Milk Intake in Early Life and Later Cancer Risk: A Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Search
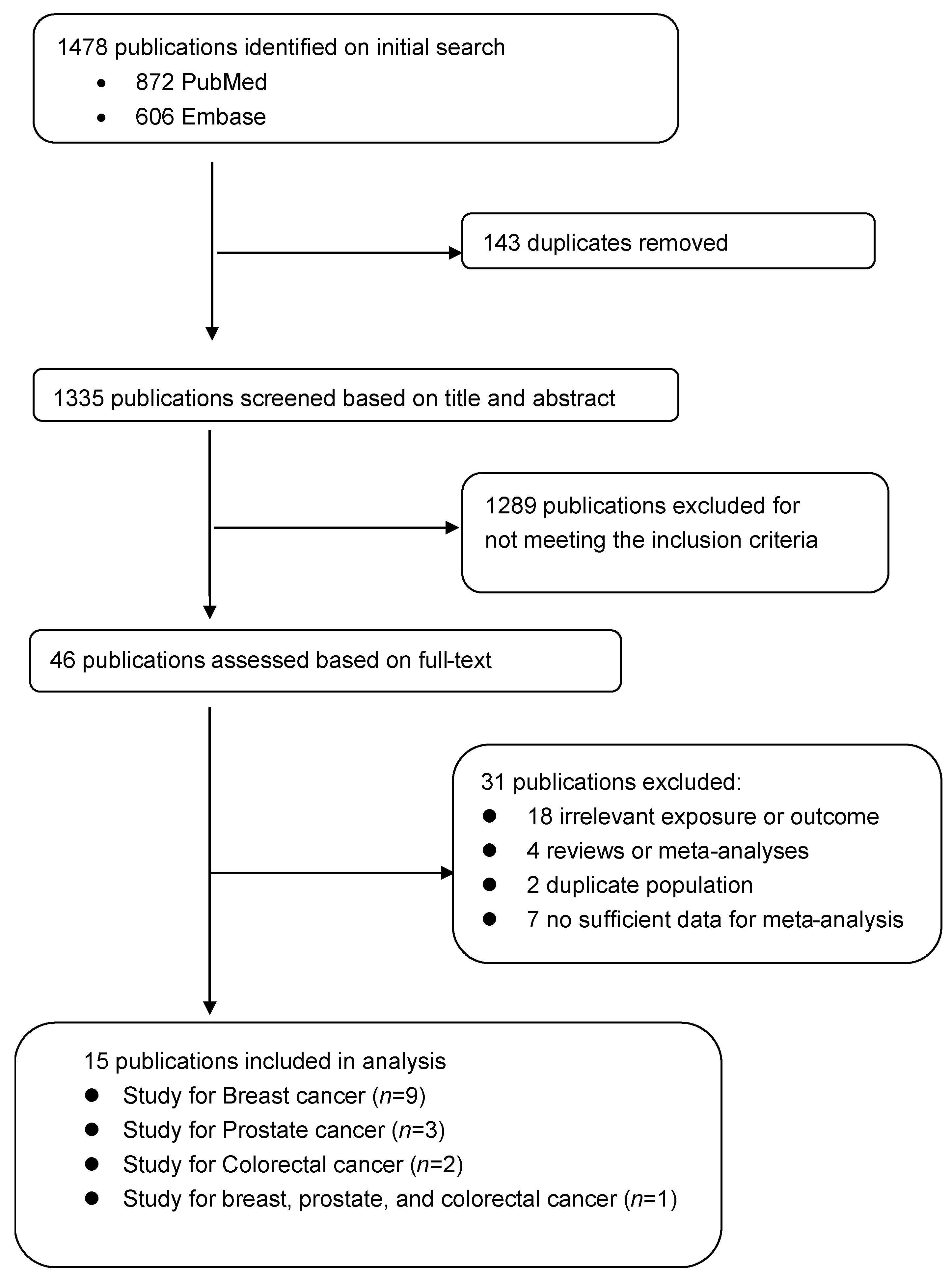
2.2. Study Selection
2.3. Data Abstraction
2.4. Statistical Analyses
3. Results
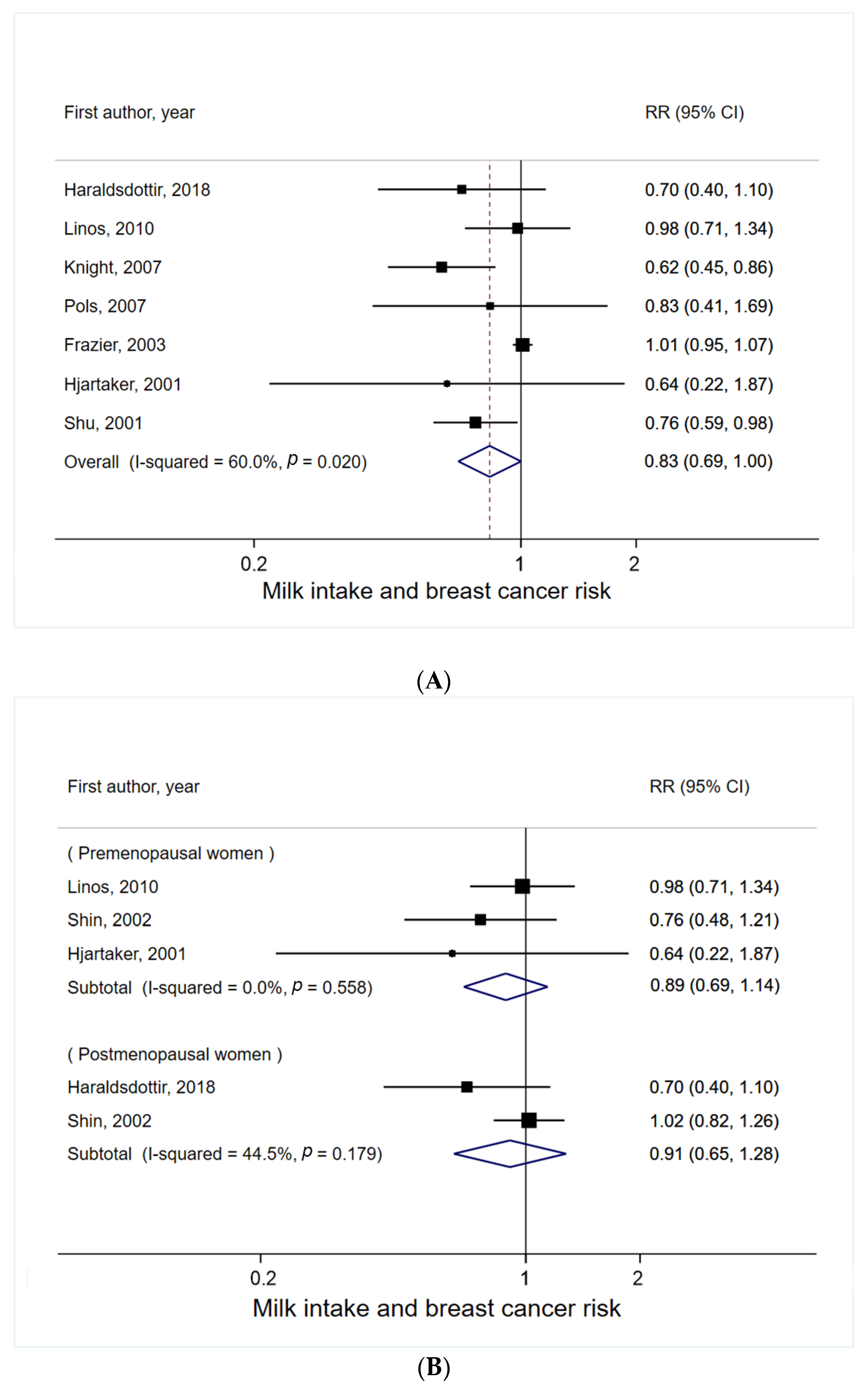
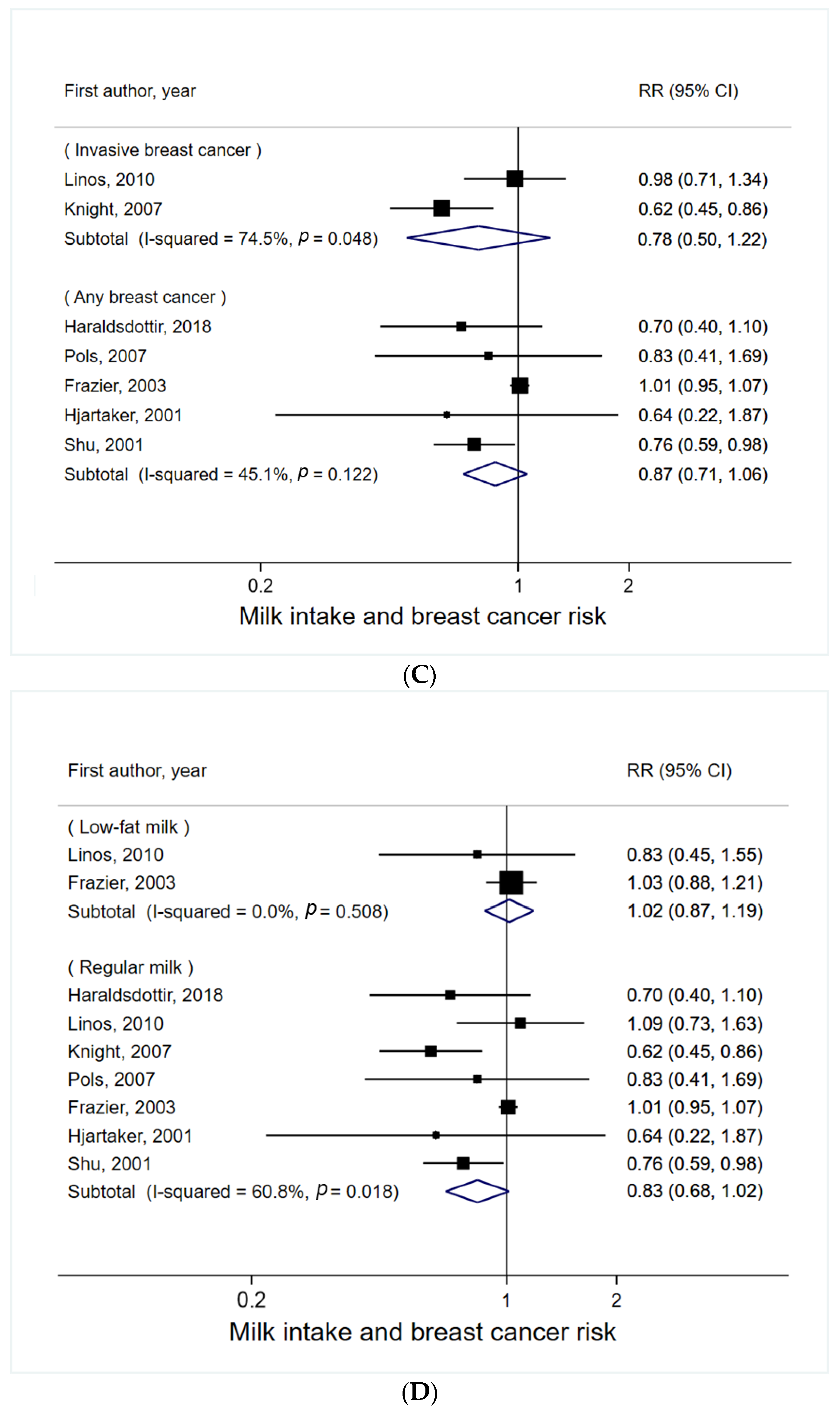
3.1. Milk Intake and Breast Cancer Risk
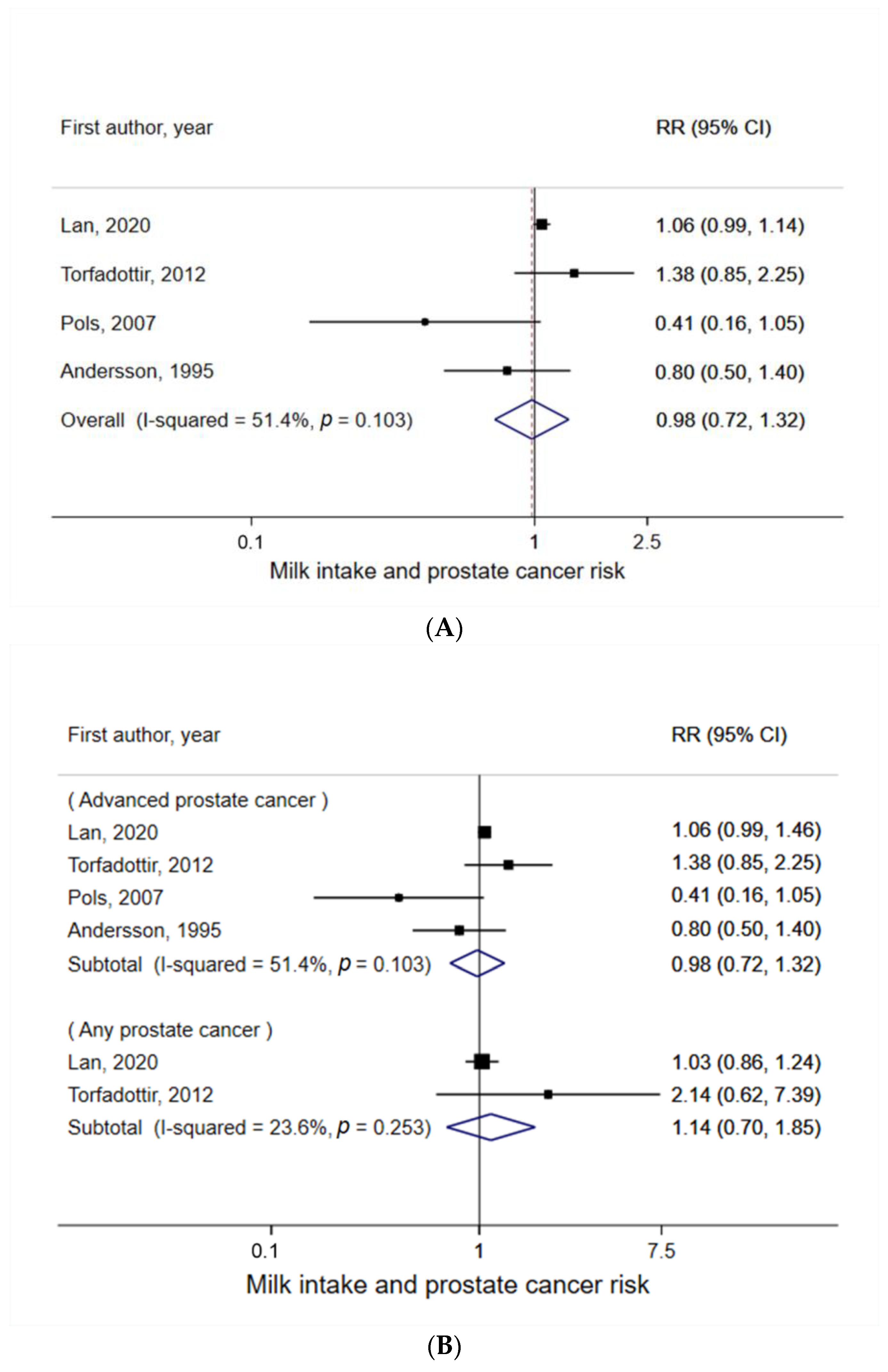
3.2. Milk Intake and Prostate Cancer Risk
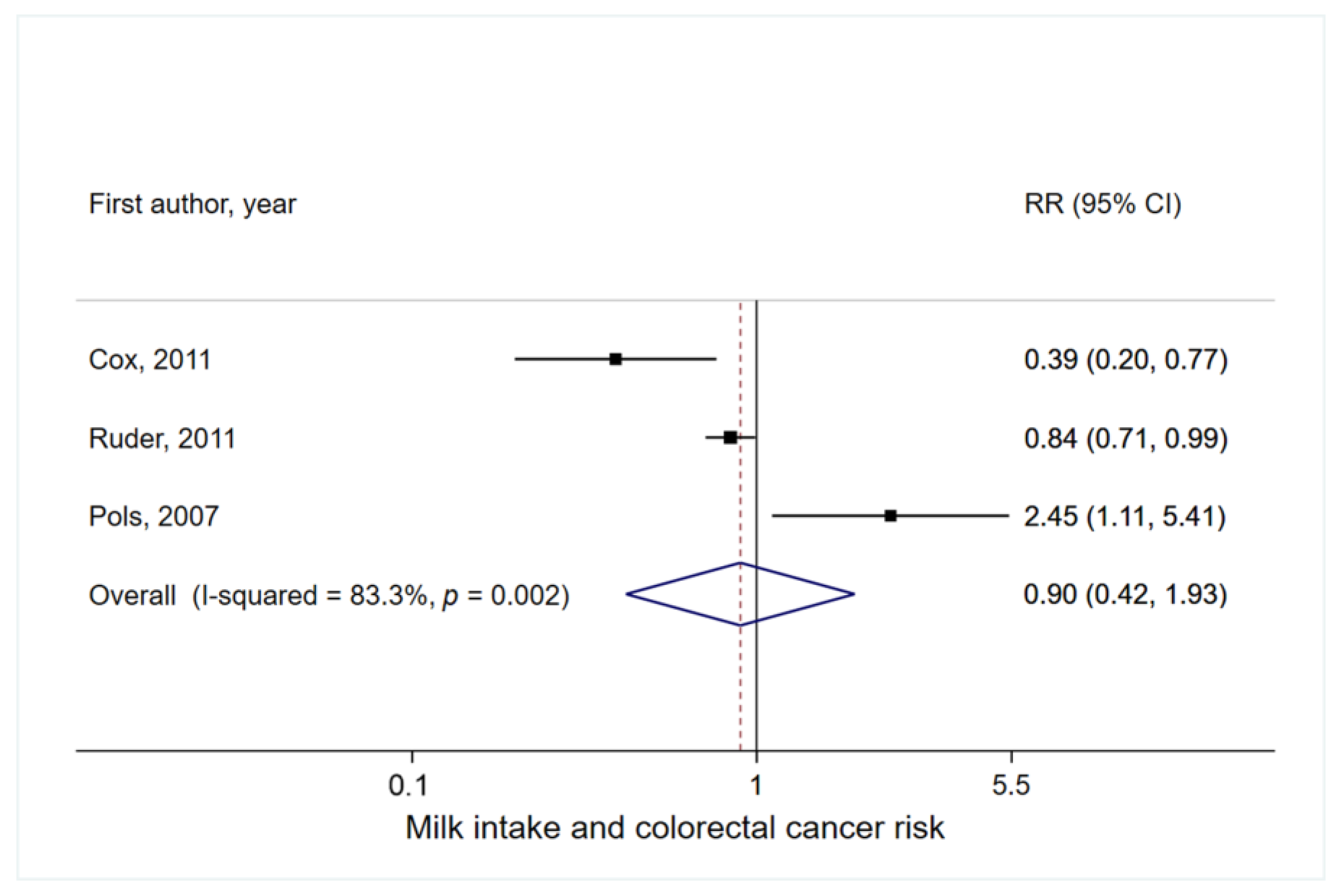
3.3. Milk Intake and Colorectal Cancer Risk
