1. Introduction
Interactions between altered gut mucosa and bacteria during HIV infection appear to contribute to chronic immune dysfunction and correlate with the route of HIV transmission [
1,
2]. However, further study is needed to understand how nutritional interventions might improve gut dysbiosis, especially during chronic diseases.
Scientific evidence has associated the more Western dietary pattern, high in saturated fatty acids, cholesterol and sugars and low in fibre and micronutrients, with various inflammatory metabolic and chronic gastrointestinal tract conditions, whereas diets rich in fibre, vitamins and antioxidants have beneficial effects on gut homeostasis by increasing microbial diversity and inducing a regulatory environment [
3,
4,
5,
6]. Although diet and dietary habits have been extensively studied in healthy people or people with different pathologies, studies referring to diet in HIV-infected patients are very scarce [
6,
7].
On the other hand, some studies have found differences in the intestinal microbiota of HIV-infected patients by transmission risk group and have suggested that these differences could be attributed to diet [
1,
8].
We aimed to analyse the dietary patterns and dietary quality of a group of 27 people living with HIV (PWH), and correlate the nutritional parameters with the gut microbiota composition and inflammatory biomarkers.
2. Materials and Methods
2.1. Study Design and Sample Collection
We conducted a cross-sectional study. Participants were recruited at the HIV unit of the Ramón y Cajal University Hospital in Madrid, Spain, between October 2017 and February 2018. Participant contact took place at the HIV unit of the hospital, where physicians informed participants about the details of the study beforehand.
The protocol was approved by the Ethics Committee of the Hospital Ramón y Cajal (Ref. 030/20) and all participants signed an informed consent form.
Stool and blood samples were collected on the same day that the dietary questionnaires were completed.
Inclusion criteria were confirmed HIV infection, age of 18 years or more, and antiretroviral therapy (ART) with undetectable plasma HIV RNA during at least 48 weeks. Exclusion criteria were failure to provide signed informed consent, acute and intercurrent health problems.
2.2. Dietary Data
A three-day dietary record [
9], including two working days and one weekend day, was used to collect all food, beverages and food supplements taken by the participant during that time period.
The questionnaire was completed by the participants. To ensure that the questionnaire was accurately completed, participants were provided with written detailed instructions on how all information was to be recorded, including food and dish ingredients (where possible), cooking techniques, brand names of products and quantities, which could be recorded in home measurements.
To minimize errors, all data were reviewed by the research group’s nutritionists to identify unrealistic servings or fluid intake or any errors in recording.
All dietary information was processed with the DIAL software version 3.0.0.12 (Alce Ingeniería, Madrid, Spain), which uses data from the Spanish Food Composition Tables [
10]. The observed energy intake, the caloric profile of the macronutrients in the participants’ diets, as well as the intake of vitamins and minerals were obtained through this programme [
11].
2.3. Gut Microbiota
2.3.1. Purification of Nucleic Acids
Faecal samples from participants were stored in Omnigene Gut kits (DNA Genotek, Kanata, ON, Canada). Faecal samples were divided into aliquots and cryopreserved at −80 °C until use.
2.3.2. Amplification of the 16 S rRNA Gene
Total DNA was extracted from faecal samples on the MagNA Pure LC Instrument robotic workstation (Roche, Basel, Switzerland) using the MagNA Pure LC III DNA isolation kit (Bacteria, Fungi) (Roche). Total DNA was quantified with a Qubit fluorometer (ThermoFisher, Waltham, MA, USA). For each sample, regions V3–V4 of the 16 S rRNA gene were amplified and amplicon libraries were constructed following Illumina instructions (Illumina, San Diego, CA, USA) [
12]. Sequencing was performed using the V3 kit (2 × 300 cycles) with MiSeq sequencer (Illumina, San Diego, CA, USA) at the Sequencing and Bioinformatics Service FISABIO, Valencia, Spain. We obtained an average of 62,939 united 16 S rRNA sequences per sample.
2.3.3. Pre-Processing and Quality Control
All sequences used in this analysis passed quality control, where the length and quality of reads were filtered using trimmomatic v0.33. To standardise the number of reads in the diversity analyses, sub-sampling methods (seqkit, sample subcommand) were used, which was performed based on the minimum number of reads per sample.
2.3.4. 16 S RNA Gene Analysis
The 16 S rRNA gene amplicon data were analysed using the Kraken taxonomic sequence classifier (v2.0.7-beta, paired-end option) [
13,
14], which examines the k-mers within a query sequence and uses the information within those k-mers to query a database. That database maps k-mers to the lowest common ancestor of all genomes known to contain a given k-mer. Taxonomic information on 16 S rDNA sequences was obtained using the Silva ribosomal RNA database (version 132) [
15] available on Kraken 2 web [
16]. After assigning taxonomic labels to the sequence reads, the Operational Taxonomic Units (OTU) table was extracted using Pavian version.
