Association of Frailty Status and Dietary Patterns in a Nationally Representative Sample of United States Adults with Olfactory Dysfunction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Population
2.2. Olfactory Assessment
2.3. Dietary Recall
2.4. Frailty
2.5. Statistical Analysis
3. Results
3.1. Participant Demographics
3.2. Dietary Patterns and Olfaction
3.3. Dietary Patterns and Frailty: Bivariate Analysis
3.4. Dietary Patterns and Frailty: Multivariate Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Murphy, C.; Schubert, C.R.; Cruickshanks, K.J.; Klein, B.E.; Klein, R.; Nondahl, D.M. Prevalence of olfactory impairment in older adults. JAMA 2002, 288, 2307–2312. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, I.A.; Roxbury, C.R.; Lin, S.Y.; Rowan, N.R. The association of frailty with olfactory and gustatory dysfunction in older adults: A nationally representative sample. Int. Forum Allergy Rhinol. 2021, 11, 866–876. [Google Scholar] [CrossRef]
- Devanand, D.P.; Lee, S.; Manly, J.; Andrews, H.; Schupf, N.; Masurkar, A.; Stern, Y.; Mayeux, R.; Doty, R.L. Olfactory identification deficits and increased mortality in the community. Ann. Neurol. 2015, 78, 401–411. [Google Scholar] [CrossRef] [PubMed]
- Ekstrom, I.; Sjolund, S.; Nordin, S.; Adolfsson, A.N.; Adolfsson, R.; Nilsson, L.-G.; Larsson, M.; Olofsson, J.K. Smell Loss Predicts Mortality Risk Regardless of Dementia Conversion. J. Am. Geriatr. Soc. 2017, 65, 1238–1243. [Google Scholar] [CrossRef]
- Pinto, J.M.; Wroblewski, K.E.; Kern, D.W.; Schumm, L.P.; McClintock, M.K. Olfactory dysfunction predicts 5-year mortality in older adults. PLoS ONE 2014, 9, e107541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harita, M.; Miwa, T.; Shiga, H.; Yamada, K.; Sugiyama, E.; Okabe, Y.; Miyake, Y.; Okuno, T.; Iritani, O.; Morimoto, S. Association of olfactory impairment with indexes of sarcopenia and frailty in community-dwelling older adults. Geriatr. Gerontol. Int. 2019, 19, 384–391. [Google Scholar] [CrossRef]
- Laudisio, A.; Navarini, L.; Margiotta, D.P.E.; Fontana, D.O.; Chiarella, I.; Spitaleri, D.; Bandinelli, S.; Gemma, A.; Ferrucci, L.; Incalzi, R.A. The Association of Olfactory Dysfunction, Frailty, and Mortality Is Mediated by Inflammation: Results from the InCHIANTI Study. J. Immunol. Res. 2019, 2019, 3128231. [Google Scholar] [CrossRef]
- Yuan, Y.; Li, C.; Luo, Z.; Simonsick, E.M.; Shiroma, E.J.; Chen, H. Olfaction and Physical Functioning in Older Adults: A Longitudinal Study. J. Gerontol. A Biol. Sci. Med. Sci 2021. Epub Ahead of Print. [Google Scholar] [CrossRef]
