Effect of Chronic Moderate Caloric Restriction on the Reproductive Function in Aged Male Wistar Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
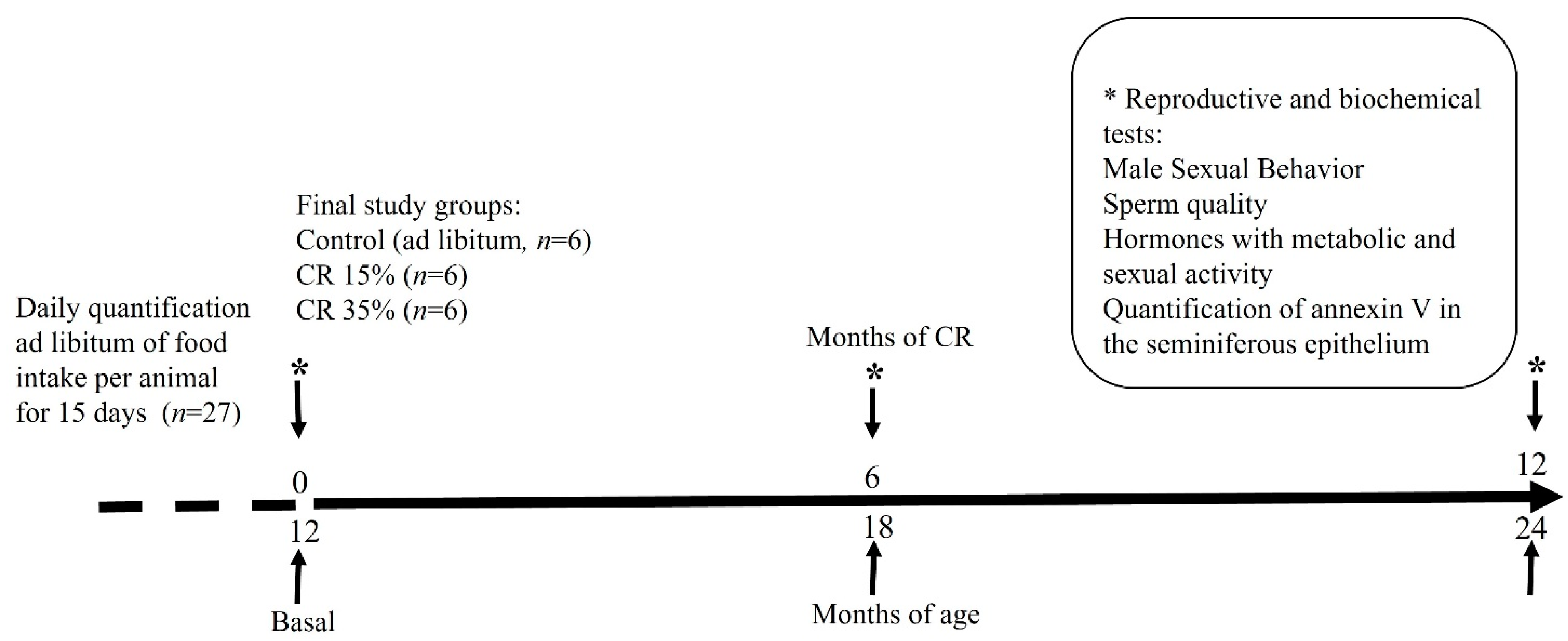
2.2. Experimental Design
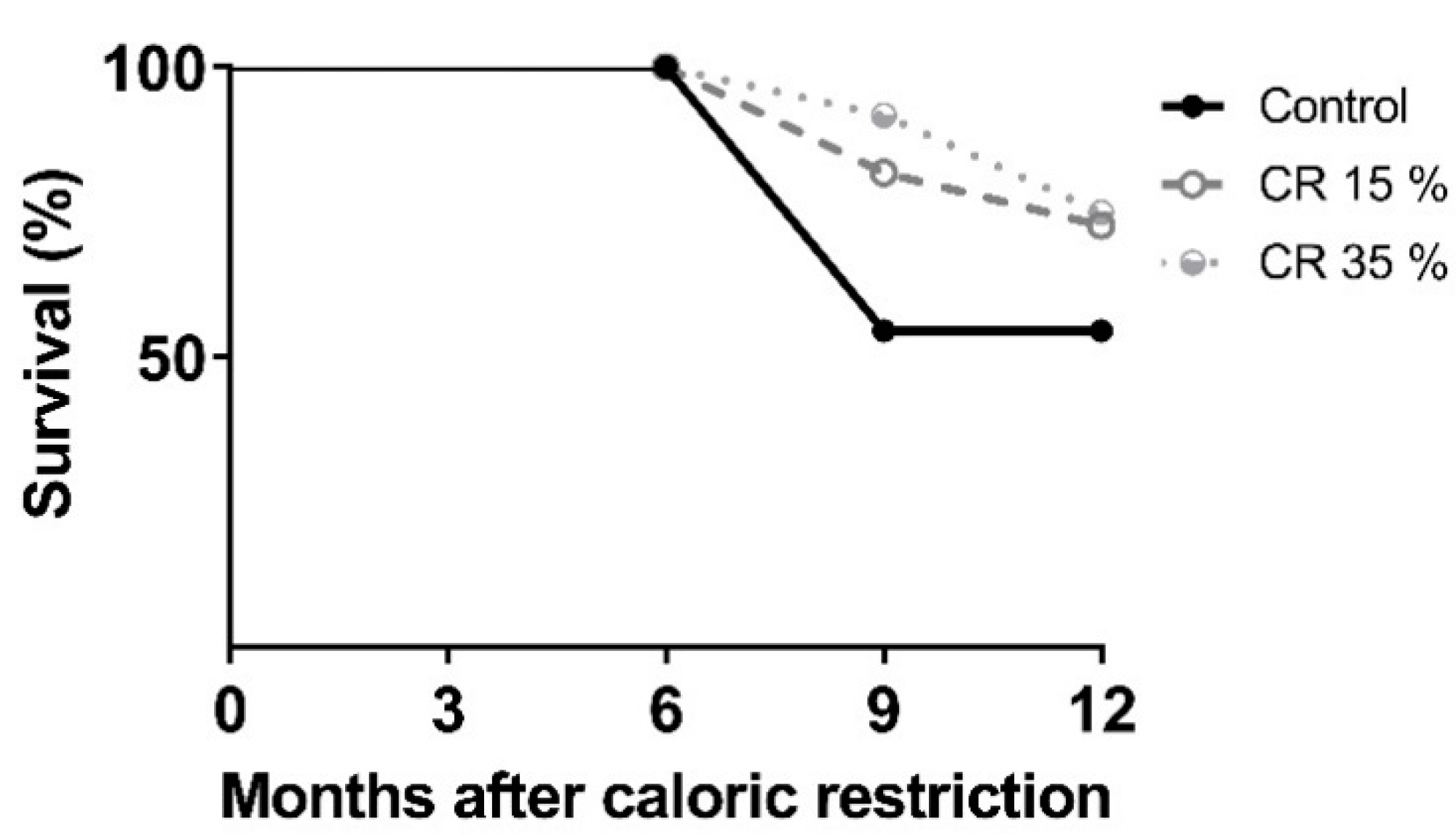
2.3. Body Weight during Caloric Restriction and Survival
2.4. Assessment of Sexual Behavior and Sperm Quality
2.5. Determination of Plasma Glucose and Hormone Levels
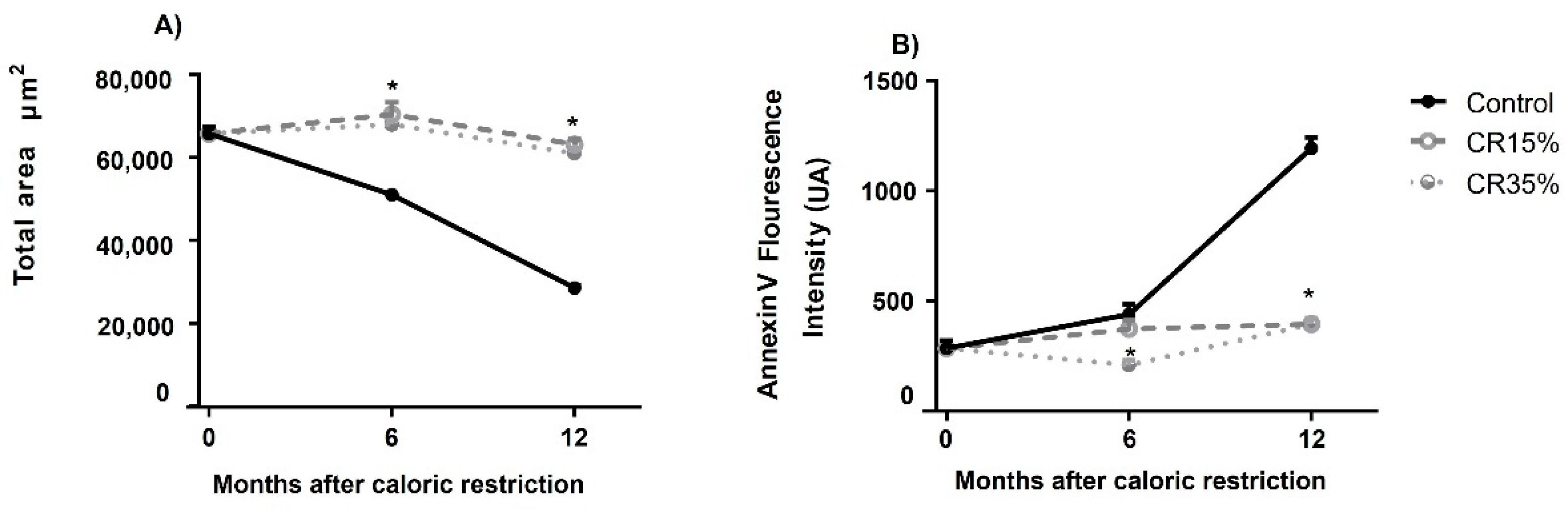
2.6. Testicular Apoptosis Analysis
2.7. Statistical Analyses
3. Results
3.1. Body Weight during Caloric Restriction and Survival
3.2. Assessment of Sexual Behavior and Sperm Quality
3.3. Sperm Quality
3.4. Metabolic Parameters
3.5. Testicular Apoptosis Measured by Annexin V Assay in Seminiferous Tubules
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Sierra, F.; Caspi, A.; Fortinsky, R.H.; Haynes, L.; Lithgow, G.J.; Moffitt, T.E.; Olshansky, S.J.; Perry, D.; Verdin, E.; Kuchel, G.A. Moving geroscience from the bench to clinical care and health policy. J. Am. Geriatr. Soc. 2021, 69, 2455–2463. [Google Scholar] [CrossRef] [PubMed]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ribarič, S. Diet and aging. Oxid. Med. Cell Longev. 2012, 2012, 741468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCay, C.M.; Maynard, L.A.; Sperling, G.; Barnes, L.L. Retarded growth, life span, ultimate body size and age changes in the albino rat after feeding diets restricted in calories. J. Nutr. 1939, 18, 1–13. [Google Scholar] [CrossRef]
- Pifferi, F.; Aujard, F. Caloric restriction, longevity and aging: Recent contributions from human and non-human primate studies. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2019, 95, 109702. [Google Scholar] [CrossRef] [PubMed]