2.4. Inflammatory Biomarkers
A fasting venous blood sample was collected from each participant and plasma levels of 8 inflammatory biomarkers were determined from the cryopreserved plasma by immunoassay in triplicate. Tumour soluble necrosis factor sTNFR2 (DRT200, R & D Systems, Bio-Techne Corporation, Minneapolis, MN, USA), C-reactive protein (CRP) (DCRP00, Quantikine ELISA kit, R & D Systems, Minneapolis, MN, USA), sCD14 (AbClonal, Wuhan, China), sCD163 (AbClonal, Wuhan, China), FABP2/IFABP (Boster Biological Technology, Wuhan, China), D-dimers (Ray Biotech, Norcross, GA, USA), LTA (Abbexa, Cambridge, UK), LBP (Boster Biolo-gical Technology, Wuhan, China).
2.5. Statistical Analysis
Results are presented as mean ± standard deviation (SD) or as re-counts and proportions in the case of categorical variables. Differences between the different dietary patterns were considered statistically significant if p < 0.05. To study the normality of the different variables within the sample and within the different analysis groups, the Shapiro–Wilk test recommended for N < 50 was used. Comparison of data between dietary patterns was performed using the Student’s t-test for two independent samples, in the case of normally distributed variables, and the Mann–Whitney U-test, in the case of non-parametric variables. For nominal variables, the chi-square test (χ2) was used.
Dietary patterns were obtained by performing a K-means cluster statistical analysis, prefixing the number of clusters to 2, and standardising (z-scores) the intake variables of 14 food groups measured in total grams per day.
Spearman correlations were used to assess the relationship of the different nutritional variables with gut microbiota and inflammatory biomarkers. The statistical programme IBM SPSS Inc. Version 20.0 (Armonk, NY, USA) was used for the analysis of the results and R software (R Core Team, Vienna, Austria) for the correlation analyses between MEDDQI and bacterial counts (libraries corrplot) and for the rest graphics Orange Data Mining Version 3.30 (University of Ljubljana, Ljubljana, Slovenia) and Ms Office Profesional Plus 2016 Version 21.12 (Microsoft, Redmond, WA, USA).
2.6. MED-DQI Dietary Quality Index
To assess the dietary quality of the dietary patterns, the value of the MED-DQI dietary quality index was calculated for each pattern, and for each participant, as it is considered one of the most suitable indices for assessing the quality of the Mediterranean diet in adults [
17,
18,
19]. The calculation was made taking into account the following components: % of saturated fatty acids in relation to total energy, cholesterol (mg), grams of meat, mL of olive oil, grams of fish and grams of fruit and vegetables.
Each nutrient or food group was assigned three scores (0, 1 and 2) based on the recommended guidelines.
MED-DQI scores between 1–4 are considered good, between 5–7 medium good, between 8–10 medium poor and 11–14 poor.
4. Discussion
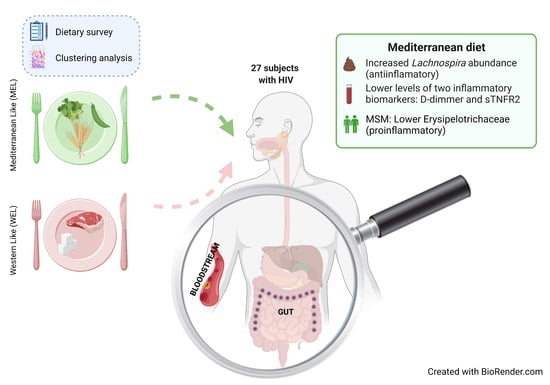
In this study in PWH, we found evidence of an intersection between dietary habits, the microbiota and systemic inflammation. We found that compared to participants with a WEL pattern, those with MEL pattern exhibited increased abundance of
Lachnospira and lower levels of D-dimers and sTNFR2, and among MSM also a lower Erysipelotrichaceae abundance and lower D-dimers. While it is now assumed that Western and Mediterranean dietary patterns and dietary fibre intake play an important role in modulating gut microbiota [
20,
21,
22,
23,
24,
25], the information in the setting of specific chronic diseases such as HIV remains scarce.
Lachnospira genus is a producer of short-chain fatty acids (SCFA), especially butyrate production, which generates energy for colonocytes, which will induce enterocyte binding proteins, promoting intestinal barrier function and favouring the increase of intestinal regulatory T cells that mitigate inflammation [
26]. Our findings regarding the association between total fibre intake and
Lachnospira abundance are in keep with previous reports outside HIV infection [
27,
28]. In our study, a higher abundance of
Lachnospira and dietary fibre intake was associated with a MEL dietary pattern, and a positive correlation was found between
Lachnospira and dietary fibre. In addition, a higher carbohydrate intake (%) was observed in the MEL versus WEL pattern, although no correlation of the latter with
Lachnospira was observed.