- Chen, X.; Mao, G.; Leng, S.X. Frailty syndrome: An overview. Clin. Interv. Aging 2014, 9, 433–441. [Google Scholar] [CrossRef] [Green Version]
- Han, L.; Clegg, A.; Doran, T.; Fraser, L. The impact of frailty on healthcare resource use: A longitudinal analysis using the Clinical Practice Research Datalink in England. Age Ageing 2019, 48, 665–671. [Google Scholar] [CrossRef]
- O’Caoimh, R.; Sezgin, D.; O’Donovan, M.R.; Molloy, D.W.; Clegg, A.; Rockwood, K.; Liew, A. Prevalence of frailty in 62 countries across the world: A systematic review and meta-analysis of population-level studies. Age Ageing 2021, 50, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, W.J.; et al. Frailty in older adults: Evidence for a phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, M146–M156. [Google Scholar] [CrossRef] [PubMed]
- Blodgett, J.; Theou, O.; Kirkland, S.; Andreou, P.; Rockwood, K. Frailty in NHANES: Comparing the frailty index and phenotype. Arch. Gerontol. Geriatr. 2015, 60, 464–470. [Google Scholar] [CrossRef] [PubMed]
- Xue, Q.L.; Tian, J.; Walston, J.D.; Chaves, P.H.M.; Newman, A.B.; Bandeen-Roche, K. Discrepancy in Frailty Identification: Move Beyond Predictive Validity. J. Gerontol. A Biol. Sci. Med. Sci. 2020, 75, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Garrison, J.L.; Knight, Z.A. Linking smell to metabolism and aging. Science 2017, 358, 718–719. [Google Scholar] [CrossRef]
- Zoon, H.F.; de Graaf, C.; Boesveldt, S. Food Odours Direct Specific Appetite. Foods 2016, 5, 12. [Google Scholar] [CrossRef]
- Kong, I.G.; Kim, S.Y.; Kim, M.S.; Park, B.; Kim, J.H.; Choi, H.G. Olfactory Dysfunction Is Associated with the Intake of Macronutrients in Korean Adults. PLoS ONE 2016, 11, e0164495. [Google Scholar] [CrossRef]
- Lowik, M.R.; Hulshof, K.F.; Brussaard, J.H. Patterns of food and nutrient intakes of Dutch adults according to intakes of total fat, saturated fatty acids, dietary fibre, and of fruit and vegetables. Br. J. Nutr. 1999, 81, S91–S98. [Google Scholar] [CrossRef] [Green Version]
- Roxbury, C.R.; Bernstein, I.A.; Lin, S.Y.; Rowan, N.R. Association between Chemosensory Dysfunction and Diet Quality in United States Adults. Am. J. Rhinol. Allergy 2021, 36, 47–56. [Google Scholar] [CrossRef]
- Bonnefoy, M.; Berrut, G.; Lesourd, B.; Ferry, M.; Gilbert, T.; Guerin, O.; Hanon, O.; Jeandel, C.; Paillauf, E.; Raynaud-Simon, A.; et al. Frailty and nutrition: Searching for evidence. J. Nutr. Health Aging 2015, 19, 250–257. [Google Scholar] [CrossRef]