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- McCray, T.; Pacheco, J.V.; Loitz, C.C.; Garcia, J.; Baumann, B.; Schlicht, M.J.; Valyi-Nagy, K.; Abern, M.R.; Nonn, L. Vitamin D sufficiency enhances differentiation of patient-derived prostate epithelial organoids. Iscience 2020, 24, 101974. [Google Scholar] [CrossRef] [PubMed]
- Van der Pols, J.C.; Bain, C.; Gunnell, D.; Davey Smith, G.; Frobisher, C.; Martin, R.M. Childhood dairy intake and adult cancer risk: 65-y follow-up of the Boyd Orr cohort. Am. J. Clin. Nutr. 2007, 86, 1722–1729. [Google Scholar] [CrossRef] [PubMed]
- Keum, N.; Giovannucci, E. Global burden of colorectal cancer: Emerging trends, risk factors and prevention strategies. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 713–732. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Giovannucci, E.; Pollak, M.; Chan, J.; Gaziano, J.M.; Willett, W.; Stampfer, M.J. Milk intake, circulating levels of insulin-like growth factor-I, and risk of colorectal cancer in men. JNCI J. Natl. Cancer Inst. 2001, 93, 1330–1336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ventura, E.R.; Konigorski, S.; Rohrmann, S.; Schneider, H.; Stalla, G.K.; Pischon, T.; Linseisen, J.; Nimptsch, K. Association of dietary intake of milk and dairy products with blood concentrations of insulin-like growth factor 1 (IGF-1) in Bavarian adults. Eur. J. Nutr. 2019, 59, 1413–1420. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, X.; Xu, Y.; Yang, J.; Du, L.; Li, K.; Zhou, Y. Milk consumption and multiple health outcomes: Umbrella review of systematic reviews and meta-analyses in humans. Nutr. Metab. 2021, 18, 7. [Google Scholar] [CrossRef]
- Chen, L.; Li, M.; Li, H. Milk and yogurt intake and breast cancer risk: A meta-analysis. Medicine 2019, 98, e14900. [Google Scholar] [CrossRef] [PubMed]
- Thorning, T.K.; Raben, A.; Tholstrup, T.; Soedamah-Muthu, S.S.; Givens, I.; Astrup, A. Milk and dairy products: Good or bad for human health? An assessment of the totality of scientific evidence. Food Nutr. Res. 2016, 60, 32527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jennan, A.; Phillips, P.; Faaohn, R.N. Dietary_Guidelines_for_Americans-2020–2025, 9th ed.; U.S. Department of Agriculture: Washington, DC, USA; U.S. Department of Health and Human Services: Washington, DC, USA, 2020; pp. 74–81.