There was also a negative correlation between the MED-DQI with
Lachnospira and a positive correlation with
Bacteroides. This latter result is consistent with numerous published studies in the general population indicating that
Bacteroides dominated microbiota [
20,
29], that is associated with long-term dietary patterns, and associated with a higher abundance of animal proteins and fats characteristic of the Western pattern [
30,
31].
In an exploratory analysis in PWH of this study, we observed a positive correlation between Lachnospira and digestive carbohydrates like fructose and glucose and non-digestive carbohydrates like dietary fibre. We also observed a negative correlation between Bacteroides and glucose, organic acids like malic and citric acid, olive oil, and vegetables.
Furthermore, we found a lower Erysipelotrichaceae abundance in MSM with MEL pattern. Although until recently, the Erysipelotrichaceae family of bacteria was not considered to play a significant role in health and disease. However, an increasing number of studies have been published attributing a potential role in numerous diseases and inflammatory processes [
32] and linking it to a high-fat Western diet [
33]. Notably, one study found that the relative abundance of
Erysipelotrichix, a bacterium belonging to the Erysipelotrichaceae family, correlated positively with levels sTNFR2 [
32] in PWH on ART. Other studies in animal models showed a positive correlation between against the abundance of Erysipelotrichaceae levels and cholesterol metabolites [
34,
35] and that supplementation with antioxidants (quercetin), which are typically abundant in the MEL pattern, inhibited the growth of against the abundance of Erysipelotrichaceae [
36].
In an exploratory analysis restricted to MSM in this study we observed a negative correlation between vitamin E, zeaxanthin and fish consumption with Erysipelotrichaceae abundance and a positive correlation with meat. For the case of MSM with MEL we observed there is a negative correlation with fish and eicosapentaonic acid (EPA) w-3 fatty acids found in oily fish with anti-inflammatory action and is clinically relevant beneficial [
37,
38] and with the carotenoide zeanxanthin. MSM with WEL pattern showed a strong negative correlation of Erysipelotrichaceae with antioxidants intake like vitamin E, dietary fibre and fruit consumption.
Importantly, we found differences in the inflammatory biomarkers D-dimmer and sTNFR2 among PWH according to the dietary pattern followed, with levels in MEL being much lower than in WEL. sTNFR2 is a proinflammatory cytokine that is used as a marker of atherogenesis and is increased in PWH despite effective ART [
37], as well as in other pro-inflammatory diseases [
39,
40,
41]. D-dimer is a marker of fibrin degradation and its levels are often increased in PWH and denote a pre-thrombotic state that may lead to clinical thrombosis [
42]. Importantly, higher levels of inflammatory biomarkers during treated HIV have consistently been linked to an excess risk of comorbidities during HIV treatment and suggested as a contributing risk factor [
43]. As chronic inflammation has become a stubbornly elusive target in HIV, pursuing the effect of dietary interventions deserve further investigations. Our findings linking the MEL pattern with microbiome shifts and lower inflammation provide further support to recent intervention studies showing that adherence to the Mediterranean diet or particular components such as extra virgin olive oil is a feasible intervention to mitigate chronic inflammation in PWH [
44,
45,
46].
Our study also has several limitations that must be taken into considerations when interpreting our results. First, we only assessed a limited number of participants, so we might have lacked statistical power to detect differences between groups in some analyses. Second, the observational design prevented us to assess causal relationships. However, in the context of the current state of the art, we can safely assume that dietary changes should explain the differences in the microbiome composition and inflammatory biomarkers, instead of the opposite.
5. Conclusions
In this observational study in PWH, compared to WEL pattern, MEL pattern was characterised by a higher intake of vegetables, cereals and legumes and a high intake of fibre, as well as a lower intake of sugars, meat and potatoes. MEL pattern determined higher Lachnospira abundance and a better score on the MED-DQI food quality index, which correlated directly with Lachnospira abundance and indirectly with Bacteroides abundance. In addition, MSM with MEL pattern exhibited lower abundance of Erysipelotrichaceae than MSM with a WEL pattern, a family of bacteria implicated in numerous inflammatory processes and which is more prevalent in Western diets characterised by a high intake of saturated fat and cholesterol and a low intake of dietary fibre, vitamins and antioxidants. Finally, MEL pattern was associated with decreased levels of D-dimer and sTNFR2, suggesting beneficial effects on long-term clinical outcomes.
Our study supports the notion that the dietary interventions should be further pursued as a feasible target to decrease inflammation in treated HIV. Larger studies with larger sample sizes assessing dietary interventions are justified to further inform the best strategies to improve chronic inflammation in PWH.