- Jayanama, K.; Theou, O.; Blodgett, J.M.; Cahill, L.; Rockwood, K. Frailty, nutrition-related parameters, and mortality across the adult age spectrum. BMC Med. 2018, 16, 188. [Google Scholar] [CrossRef] [Green Version]
- Jacobs, D.R., Jr.; Gross, M.D.; Tapsell, L.C. Food synergy: An operational concept for understanding nutrition. Am. J. Clin. Nutr. 2009, 89, 1543S–1548S. [Google Scholar] [CrossRef] [PubMed]
- Schulze, M.B.; Hoffmann, K.; Kroke, A.; Boeing, H. An approach to construct simplified measures of dietay patterns from exploratory factor analysis. Br. J. Nutr. 2003, 89, 409–419. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, H.J.; Rawal, S.; Li, C.M.; Duffy, V.B. New chemosensory component in the U.S. National Health and Nutrition Examination Survey (NHANES): First-year results for measured olfactory dysfunction. Rev. Endocr. Metab. Disord. 2016, 17, 221–240. [Google Scholar] [CrossRef] [Green Version]
- Kurnat-Thoma, E.L.; Murray, M.T.; Juneau, P. Frailty and Determinants of Health among Older Adults in the United States 2011–2016. J. Aging Health 2021, In Press. 08982643211040706. [Google Scholar] [CrossRef]
- Fransen, H.P.; May, A.M.; Stricker, M.D.; Boer, J.M.A.; Hennig, C.; Rossel, Y.; Ocke, M.C.; Peeters, P.H.M.; Beulens, J.W.J. A posteriori dietary patterns: How many patterns to retain? J. Nutr. 2014, 144, 1274–1282. [Google Scholar] [CrossRef]
- Willett, W.C.; Howe, G.R.; Kushi, L.H. Adjustment for total energy intake in epidemiologic studies. Am. J. Clin. Nutr. 1997, 65, 1220S–1228S. [Google Scholar] [CrossRef]
- Beasley, J.M.; LaCroix, A.Z.; Neuhouser, M.L.; Huang, Y.; Tinker, L.; Woods, N.; Michael, Y.; Curb, J.D.; Prentice, R.L. Protein intake and incident frailty in the Women’s Health Initiative observational study. J. Am. Geriatr. Soc. 2010, 58, 1063–1071. [Google Scholar] [CrossRef] [Green Version]
- Sandoval-Insausti, H.; Perez-Tasigchana, R.F.; Lopez-Garcia, E.; Garcia-Esquinas, E.; Rodriguez-Artalejo, F.; Guallar-Castillon, P. Macronutrients Intake and Incident Frailty in Older Adults: A Prospective Cohort Study. J. Gerontol. A Biol. Sci. Med. Sci. 2016, 71, 1329–1334. [Google Scholar] [CrossRef]
- Beck, J.; Ferrucci, L.; Sun, K.; Walston, J.; Fried, L.P.; Varadhan, R.; Guralnik, J.M.; Semba, R.D. Low serum selenium concentrations are associated with poor grip strength among older women living in the community. Biofactors 2007, 29, 37–44. [Google Scholar] [CrossRef] [Green Version]