- Weindruch, R.; Sohal, R.S. Seminars in medicine of the Beth Israel Deaconess Medical Center. Caloric intake and aging. N. Engl. J. Med. 1997, 337, 986–994. [Google Scholar] [CrossRef] [PubMed]
- Belsky, D.W.; Huffman, K.M.; Pieper, C.F.; Shalev, I.; Kraus, W.E.; Anderson, R. Change in the rate of biological aging in response to caloric restriction: Calerie biobank analysis. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2018, 73, 4–10. [Google Scholar] [CrossRef] [Green Version]
- Fontana, L.; Partridge, L. Promoting health and longevity through diet: From model organisms to humans. Cell 2015, 161, 106–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niedernhofer, L.J.; Kirkland, J.L.; Ladiges, W. Molecular pathology endpoints useful for aging studies. Ageing Res. Rev. 2017, 35, 241–249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kemnitz, J.W. Calorie restriction and aging in nonhuman primates. ILAR J. 2011, 52, 66–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marchal, J.; Perret, M.; Aujard, F. La restriction calorique chez les primates. Caloric restriction in primates: How efficient as an anti-aging approach? Med. Sci. 2012, 28, 1081–1086. [Google Scholar] [CrossRef]
- Le Bourg, E. Does Calorie Restriction in Primates Increase Lifespan? Revisiting Studies on Macaques (Macaca mulatta) and Mouse Lemurs (Microcebus murinus). Bioessays 2018, 40, e1800111. [Google Scholar] [CrossRef] [PubMed]
- Hajializadeh, Z.; Khaksari, M.; Najafipour, H.; Sanjari, M.; Mahani, F.D.; Raji-Amirhasani, A. Substitution of calorie restriction for protective effects of estrogen on cardiometabolic risk factors and oxidative stress in obese postmenopausal rat model. Life Sci. 2022, 294, 120367. [Google Scholar] [CrossRef]
