vitamin D deficiency in Parkinson’s disease patients with orthostatic hypotension. Acta Neurol. Scand. 2015, 132, 242–250. [Google Scholar] [CrossRef]
- Kwon, K.Y.; Jo, K.D.; Lee, M.K.; Oh, M.; Kim, E.N.; Park, J.; Kim, J.S.; Youn, J.; Oh, E.; Kim, H.T.; et al. Low serum Vitamin D levels may contribute to gastric dysmotility in de novo Parkinson’s disease. Neurodegener. Dis. 2016, 16, 199–205. [Google Scholar] [CrossRef]
- Garcion, E.; Sindji, L.; Leblondel, G.; Brachet, P.; Darcy, F. 1,25-dihydroxyvitamin D3 regulates the synthesis of γ-glutamyl transpeptidase and glutathione levels in rat primary astrocytes. J. Neurochem. 1999, 73, 859–866. [Google Scholar] [CrossRef]
- Shinpo, K.; Kikuchi, S.; Sasaki, H.; Moriwaka, F.; Tashiro, K. Effect of 1,25-dihydroxyvitamin D3 on cultured mesencephalic dopaminergic neurons to the combined toxicity caused by L-buthionine sulfoximine and 1-methyl-4-phenylpyridine. J. Neurosci. Res. 2000, 62, 374–382. [Google Scholar] [CrossRef]
- Luthra, N.S.; Kim, S.; Zhang, Y.; Christine, C.W. Characterization of vitamin D supplementation and clinical outcomes in a large cohort of early Parkinson’s disease. J. Clin. Mov. Disord. 2018, 5, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, X.; Dolnikowski, G.; Patterson, W.; Dawson-Hughes, B.; Zheng, T.; Morris, M.; Holland, T.; Booth, S. Determination of Vitamin D and Its Metabolites in Human Brain Using an Ultra-Pressure LC–Tandem Mass Spectra Method. Curr. Dev. Nutr. 2019, 3, nzz074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naveilhan, P.; Neveu, I.; Baude, C.; Ohyama, K.Y.; Brachet, P.; Wion, D. Expression of 25(OH) vitamin d3 24-hydroxylase gene in glial cells. NeuroReport 1993, 5, 255–257. [Google Scholar] [CrossRef] [PubMed]
- Prüfer, K.; Veenstra, T.D.; Jirikowski, G.F.; Kumar, R. Distribution of 1,25-dihydroxyvitamin D3 receptor immunoreactivity in the rat brain and spinal cord. J. Chem. Neuroanat. 1999, 16, 135–145. [Google Scholar] [CrossRef]
| Study Type | Authors | Year | Main Conclusion |
|---|---|---|---|
| SNP | Butler et al. [68] | 2011 | VDR as a potential susceptibility gene and support an essential role of vitamin D in PD |
| Gatto et al. [78] | 2015 | VDR polymorphisms may modulate risk of PD in a population highly exposed to UVR throughout lifetime | |
| Cui et al. [66] | 2015 | VDR is present in the nucleus of tyrosine hydroxylase (TH)-positive neurons in both human and rat substantia nigra | |
| Fu et al. [102] | 2019 | No vitamin D3 was detected in metabolites in the prefrontal cortex, middle frontal cortex, middle temporal cortex, cerebellum, corpus callosum, medulla, and pons of this single human brain | |
| post-mortem | Eyles et al. [8] | 2005 | 1a-OHase and VDR are widespread distributed in human brain |
| Shirazi et al. [45] | 2015 | 1,25OH2D3. This vitamin significantly enhanced proliferation of NSCs, and enhanced their differentiation into neurons and oligodendrocytes, but not astrocytes | |
| in vitro | Puchacz et al. [73] | 2013 | 1,25OH2D3 regulates catecholamine production in adrenal chromaffin cells providing response and adaptation to stress |
| Cui et al. [20] | 2007 | DVD deficiency has been shown to alter brain structure and function | |
| Shinpo et al. [100] | 2000 | 1,25OH2D3 increases the intracellular glutathione determining ROS suppression with antioxidative function and defending mesencephalic dopaminergic neurons against BSO/MPP(+)-induced toxicity | |
| Garcion et al. [99] | 1999 | 1,25-D3 play a fundamental role in astrocyte detoxification pathways | |
| Musiol et al. [44] | 1997 | 1,25OH2D3 treatment increased the NGF concentration | |