- Lauretani, F.; Semba, R.D.; Bandinelli, S.; Ray, A.L.; Guralnik, J.M.; Ferrucci, L. Association of low plasma selenium concentrations with poor muscle strength in older community-dwelling adults: The InCHIANTI Study. Am. J. Clin. Nutr. 2007, 86, 347–352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hickson, M. Nutritional interventions in sarcopenia: A critical review. Proc. Nutr. Soc. 2015, 74, 378–386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balboa-Castillo, T.; Struijk, E.A.; Lopez-Garcia, E.; Banegas, J.R.; Rodriguez-Artalejo, F.; Guallar-Castillon, P. Low vitamin intake is associated with risk of frailty in older adults. Age Ageing 2018, 47, 872–879. [Google Scholar] [CrossRef] [Green Version]
- Fletcher, J. Low intake of vitamins B6, C, E and folate from dietary sources may lead to a higher risk of developing frailty in older adults. Evid. Based Nurs. 2019, 22, 88. [Google Scholar] [CrossRef]
- Kobayashi, S.; Asakura, K.; Suga, H.; Sasaki, S. Three-generation Study of Women on Diets and Health Study Groups. Inverse association between dietary habits with high total antioxidant capacity and prevalence of frailty among elderly Japanese women: A multicenter cross-sectional study. J. Nutr. Health Aging 2014, 18, 827–839. [Google Scholar] [CrossRef] [PubMed]
- Lochlainn, M.N.; Cox, N.J.; Wilson, T.; Hayhoe, R.P.G.; Ramsay, S.E.; Granic, A.; Isanejad, M.; Roberts, H.C.; Wilson, D.; Welch, C.; et al. Nutrition and Frailty: Opportunities for Prevention and Treatment. Nutrients 2021, 13, 2349. [Google Scholar] [CrossRef] [PubMed]
- Soysal, P.; Isik, A.T.; Carvalho, A.F.; Fernandes, B.S.; Solmi, M.; Schofield, P.; Veronese, N.; Stubbs, B. Oxidative stress and frailty: A systematic review and synthesis of the best evidence. Maturitas 2017, 99, 66–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kochlik, B.; Stuetz, W.; Peres, K.; Pilleron, S.; Feart, C.; Garcia, F.J.; Bandinelli, S.; Gomez-Cambrero, D.; Rodriguez-Manas, L.; Grune, T.; et al. Associations of fat-soluble micronutrients and redox biomarkers with frailty status in the FRAILOMIC initiative. J. Cachex. Sarcopenia Muscle 2019, 10, 1339–1346. [Google Scholar] [CrossRef] [Green Version]
- Nomura, Y.; Ishii, Y.; Suzuki, S.; Morita, K.; Suzuki, A.; Suzuki, S.; Tanabe, J.; Ishiwata, Y.; Yamakawa, K.; Chiba, Y.; et al. Nutritional Status and Oral Frailty: A Community Based Study. Nutrients 2020, 12, 2886. [Google Scholar] [CrossRef]