- Chakraborty, A.; Banerjee, S.; Mukherjee, B.; Poddar, M.K.; Ali, N. Calorie restriction modulates neuro-immune system differently in young and aged rats. Int. Immunopharmacol. 2021, 100, 108141. [Google Scholar] [CrossRef] [PubMed]
- Saito, K.; Ito, M.; Chiba, T.; Jia, H.; Kato, H. A Comparison of Gene Expression Profiles of Rat Tissues after Mild and Short-Term Calorie Restrictions. Nutrients 2021, 13, 2277. [Google Scholar] [CrossRef] [PubMed]
- Senesi, P.; Luzi, L.; Terruzzi, I. Adipokines, Myokines, and Cardiokines: The Role of Nutritional Interventions. Int. J. Mol. Sci. 2020, 21, 8372. [Google Scholar] [CrossRef]
- Vasconcelos, A.R.; Cabral-Costa, J.V.; Mazucanti, C.H.; Scavone, C.; Kawamoto, E.M. The Role of Steroid Hormones in the Modulation of Neuroinflammation by Dietary Interventions. Front. Endocrinol. 2016, 7, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jennings, K.J.; de Lecea, L. Neural and Hormonal Control of Sexual Behavior. Endocrinology 2020, 161, bqaa150. [Google Scholar] [CrossRef]
- Hull, E.M.; Dominguez, J.M. Sexual behavior in male rodents. Horm. Behav. 2007, 52, 45–55. [Google Scholar] [CrossRef] [Green Version]
- Pascual-Mathey, L.I.; Rodríguez Romero, S.; Becerra Lara, P.; Manzo, J.; Hernández, M.E. Effect of the induction of moderate hyperprolactinemia on sexual behavior in Wistar male rats. J. Behav. Health Soc. Issues 2015, 7, 9–17. [Google Scholar] [CrossRef]
- Moralí, G.; Asunción Pía Soto, M.; Luis Contreras, J.; Arteaga, M.; González-Vidal, M.D.; Beyer, C. Detailed analysis of the male copulatory motor pattern in mammals: Hormonales bases. Scand. J. Psychol. 2003, 44, 279–288. [Google Scholar] [CrossRef]