- Best, O.; Ban, S. Adolescence: Physical changes and neurological development. Br. J. Nurs. 2021, 30, 272–275. [Google Scholar] [CrossRef]
- Michels, K.B.; Rosner, B.A.; Chumlea, W.C.; Colditz, G.; Willett, W.C. Preschool diet and adult risk of breast cancer. Int. J. Cancer 2006, 118, 749–754. [Google Scholar] [CrossRef] [PubMed]
- Herber, C.; Bogler, L.; Subramanian, S.V.; Vollmer, S. Association between milk consumption and child growth for children aged 6–59 months. Sci. Rep. 2020, 10, 6730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andersson, O.S.; Baron, J.; Wolk, A.; Lindgren, C.; Bergström, R.; Adami, O.H. Early life risk factors for prostate cancer: A population-based case-control study in Sweden. Cancer Epidemiol. Biomark. Prev. 1995, 4, 187–192. [Google Scholar]
- Lan, T.; Park, Y.; Colditz, G.; Liu, J.; Wang, M.; Wu, K.; Giovannucci, E.; Sutcliffe, S. Adolescent dairy product and calcium intake in relation to later prostate cancer risk and mortality in the NIH-AARP Diet and Health Study. Cancer Causes Control 2020, 31, 891–904. [Google Scholar] [CrossRef] [PubMed]
- Torfadottir, J.E.; Steingrimsdottir, L.; Mucci, L.; Aspelund, T.; Kasperzyk, J.L.; Olafsson, O.; Fall, K.; Tryggvadottir, L.; Harris, T.B.; Launer, L.; et al. Milk Intake in Early Life and Risk of Advanced Prostate Cancer. Am. J. Epidemiol. 2011, 175, 144–153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cox, B.; Sneyd, M.J. School Milk and Risk of Colorectal Cancer: A National Case-Control Study. Am. J. Epidemiol. 2011, 173, 394–403. [Google Scholar] [CrossRef] [Green Version]
- Nimptsch, K.; Lee, D.H.; Zhang, X.; Song, M.; Farvid, M.S.; Rezende, L.F.M.; Cao, Y.; Chan, A.T.; Fuchs, C.; Meyerhardt, J.; et al. Dairy intake during adolescence and risk of colorectal adenoma later in life. Br. J. Cancer 2021, 124, 1160–1168. [Google Scholar] [CrossRef]
- Ruder, E.H.; Thiébaut, A.C.M.; Thompson, E.F.; Potischman, N.; Subar, A.F.; Park, Y.; Graubard, I.B.; Hollenbeck, A.R.; Cross, A.J. Adolescent and mid-life diet: Risk of colorectal cancer in the NIH-AARP Diet and Health Study. Am. J. Clin. Nutr. 2011, 94, 1607–1619. [Google Scholar] [CrossRef] [PubMed]
- Frazier, A.L.; Ryan, C.T.; Rockett, H.; Willett, W.C.; Colditz, G. Adolescent diet and risk of breast cancer. Breast Cancer Res. 2003, 5, R59–R64. [Google Scholar] [CrossRef] [Green Version]
- Shin, M.-H.; Holmes, M.D.; Hankinson, S.E.; Wu, K.; Colditz, G.A.; Willett, W.C. Intake of dairy products, calcium, and vitamin d and risk of breast cancer. JNCI J. Natl. Cancer Inst. 2002, 94, 1301–1310. [Google Scholar] [CrossRef] [Green Version]
- Farvid, M.S.; Eliassen, A.H.; Cho, E.; Chen, W.Y.; Willett, W.C. Dairy Consumption in Adolescence and Early Adulthood and Risk of Breast Cancer. Cancer Epidemiol. Biomark. Prev. 2018, 27, 575–584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Linos, E.; Willett, W.C.; Cho, E.; Frazier, L. Adolescent Diet in Relation to Breast Cancer Risk among Premenopausal Women. Cancer Epidemiol. Biomark. Prev. 2010, 19, 689–696. [Google Scholar] [CrossRef] [Green Version]
- Laake, P.; Lund, E. Childhood and adult milk consumption and risk of premenopausal breast cancer in a cohort of 48,844 women? The Norwegian women and cancer study. Int. J. Cancer 2001, 93, 888–893. [Google Scholar] [CrossRef]