- Dong, J.; Pinto, J.M.; Guo, X.; Alonso, A.; Tranah, G.; Cauley, J.A.; Garcia, M.; Satterfield, S.; Huang, X.; Harris, T.; et al. The Prevalence of Anosmia and Associated Factors among, U.S. Black and White Older Adults. J. Gerontol. A Biol. Sci. Med. Sci. 2017, 72, 1080–1086. [Google Scholar] [CrossRef] [Green Version]
- Hummel, T.; Whitcroft, K.L.; Rueter, G.; Haehner, A. Intranasal vitamin A is beneficial in post-infectious olfactory loss. Eur. Arch. Otorhinolaryngol. 2017, 274, 2819–2825. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Gomez, M.E.; Zapico, S.C. Frailty, Cognitive Decline, Neurodegenerative Diseases and Nutrition Interventions. Int. J. Mol. Sci. 2019, 20, 2842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kovacs, T. Mechanisms of olfactory dysfunction in aging and neurodegenerative disorders. Ageing Res. Rev. 2004, 3, 215–232. [Google Scholar] [CrossRef] [PubMed]
- Van Regemorter, V.; Hummel, T.; Rosenzweig, F.; Mouraux, A.; Rombaux, P.; Huart, C. Mechanisms Linking Olfactory Impairment and Risk of Mortality. Front. Neurosci. 2020, 14, 140. [Google Scholar] [CrossRef] [Green Version]
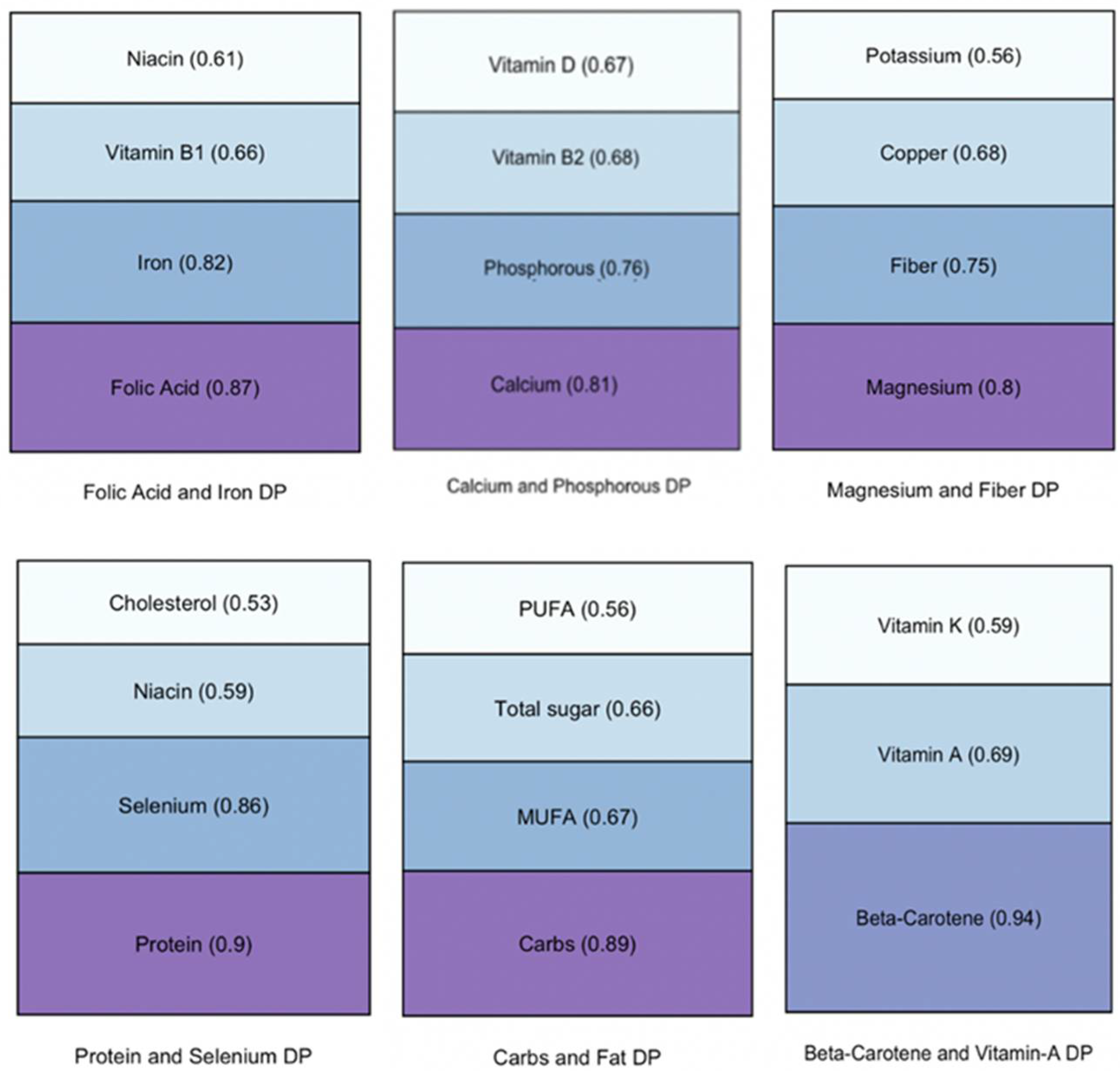
| Total (n = 350) | Frail (n = 155) | Non-Frail (n = 195) | p-Value | |
|---|---|---|---|---|
| Age | 69.6 ± 0.71 | 71.8 ± 0.89 | 68.0 ± 0.0.91 | <0.001 *** |
| Gender | <0.001 *** | |||
| Male | 203 (0.58) | 80 (0.52) | 123 (0.63) | |
| Female | 147 (0.42) | 75 (0.48) | 72 (0.37) | |
| Body mass index | 29.7 ± 0.41 | 30.5 ± 0.89 | 29.05 ± 0.4 | 0.52 |
| Race | ||||
| Non-Hispanic White | 163 (0.47) | 83 (0.54) | 80 (0.41) | 0.431 |
| Mexican American | 35 (0.1) | 16 (0.1) | 19 (0.1) | |
| Other Hispanic | 35 (0.1) | 11 (0.07) | 24 (0.12) | |
| Non-Hispanic Black | 86 (0.25) | 36 (0.23) | 50 (0.26) | |
| Non-Hispanic Asian | 27 (0.08) | 7 (0.05) | 20 (0.1) | |
| Other Race | 4 (0.01) | 2 (0.01) | 2 (0.01) | |
| Income-to-poverty ratio | 2.71 ± 0.16 | 2.36 ± 0.18 | 2.99 ± 0.22 | 0.04 * |
| Current smoker | 0.147 | |||
| No | 303 (0.87) | 132 (0.85) | 171 (0.88) | |
| Yes | 47 (0.13) | 23 (0.15) | 24 (0.12) | |
| Energy (kcal) | 1790.8 ± 46.7 | 1663.7 ± 79.3 | 1886.9 ± 47.18 | 0.017 * |
| Frailty index score | 0.21 ± 0.12 | 0.34 ± 0.013 | 0.12 ± 0.05 | <0.001 *** |
| Frail (n = 38) | Non-Frail (n = 312) | p-Value | |
|---|---|---|---|
| Age | 68.76 ± 1.58 | 69.7 ± 0.84 | 0.7 |
| Gender | 0.067 | ||
| Male | 14 (0.37) | 189 (0.61) | |
| Female | 24 (0.63) | 123 (0.39) | |
| Body mass index | 33.9 ± 1.67 | 28.52 ± 0.52 | 0.04 * |
| Race | 0.32 | ||