- Bonilla-Jaime, H.; Vázquez-Palacios, G.; Arteaga-Silva, M.; Retana-Márquez, S. Hormonal responses to different sexually related conditions in male rats. Horm. Behav. 2006, 49, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Corona, G.; Isidori, A.M.; Aversa, A.; Burnett, A.L.; Maggi, M. Endocrinologic Control of Men’s Sexual Desire and Arousal/Erection. J. Sex. Med. 2016, 13, 317–337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kahn, B.E.; Brannigan, R.E. Obesity and male infertility. Curr. Opin. Urol. 2017, 27, 441–445. [Google Scholar] [CrossRef]
- Khodamoradi, K.; Parmar, M.; Khosravizadeh, Z.; Kuchakulla, M.; Manoharan, M.; Arora, H. The role of leptin and obesity on male infertility. Curr. Opin. Urol. 2020, 30, 334–339. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.R.; Stefanick, M.L.; Clark, J.T.; Davidson, J.M. Hormones and sexual behavior in relationship to aging in male rats. Horm. Behav. 1992, 26, 110–135. [Google Scholar] [CrossRef]
- Wu, D.; Gore, A.C. Changes in androgen receptor, estrogen receptor alpha, and sexual behavior with aging and testosterone in male rats. Horm. Behav. 2010, 58, 306–316. [Google Scholar] [CrossRef] [Green Version]
- Lucio, R.A.; Tlachi-López, J.L.; Eguibar, J.R.; Ågmo, A. Sperm count and sperm motility decrease in old rats. Physiol. Behav. 2013, 110, 73–79. [Google Scholar] [CrossRef]
- Bhasin, S.; Valderrábano, R.J.; Gagliano-Jucá, T. Age-Related Changes in the Male Reproductive System. In Endotext. [Internet]; Feing, K.R., Anwalt, B., Boyce, A., Chrousos, J., de Herder, W.W., Dhatariya, K., Dungan, K., Hershman, J.M., Hofland, J., Kalra, S., et al., Eds.; MDText.com, Inc.: Hanover, MA, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK278998 (accessed on 10 December 2021).