- Potischman, N.; Swanson, C.A.; Hoover, R.N.; Brinton, L.A.; Weiss, H.A.; Coates, R.J.; Gammon, M.D.; Brogan, D.; Malone, K.E.; Stanford, J.L. Diet during adolescence and risk of breast cancer among young women. JNCI J. Natl. Cancer Inst. 1998, 90, 226–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haraldsdottir, A.; Torfadottir, J.E.; Valdimarsdottir, U.A.; Adami, H.O.; Aspelund, T.; Tryggvadottir, L.; Thordardottir, M.; Birgisdottir, B.E.; Harris, T.B.; Launer, L.J.; et al. Dietary habits in adolescence and midlife and risk of breast cancer in older women. PLoS ONE 2018, 13, e0198017. [Google Scholar]
- Knight, J.A.; Lesosky, M.; Barnett, H.; Raboud, J.M.; Vieth, R. Vitamin D and Reduced Risk of Breast Cancer: A Population-Based Case-Control Study. Cancer Epidemiol. Biomark. Prev. 2007, 16, 422–429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shu, O.X.; Jin, F.; Dai, Q.; Wen, W.; Potter, J.; Kushi, L.; Ruan, Z.; Gao, Y.T.; Zheng, W. Soyfood intake during adolescence and subsequent risk of breast cancer among Chinese women. Cancer Epidemiol. Biomark. Prev. 2001, 10, 483–488. [Google Scholar]
- Stroup, D.F.; Berlin, J.A.; Morton, S.C.; Olkin, I.; Williamson, G.D.; Rennie, D.; Moher, D.; Becker, B.J.; Sipe, T.A.; Thacker, S.B. Meta-analysis of observational studies in epidemiology: A proposal for reporting. Meta-analysis of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000, 283, 2008–2012. [Google Scholar] [CrossRef] [PubMed]
- Frazier, A.L.; Li, L.; Cho, E.; Willett, W.C.; Colditz, G.A. Adolescent diet and risk of breast cancer. Cancer Causes Control 2004, 15, 73–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- White, I.R. Multivariate Random-effects Meta-analysis. Stata J. Promot. Commun. Stat. Stata 2009, 9, 40–56. [Google Scholar] [CrossRef] [Green Version]
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, L.; Chu, H. Quantifying publication bias in meta-analysis. Biometrics 2018, 74, 785–794. [Google Scholar] [CrossRef] [PubMed]
- Peters, J.L.; Sutton, A.J.; Jones, D.R.; Abrams, K.R.; Rushton, L. Contour-enhanced meta-analysis funnel plots help distinguish publication bias from other causes of asymmetry. J. Clin. Epidemiol. 2008, 61, 991–996. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grimberg, A. Mechanisms by which IGF-I may promote cancer. Cancer Biol. Ther. 2003, 2, 630–635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srinivasan, V.; Nimptsch, K.; Rohrmann, S. Associations of Current, Childhood, and Adolescent Milk Intake with Serum Insulin-like Growth Factor (IGF)-1 and IGF Binding Protein 3 Concentrations in Adulthood. Nutr. Cancer 2019, 71, 931–938. [Google Scholar] [CrossRef] [PubMed]
- Zang, J.; Shen, M.; Du, S.; Chen, T.; Zou, S. The Association between Dairy Intake and Breast Cancer in Western and Asian Populations: A Systematic Review and Meta-Analysis. J. Breast Cancer 2015, 18, 313–322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bissell, M.J.; Hall, H.; Parry, G. How does the extracellular matrix direct gene expression? J. Theor. Biol. 1982, 99, 31–68. [Google Scholar] [CrossRef]
- Streuli, C.H.; Bailey, N.; Bissell, M.J. Control of mammary epithelial differentiation: Basement membrane induces tis-sue-specific gene expression in the absence of cell-cell interaction and morphological polarity. J. Cell Biol. 1991, 115, 1383–1395. [Google Scholar] [CrossRef] [Green Version]
- Higgins, P.J. Antigenic and cyto-architectural ‘markers’ of differentiation pathways in normal and malignant colon epithelial cells. In Cell and Molecular Biology of Colon Cancer; Augenlicht, L.H., Ed.; CRC Press: Boca Raton, FL, USA, 1988; pp. 111–147. [Google Scholar]