| Non-Hispanic White | 21 (0.55) | 142 (0.46) | |
| Mexican American | 3 (0.08) | 32 (0.1) | |
| Other Hispanic | 1 (0.03) | 34 (0.11) | |
| Non-Hispanic black | 11 (0.29) | 75 (0.24) | |
| Non-Hispanic Asian | 1 (0.03) | 26 (0.08) | |
| Other Race | 1 (0.03) | 3 (0.01) | |
| Income-to-poverty ratio | 2.35 ± 0.36 | 2.78 ± 0.2 | 0.3 |
| Current smoker | 0.057 | ||
| No | 29 (0.76) | 274 (0.88) | |
| Yes | 9 (0.24) | 38 (0.12) | |
| Energy (kcal) | 1559.83 ± 125.24 | 1821.2 ± 42.13 | 0.039 * |
| Frailty phenotype score | 0.39 ± 0.01 | 0.19 ± 0.01 | <0.001 *** |
| Model 1 a: β-Estimate [95% CI] | Model 2: β-Estimate [95% CI] | |
|---|---|---|
| DP 1 (folate and iron) | −0.19 [−0.32 to −0.07] ** | −0.11 [−0.24 to 0.01] |
| DP 2 (calcium and phosphorus) | −0.16 [−0.29 to −0.04] * | −0.103 [−0.23 to 0.02] |
| DP 3 (magnesium and fiber) | −0.25 [−0.37 to −0.12] *** | −0.16 [−0.29 to −0.04] * |
| DP 4 (protein and selenium) | −0.23 [−0.35 to −0.1] *** | −0.18 [−0.3 to −0.05] ** |
| DP 5 (carbs and fat) | −0.14 [−0.27 to −0.01] * | −0.096 [−0.22 to 0.03] |
| DP 6 (β-carotene and vitamin A) | −0.19 [−0.32 to −0.06] ** | −0.22 [−0.34 to −0.09] *** |
| Frailty Index | Physical Frailty | |||||
|---|---|---|---|---|---|---|
| Frail (n = 155) | Non-Frail (n = 195) | p-Value | Frail (n = 38) | Non-Frail (n = 312) | p-Value | |
| DP 1 (folate and iron) | 0.06 | 0.36 | ||||
| Low | 69 (0.45) | 70 (0.36) | 19 (0.5) | 120 (0.38) | ||
| Middle | 48 (0.31) | 65 (0.33) | 11 (0.29) | 102 (0.33) | ||
| High | 38 (0.25) | 60 (0.31) | 8 (0.21) | 90 (0.29) | ||
| DP 2 (calcium and phosphorous) | 0.018 * | 0.38 | ||||
| Low | 74 (0.48) | 73 (0.37) | 21 (0.55) | 126 (0.4) | ||
| Middle | 41 (0.26) | 65 (0.33) | 8 (0.21) | 98 (0.31) | ||
| High | 40 (0.26) | 57 (0.29) | 9 (0.24) | 88 (0.28) | ||
| DP 3 (magnesium and fiber) | 0.004 ** | 0.06 | ||||
| Low | 71 (0.46) | 66 (0.34) | 19 (0.5) | 118 (0.38) | ||
| Middle | 52 (0.34) | 61 (0.31) | 15 (0.39) | 98 (0.31) | ||
| High | 32 (0.21) | 68 (0.35) | 4 (0.11) | 96 (0.31) | ||
| DP 4 (protein and selenium) | <0.01 ** | 0.03 * | ||||
| Low | 88 (0.57) | 77 (0.39) | 25 (0.66) | 140 (0.45) | ||
| Middle | 35 (0.23) | 65 (0.33) | 8 (0.21) | 92 (0.29) | ||
| High | 32 (0.21) | 53 (0.27) | 5 (0.13) | 80 (0.26) | ||
| DP 5 (carbs and fat) | 0.07313 | 0.12 | ||||
| Low | 66 (0.43) | 74 (0.38) | 20 (0.53) | 120 (0.38) | ||
| Middle | 50 (0.32) | 70 (0.36) | 13 (0.34) | 107 (0.34) | ||
| High | 39 (0.25) | 51 (0.26) | 5 (0.13) | 85 (0.27) | ||