- Chen, H.; Hardy, M.P.; Zirkin, B.R. Age-Related Decreases in Leydig Cell Testosterone Production Are Not Restored by Exposure to LH in Vitro. Endocrinology 2004, 143, 1637–1642. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.M.; Shanmuganayagam, D.; Weindruch, R. Caloric restriction and aging: Studies in mice and monkeys. Toxicol. Pathol. 2009, 37, 47–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Govic, A.; Levay, E.A.; Hazi, A.; Penman, J.; Kent, S.; Paolini, A.G. Alterations in male sexual behaviour, attractiveness and testosterone levels induced by an adult-onset calorie restriction regimen. Behav. Brain Res. 2008, 190, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Quirós Cognuck, S.; Reis, W.L.; Silva, M.; Debarba, L.K.; Mecawi, A.S.; de Paula, F.; Rodrigues Franci, C.; Elias, L.; Antunes-Rodrigues, J. Sex differences in body composition, metabolism-related hormones, and energy homeostasis during aging in Wistar rats. Physiol. Rep. 2020, 8, e14597. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Rodríguez, J.; Arenas-Ríos, E.; Jiménez-Morales, I.; Cortés-Barberena, E.; Montes, S.; Vigueras-Villaseñor, R.M.; Arteaga-Silva, M. Postnatal cadmium administration affects the presence and distribution of carbohydrates in the sperm membrane during maturation in the epididymis in adult Wistar rats. Reprod. Fertil. Dev. 2021, 33, 349–362. [Google Scholar] [CrossRef] [PubMed]
- Simon, L.; Carrell, D.T. Sperm DNA damage measured by comet assay. Methods Mol. Biol. 2013, 927, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Moghadam, M.T.; Hosseini, G.; Absalan, F.; Tabar, M.H.; Nikbakht, R. Effects of Vitamin D on Apoptosis and Quality of Sperm in Asthenozoospermia. JBRA Assist. Reprod. 2020, 24, 316–323. [Google Scholar] [CrossRef]
- Azzu, V.; Valencak, T.G. Energy Metabolism and Ageing in the Mouse: A Mini-Review. Gerontology 2017, 63, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Chang, A.M.; Halter, J.B. Aging and insulin secretion. Am. J. Physiol. Endocrinol. Metab. 2003, 284, E7–E12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gulcelik, N.E.; Halil, M.; Ariogul, S.; Usman, A. Adipocytokines and aging: Adiponectin and leptin. Minerva Endocrinol. 2013, 38, 203–210. [Google Scholar]
- Shortliffe, L.M.; Ye, Y.; Behr, B.; Wang, B. Testosterone changes bladder and kidney structure in juvenile male rats. J. Urol. 2014, 191, 1913–1919. [Google Scholar] [CrossRef] [PubMed]
- Sitzmann, B.D.; Brown, D.I.; Garyfallou, V.T.; Kohama, S.G.; Mattison, J.A.; Ingram, D.K.; Roth, G.S.; Ottinger, M.A.; Urbanski, H.F. Impact of moderate calorie restriction on testicular morphology and endocrine function in adult rhesus macaques (Macaca mulatta). Age 2014, 36, 183–197. [Google Scholar] [CrossRef] [Green Version]