| Garcion et al. [39] | 1997 | 1,25D3 has an inhibitory effect on iNOS expression and could be synthesized by macrophages or microglia controlling CNS-specific immune responses. | |
| Furman et al. [40] | 1996 | 1,25OH2D3 play a role in regulation on CNS immune response, by modification of astrocytes response to an inflammatory stimulus | |
| Garcion et al. [41] | 1996 | 1,25D3 could be an effector controlling detoxification processes in the brain. | |
| Naveilhan et al. [9] | 1996 | 1,25OH2D3 is a potent inducer of GDNF expression | |
| Neveu et al. [43] | 1994 | Activated brain macrophages may be committed to synthesize 1,25OHD, in vitro. | |
| Naveilhan et al. [103] | 1993 | vitamin D3 metabolites are involved in brain function | |
| De Viragh et al. [38] | 1989 | Vitamin D influences the concentration of calcium-binding-proteins in the periphery and brain | |
| Moghaddasi et al. [53] | 2013 | Non-PD patients were detected lower 25OHD level and it was significantly associated with FOG, postural instability and abnormal postures | |
| in vivo | Calvello et al. [69] | 2017 | Vitamin D exhibits substantial neuroprotective effects in this PD animal model, by attenuating pro-inflammatory and up-regulating anti-inflammatory processes |
| Cass et al. [70] | 2014 | Calcitriol can upregulate GDNF and dopaminergic release in striatum, increasing DA levels in the substantia nigra | |
| Cass et al. [71] | 2012 | In animals treated with 6-OHDA followed by calcitriol there was significantly greater potassium and amphetamine evoked overflow of DA from the lesioned striatum compared to that from the control animals | |
| Cui et al. [16] | 2010 | the DVD-deficient embryos had a significant reduction in factors crucial in specifying dopaminergic phenotype, such as Nurr1 and p57Kip2 | |
| Smith et al. [74] | 2006 | Long-treatment with calcitriol can provide partial protection for dopaminergic neurons against the effects of 6-OHDA | |
| Burne et al. [67] | 2005 | VDR mice knockout have motor impairments but seemingly no compromission in cognition | |
| Kalueff et al. [75] | 2004 | VDR genetic ablation produces severe motor impairment | |
| Eyles et al. [18] | 2003 | Rats born to vitamin D3-deficient mothers had alterations in the brain at birth: lateral ventricles were enlarged, the cortex was thinner | |
| Wang et al. [72] | 2001 | D3 pretreatment reduces the hypokinesia and DA neuronal toxicity induced by 6-OHDA | |
| Prüfer et al. [104] | 1999 | The widespread distribution of vitamin D3 receptor suggests multiple functions of 1,25OHD3 in the CNS. | |
| Fahmy et al. [52] | 2020 | Serum 25OHD3 was lower in PD patients and was negatively correlated with age and age at onset of disease, but not with disease duration and PD severity. Serum 25OHD3 was not found to be predictor for severity of PD | |
| case–control | Zhang et al. [93] | 2019 | Vitamin D levels significantly correlated with falls and some non-motor symptoms |
| Kim et al. [96] | 2018 | The serum 25OHD3 level was independently associated with odor identification score in patients with PD | |
| Alfieri et al. [42] | 2017 | 25OHD levels were negatively correlated with mRS after three-month follow-up | |
| Sleeman et al. [83] | 2017 | PD patients have significantly lower serum 25OHD concentrations than controls, which may have implications in terms of bone health and fracture risk | |
| Kwon et al. [98] | 2016 | Vitamin D status may play a role in the pathogenesis of delayed gastric emptying in drug-naive PD. | |