| DP 6 (β-carotene and vitamin A) | <0.001 *** | 0.06 | ||||
| Low | 77 (0.5) | 70 (0.36) | 21 (0.55) | 126 (0.4) | ||
| Middle | 44 (0.28) | 53 (0.27) | 13 (0.34) | 84 (0.27) | ||
| High | 34 (0.22) | 72 (0.37) | 4 (0.11) | 102 (0.33) | ||
| Frailty Index | Physical Frailty | |||||
|---|---|---|---|---|---|---|
| AOR | 95% CI | p-Value | AOR | 95% CI | p-Value | |
| DP 1 (folate and iron) | 0.93 | 0.88 to 0.98 | 0.07 | 0.97 | 0.81 to 1.17 | 0.79 |
| DP 2 (calcium and phosphorus) | 0.9 | 0.85 to 0.96 | 0.045 | 0.95 | 0.86 to 1.05 | 0.41 |
| DP 3 (magnesium and fiber) | 0.89 | 0.82 to 0.96 | 0.06 | 0.91 | 0.78 to 1.06 | 0.31 |
| DP 4 (protein and selenium) | 0.82 | 0.74 to 0.92 | 0.041 * | 0.76 | 0.66 to 0.88 | 0.037 * |
| DP 5 (carbs and fat) | 0.88 | 0.73 to 1.06 | 0.27 | 0.84 | 0.66 to 1.07 | 0.26 |
| DP 6 (β-carotene and vitamin A) | 0.75 | 0.65 to 0.86 | 0.027 * | 0.84 | 0.62 to 1.15 | 0.36 |
| AOR | 95% CI | p-Value | |
|---|---|---|---|
| DP 1 (folate and iron) | 0.92 | 0.83 to 1.03 | 0.23 |
| DP 2 (calcium and phosphorus) | 0.91 | 0.81 to 1.01 | 0.19 |
| DP 3 (magnesium and fiber) | 0.89 | 0.81 to 0.98 | 0.1 |
| DP 4 (protein and selenium) | 0.92 | 0.82 to 1.01 | 0.18 |
| DP 5 (carbs and fat) | 0.95 | 0.83 to 1.08 | 0.46 |
| DP 6 (β-carotene and vitamin A) | 0.76 | 0.65 to 0.90 | 0.043 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vohra, V.; Leland, E.M.; Schlosser, R.J.; Kamath, V.; Rowan, N.R. Association of Frailty Status and Dietary Patterns in a Nationally Representative Sample of United States Adults with Olfactory Dysfunction. Nutrients 2022, 14, 1238. https://doi.org/10.3390/nu14061238
Vohra V, Leland EM, Schlosser RJ, Kamath V, Rowan NR. Association of Frailty Status and Dietary Patterns in a Nationally Representative Sample of United States Adults with Olfactory Dysfunction. Nutrients. 2022; 14(6):1238. https://doi.org/10.3390/nu14061238
Chicago/Turabian StyleVohra, Varun, Evelyn M. Leland, Rodney J. Schlosser, Vidyulata Kamath, and Nicholas R. Rowan. 2022. "Association of Frailty Status and Dietary Patterns in a Nationally Representative Sample of United States Adults with Olfactory Dysfunction" Nutrients 14, no. 6: 1238. https://doi.org/10.3390/nu14061238
APA StyleVohra, V., Leland, E. M., Schlosser, R. J., Kamath, V., & Rowan, N. R. (2022). Association of Frailty Status and Dietary Patterns in a Nationally Representative Sample of United States Adults with Olfactory Dysfunction. Nutrients, 14(6), 1238. https://doi.org/10.3390/nu14061238