- Brooks, D.C.; Coon, V.J.S.; Ercan, C.M.; Xu, X.; Dong, H.; Levine, J.E.; Bulun, S.E.; Zhao, H. Brain Aromatase and the Regulation of Sexual Activity in Male Mice. Endocrinology 2020, 161, bqaa137. [Google Scholar] [CrossRef] [PubMed]
- Salais-López, H.; Agustín-Pavón, C.; Lanuza, E.; Martínez-García, F. The maternal hormone in the male brain: Sexually dimorphic distribution of prolactin signalling in the mouse brain. PLoS ONE 2018, 13, e0208960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeap, B.B. Testosterone and ill-health in aging men. Nat. Clin. Pract. Endocrinol Metab. 2009, 5, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Rana, M.M.; Shiozawa, K.; Mukai, K.; Takayanagi, K.; Eguchi, K.; Sultana, H.; Ohsaki, Y.; Komai, M.; Shirakawa, H. S-allyl Cysteine Enhances Testosterone Production in Mice and Mouse Testis-Derived I-10. Molecules 2021, 26, 1697. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, E.C.; Leonelli, C.; Pereira, O.; Bittencourt, J.C.; Carvalho, H.F. Estrogen imprinting compromises male sexual behavior and affects the number of androgen-receptor-expressing hypothalamic neurons†. Biol. Reprod. 2019, 100, 737–744. [Google Scholar] [CrossRef] [PubMed]
- Sitzmann, B.D.; Leone, E.H.; Mattison, J.A.; Ingram, D.K.; Roth, G.S.; Urbanski, H.F.; Zelinski, M.B.; Ottinger, M.A. Effects of moderate calorie restriction on testosterone production and semen characteristics in young rhesus macaques (Macaca mulatta). Biol. Reprod. 2010, 83, 635–640. [Google Scholar] [CrossRef] [Green Version]
- Qiu, Y.; Yang, H.; Li, C.; Xu, C. Progress in Research on Sperm DNA Fragmentation. Med. Sci. Monit. 2020, 26, e918746. [Google Scholar] [CrossRef]
- Vendramini, V.; Cedenho, A.P.; Miraglia, S.M.; Spaine, D.M. Reproductive function of the male obese Zucker rats: Alteration in sperm production and sperm DNA damage. Reprod. Sci. 2014, 21, 221–229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esteves, S.C.; Agarwal, A.; Cho CLMajzoub, A. A Strengths-Weaknesses-Opportunities-Threats (SWOT) analysis on the clinical utility of sperm DNA fragmentation testing in specific male infertility scenarios. Transl. Androl. Urol. 2017, 6, S734–S760. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Zong, X.; Wu, G.; Lin, S.; Feng, Y.; Hu, J. Taurine increases testicular function in aged rats by inhibiting oxidative stress and apoptosis. Amino Acids 2015, 47, 1549–1558. [Google Scholar] [CrossRef] [PubMed]
- Jeremy, M.; Gurusubramanian, G.; Roy, V.K. Vitamin D3 regulates apoptosis and proliferation in the testis of D-galactose-induced aged rat model. Sci. Rep. 2019, 9, 14103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, C.; Zhao, C.; Zhang, S.; Liu, S. Effect of Moxibustion on Testosterone Secretion and Apoptosis of Spermatogenic Cells in Aging Rats. Evid.-Based Complement. Altern. Med. 2019, 24, 5186408. [Google Scholar] [CrossRef]