- Xue, L.; Lipkin, M.; Newmark, H.; Wang, J. Influence of dietary calcium and vitamin D on diet-induced epithelial cell hyperproliferation in mice. JNCI J. Natl. Cancer Inst. 1999, 91, 176–181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atoum, M.; Alzoughool, F. Vitamin D and Breast Cancer: Latest Evidence and Future Steps. Breast Cancer Basic Clin. Res. 2017, 11, 1178223417749816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rautiainen, S.; Gaziano, J.M.; Christen, W.G.; Bubes, V.; Kotler, G.; Glynn, R.J.; Manson, J.E.; Buring, J.E.; Sesso, H.D. Effect of Baseline Nutritional Status on Long-term Multivitamin Use and Cardiovascular Disease Risk: A Secondary Analysis of the Physicians’ Health Study II Randomized Clinical Trial. JAMA Cardiol. 2017, 2, 617–625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clinton, S.K.; Giovannucci, E.L.; Hursting, S.D. The World Cancer Research Fund/American Institute for Cancer Research Third Expert Report on Diet, Nutrition, Physical Activity, and Cancer: Impact and Future Directions. J. Nutr. 2019, 150, 663–671. [Google Scholar] [CrossRef] [PubMed]
- Melnik, B.C.; Schmitz, G. Role of insulin, insulin-like growth factor-1, hyperglycaemic food and milk consumption in the pathogenesis of acne vulgaris. Exp. Dermatol. 2009, 18, 833–841. [Google Scholar] [CrossRef] [Green Version]
- Rowlands, M.A.; Gunnell, D.; Harris, R.; Vatten, L.J.; Holly, J.M.; Martin, R.M. Circulating insulin-like growth factor peptides and prostate cancer risk: A systematic review and meta-analysis. Int. J. Cancer 2009, 124, 2416–2419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Sebastiano, K.M.; Mourtzakis, M. The Role of Dietary Fat throughout the Prostate Cancer Trajectory. Nutrients 2014, 6, 6095–6109. [Google Scholar] [CrossRef] [PubMed]
- Benmoussa, A.; Laugier, J.; Beauparlant, C.J.; Lambert, M.; Droit, A.; Provost, P. Complexity of the microRNA transcriptome of cow milk and milk-derived extracellular vesicles isolated via differential ultracentrifugation. J. Dairy Sci. 2020, 103, 16–29. [Google Scholar] [CrossRef]
- Melnik, B.C. Milk disrupts p53 and DNMT1, the guardians of the genome: Implications for acne vulgaris and prostate cancer. Nutr. Metab. 2017, 14, 55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aune, D.; Rosenblatt, D.A.N.; Chan, D.S.M.; Vieira, A.R.; Vieira, R.; Greenwood, D.C.; Vatten, L.J.; Norat, T. Dairy products, calcium, and prostate cancer risk: A systematic review and meta-analysis of cohort studies. Am. J. Clin. Nutr. 2014, 101, 87–117. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gil, H.; Chen, Q.-Y.; Khil, J.; Park, J.; Na, G.; Lee, D.; Keum, N. Milk Intake in Early Life and Later Cancer Risk: A Meta-Analysis. Nutrients 2022, 14, 1233. https://doi.org/10.3390/nu14061233
Gil H, Chen Q-Y, Khil J, Park J, Na G, Lee D, Keum N. Milk Intake in Early Life and Later Cancer Risk: A Meta-Analysis. Nutrients. 2022; 14(6):1233. https://doi.org/10.3390/nu14061233
Chicago/Turabian StyleGil, Hyeonmin, Qiao-Yi Chen, Jaewon Khil, Jihyun Park, Gyumi Na, Donghoon Lee, and Nana Keum. 2022. "Milk Intake in Early Life and Later Cancer Risk: A Meta-Analysis" Nutrients 14, no. 6: 1233. https://doi.org/10.3390/nu14061233
APA StyleGil, H., Chen, Q.-Y., Khil, J., Park, J., Na, G., Lee, D., & Keum, N. (2022). Milk Intake in Early Life and Later Cancer Risk: A Meta-Analysis. Nutrients, 14(6), 1233. https://doi.org/10.3390/nu14061233