| Wang et al. [54] | 2015 | Association between vitamin D levels and PD is not simply due to lack of sunlight exposure PD patients with impaired mobility | |
| Jang et al. [97] | 2015 | Low vitamin D status is associated with OH in patients with PD | |
| Zhu et al. [61] | 2014 | Outdoor activity and total vitamin D intake were inversely associated with PD | |
| Ding et al. [55] | 2013 | Lower levels of 25OHD3 are correlated with higher total UPDRS scores at baseline and during follow-up | |
| Török et al. [76] | 2013 | The frequency of FokI C allele was significantly higher in PD patients than in controls, suggesting that this polymorphism may have a role in the development of PD in these patients | |
| Peterson et al. [92] | 2013 | Vitamin D plays a role in balance in PD | |
| Han et al. [77] | 2012 | VDR FokI T/C polymorphism is related to PD and it may change genetic susceptibility to sporadic PD | |
| Suzuki et al. [35] | 2012 | Higher 25OHD levels and vitamin D receptor FokICC genotype may be independently associated with milder forms of PD | |
| Abou-Raya et al. [59] | 2009 | PD is associated with increased risk of falls, fractures and osteoporosis | |
| Di Monaco et al. [90] | 2006 | BMD expressed as a T score did not differ significantly between PD patients and controls | |
| Kim et al. [81] | 2005 | association between PD and a VDRG BsmI polymorphism, which might be involved in the pathogenesis of PD | |
| Meamar et al. [82] | 2015 | Negative correlation between interaction of serum vitamin D3 and UA with severity of PD | |
| cross sectional | Luthra et al. [101] | 2018 | Vitamin D administration does not influence disease progression in PD patients |
| cohort | Gezen et al. [86] | 2017 | PD patients with slower progression had significantly higher levels of serum 25OHD |
| Gatto et al. [94] | 2016 | Fokl, a functional VDR polymorphism, as being associated with cognitive decline in PD. | |
| Shrestha et al. [63] | 2016 | Vitamin D may reduce the risk of PD | |
| Peterson et al. [87] | 2013 | Higher plasma vitamin D is associated with better cognition and better mood in this sample of PD patients without dementia | |
| Evatt et al. [85] | 2011 | Vitamin D concentrations did not decline during progression of PD | |
| Knekt et al. [51] | 2010 | higher serum vitamin D concentrations showed a reduced risk of Parkinson disease | |
| Evatt et al. [56] | 2008 | Higher prevalence of hypovitaminosis in PD respect both healthy controls and patients with AD | |
| Van de Bos et al. [58] | 2013 | More than half of the patients with early stage PD had an abnormal BMD. Vit. D concentrations were reduced in PD underscoring the importance of proactive screening for bone loss and vitamin D deficiency, even in early stages of PD | |
| RCT | Suzuki et al. [34] | 2013 | Vitamin D3 supplementation may stabilize PD for a short period in patients with FokI TT or CT genotypes. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pignolo, A.; Mastrilli, S.; Davì, C.; Arnao, V.; Aridon, P.; dos Santos Mendes, F.A.; Gagliardo, C.; D’Amelio, M. Vitamin D and Parkinson’s Disease. Nutrients 2022, 14, 1220. https://doi.org/10.3390/nu14061220
Pignolo A, Mastrilli S, Davì C, Arnao V, Aridon P, dos Santos Mendes FA, Gagliardo C, D’Amelio M. Vitamin D and Parkinson’s Disease. Nutrients. 2022; 14(6):1220. https://doi.org/10.3390/nu14061220
Chicago/Turabian StylePignolo, Antonia, Sergio Mastrilli, Chiara Davì, Valentina Arnao, Paolo Aridon, Felipe Augusto dos Santos Mendes, Cesare Gagliardo, and Marco D’Amelio. 2022. "Vitamin D and Parkinson’s Disease" Nutrients 14, no. 6: 1220. https://doi.org/10.3390/nu14061220