- Uriondo, H.; Álvarez, S.C.; Gil, M.V.; Frazer, P.; Serna, J.; Nodar, F. Niveles de correlación entre la externalización de fosfatidilserina y apoptosis espermática en pacientes con infertilidad masculina. Reproducción 2011, 26, 3. [Google Scholar]
- Mueller, A.; Hermo, L.; Robaire, B. The effects of aging on the expression of glutathione S-transferases in the testis and epididymis of the Brown Norway rat. J. Androl. 1998, 19, 450–465. [Google Scholar] [PubMed]
- Sastre, J.; Pallardó, F.V.; García de la Asunción, J.; Viña, J. Mitochondria, oxidative stress and aging. Free Radic. Res. 2000, 32, 189–198. [Google Scholar] [CrossRef]
- Luo, L.; Chen, H.; Trush, M.A.; Show, M.D.; Anway, M.D.; Zirkin, B.R. Aging and the brown Norway rat leydig cell antioxidant defense system. J. Androl. 2006, 27, 240–247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colin-Valenzuela, A.; Gómez López, N.; Avila Lombardo, R.; Barroso Villa, G. Impacto del envejecimiento masculino en la capacidad funcional del espermatozoide a través de la expresión de fosfatidil serina y oligonucleomas. Ginecol. Obstet. México 2010, 78, 669–676. [Google Scholar]
| 6 Months after CR | 12 Months after CR | ||||||
|---|---|---|---|---|---|---|---|
| Basal | Control | CR 15% | CR 35% | Control | CR 15% | CR 35% | |
| Body Weight | 541.6 ± 6.7 | 640.2 ± 10.6 # | 447.0 ± 19.6 * | 437.7 ± 7.2 * | 630.3 ± 15.3 # | 443.2 ± 15.3 * | 446.7 ± 17.0 * |
| After 6 Months of CR | ||||
| Basal | Control | CR 15% | CR 35% | |
|---|---|---|---|---|
| Mount latency (s) | 56.5 ± 7.37 | 200.0 ± 47.70 # | 83.0 ± 55.33 * | 40.83 ± 7.50 * |
| Number of mounts | 3.4 ± 0.77 | 5.5 ± 1.25 | 4.16 ± 1.35 * | 3.16 ± 0.47 * |
| Intromission latency (s) | 73.6 ± 52.5 | 142.5 ± 62.08 # | 78.0 ± 56.5 * | 67.5 ± 28.03 * |
| Number of intromissions | 8.5 ± 0.43 | 14.5 ± 1.89 # | 17.8 ± 2.44 # | 14.0 ± 1.61 # |
| Ejaculation latency (s) | 521.0 ± 145.0 | 624.0 ± 235.0 | 738.0 ± 165 # | 638.0 ± 98.0 |
| Post-ejaculatory interval (s) | 443.3 ± 103.4 | 414.8 ± 155.4 | 670.8 ± 203.6 | 442.0 ± 48.13 |
| Number of ejaculations | 2.3 ± 0.2 | 1.0 ± 0.0 # | 2.0 ± 1.0 | 3.0 ± 1.0 * |
| Hit rate | 0.77 ± 0.26 | 0.67 ± 0.007 # | 0.79 ± 0.033 * | 0.82 ± 0.25 * |
| After 6 Months of CR | After 12 Months of CR | ||||||
|---|---|---|---|---|---|---|---|
| Basal | Control | CR 15% | CR 35% | Control | CR 15% | CR 35% | |
| Motility (%) | 64.0 ± 0.9 | 43.8 ± 0.9 | 50.0 ± 2.0 *# | 56.6 ± 0.8 *# | 25.3 ± 2.0 # | 40.2 ± 0.4 *# | 43.0 ± 2.5 *# |
| Viability (%) | 89.0 ± 2.1 | 80.3 ± 0.9 | 81.1 ± 1.6 | 86.0 ± 1.6 | 44.1 ± 4.7 # | 50.8 ± 6.9 # | 55.0 ± 8.4 # |
| Sperm count (106/mL) | 245.0 ± 16.9 | 220.0 ± 16.6 | 257.0 ± 8.3 | 245.0 ± 12.7 | 148.2 ± 6.2 # | 218.0 ± 15.2 * | 178.0 ± 9.0 * |
| Fragmentation (%) | 96.4 ± 0.3 | 97.0 ± 0.4 | 97.0 ± 0.3 | 97.0 ± 0.2 | 96.0 ± 0.3 | 96.0 ± 0.2 | 96.0 ± 0.3 |
| After 6 Months of CR | After 12 Months of CR | ||||||
|---|---|---|---|---|---|---|---|
| Basal | Control | CR 15% | CR 35% | Control | CR 15% | CR 35% | |
| Glucose (mmol/L) | 8.87 ± 0.52 | 11.88 ± 0.42 | 6.17 ± 1.23 * | 5.72 ± 1.12 * | 11.90 ± 0.92 | 6.07 ± 1.22 * | 5.22 ± 0.99 * |
| Leptin (pg/mL) | 2680.0 ± 398.0 | 3004.0 ± 341.0 | 773.0 ± 114.0 * | 481.0 ± 87.0 * | 1427.0 ± 119 | 466.0 ± 559.0 * | 336.0 ± 67.0 * |
| Adiponectin (ng/mL) | 12.18 ± 0.23 | 12.60 ± 0.80 | 13.80 ± 0.69 | 12.30 ± 0.20 | 10.80 ± 0.18 | 12.27 ± 0.75 | 11.60 ± 1.08 |
| Insulin (ng/mL) | 0.719 ± 0.11 | 1.52 ± 0.16 | 0.532 ± 0.79 * | 0.559 ± 0.075 * | 4.371 ± 0.56 # | 0.513 ± 0.12 * | 0.650 ± 0.58 * |
| Glucagon (pg/mL) | 199.0 ± 22.0 | 237.0 ± 55.0 | 56.0 ± 8.9 # | 63.0 ± 7.1 # | 161.0 ± 16.0 | 74.0 ± 11.0 # | 36.0 ± 6.3 # |
| Testosterone (ng/mL) | 1.95 ± 0.04 | 0.57 ± 0.03 # | 1.75 ± 0.14 * | 2.10 ± 0.15 * | 0.17 ± 0.02 # | 1.47 ± 0.07 * | 1.17 ± 0.08 * |
| Estradiol (pg/mL) | 1.68 ± 0.03 | 1.99 ± 0.05 # | 1.80 ± 0.03 | 1.78 ± 0.04 * | 1.96 ± 0.06 # | 1.76 ± 0.03 * | 01.74 ± 0.04 * |
| Prolactin (pg/mL) | 65.0 ± 1.56 | 80.0 ± 5.07 | 69.0 ± 4.60 | 71.0 ± 6.01 | 92.0 ± 13.16 # | 65.0 ± 3.75 | 66.0 ± 3.63 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jesús, P.L.d.; Arenas-Ríos, E.; Ruíz-Ramos, M.; Flores-Alonso, J.C.; Mendoza-Núñez, V.M.; Arrieta-Cruz, I.; Arteaga-Silva, M. Effect of Chronic Moderate Caloric Restriction on the Reproductive Function in Aged Male Wistar Rats. Nutrients 2022, 14, 1256. https://doi.org/10.3390/nu14061256
Jesús PLd, Arenas-Ríos E, Ruíz-Ramos M, Flores-Alonso JC, Mendoza-Núñez VM, Arrieta-Cruz I, Arteaga-Silva M. Effect of Chronic Moderate Caloric Restriction on the Reproductive Function in Aged Male Wistar Rats. Nutrients. 2022; 14(6):1256. https://doi.org/10.3390/nu14061256
Chicago/Turabian StyleJesús, Pablo López de, Edith Arenas-Ríos, Mirna Ruíz-Ramos, Juan Carlos Flores-Alonso, Víctor Manuel Mendoza-Núñez, Isabel Arrieta-Cruz, and Marcela Arteaga-Silva. 2022. "Effect of Chronic Moderate Caloric Restriction on the Reproductive Function in Aged Male Wistar Rats" Nutrients 14, no. 6: 1256. https://doi.org/10.3390/nu14061256
APA StyleJesús, P. L. d., Arenas-Ríos, E., Ruíz-Ramos, M., Flores-Alonso, J. C., Mendoza-Núñez, V. M., Arrieta-Cruz, I., & Arteaga-Silva, M. (2022). Effect of Chronic Moderate Caloric Restriction on the Reproductive Function in Aged Male Wistar Rats. Nutrients, 14(6), 1256. https://doi.org/10.3390/nu14061256







