Probiotic Strains Isolated from an Olympic Woman’s Weightlifting Gold Medalist Increase Weight Loss and Exercise Performance in a Mouse Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Probiotics
2.2. Animals and Experimental Design
2.3. Evaluation of Exercise Performance
2.3.1. Forelimb Grip Strength
2.3.2. Exhaustive Running Test
2.3.3. Swimming Test
2.4. Glycogen Levels in the Muscle and Liver
2.5. Body Fat, Blood Lipids, and Biochemical Variables
2.6. Tissue Sectioning and Histology
2.7. Fatty-Acid Accumulation in Intestinal Caco-2 Cells
2.8. Statistical Analysis
3. Results
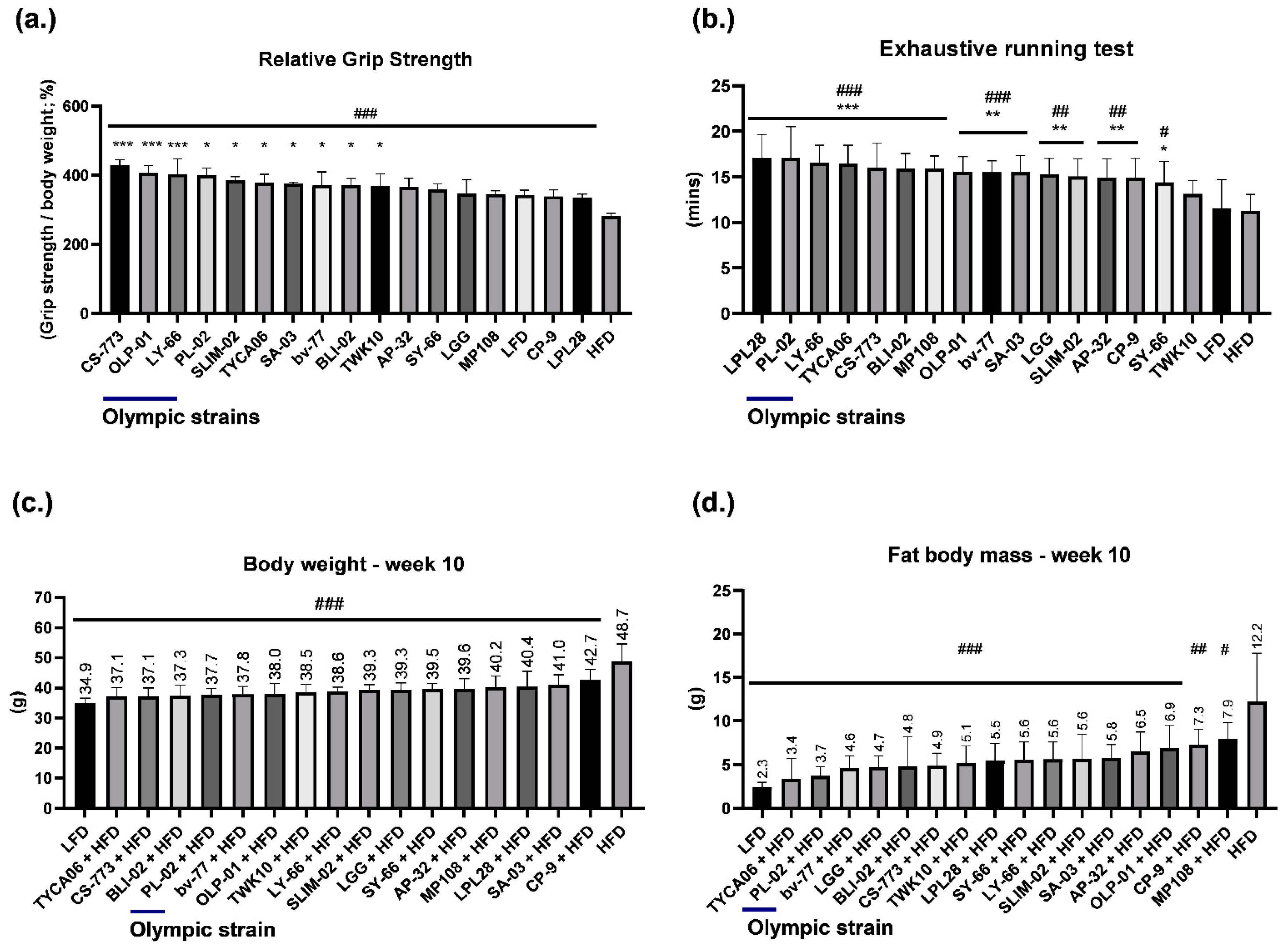
3.1. Healthy Human Gut Strain CS-773 and Gold Medalist Strains OLP-01, PL-02, and LY-66 Facilitated Excellent Grip Strength Performance in HFD-Fed Mice
3.2. Healthy Human Gut Strains TYCA06 and CS-773 and Gold Medalist Strain PL-02 Alleviated Body Weight Gain in HFD-Fed Mice
3.3. CS-773, TYCA06, OLP-01, PL-02, and LY-66 Reduced Fatty-Acid Accumulation in Intestinal Caco-2 Cells
3.4. Effects of Probiotics on Glycogen Levels
3.5. Gold Medalist-Derived Probiotic Strains OLP-01, PL-02, and LY-66 Reduced Serum Metabolite Levels of Lactate, BUN, Ammonia, and CK in the Swimming Test
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kopelman, P.G. Obesity as a medical problem. Nature 2000, 404, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Zibellini, J.; Seimon, R.V.; Lee, C.M.Y.; Gibson, A.A.; Hsu, M.S.H.; Sainsbury, A. Effect of diet-induced weight loss on muscle strength in adults with overweight or obesity—A systematic review and meta-analysis of clinical trials. Obes. Rev. 2016, 17, 647–663. [Google Scholar] [CrossRef] [PubMed]
- Ogden, C.L.; Yanovski, S.Z.; Carroll, M.D.; Flegal, K.M. The epidemiology of obesity. Gastroenterology 2007, 132, 2087–2102. [Google Scholar] [CrossRef] [PubMed]
- von Deneen, K.; Wei, Q.; Tian, J.; Liu, Y. Obesity in China: What are the causes? Curr. Pharm. Pharm. Des. 2011, 17, 1132–1139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torres-Fuentes, C.; Schellekens, H.; Dinan, T.G.; Cryan, J.F. The microbiota–gut–brain axis in obesity. Lancet Gastroenterol. Hepatol. 2017, 2, 747–756. [Google Scholar] [CrossRef]
- Koliada, A.; Syzenko, G.; Moseiko, V.; Budovska, L.; Puchkov, K.; Perederiy, V.; Gavalko, Y.; Dorofeyev, A.; Romanenko, M.; Tkach, S.; et al. Association between body mass index and Firmicutes/Bacteroidetes ratio in an adult Ukrainian population. BMC Microbiol. 2017, 17, 120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Didari, T.; Solki, S.; Mozaffari, S.; Nikfar, S.; Abdollahi, M. A systematic review of the safety of probiotics. Expert Opin. Drug Saf. 2013, 13, 227–239. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Bae, J.-H. Probiotics for weight loss: A systematic review and meta-analysis. Nutr. Res. 2015, 35, 566–575. [Google Scholar] [CrossRef] [PubMed]
- Bron, P.A.; Kleerebezem, M.; Brummer, R.-J.; Cani, P.D.; Mercenier, A.; Macdonald, T.T.; Garcia-Ródenas, C.L.; Wells, J.M. Can probiotics modulate human disease by impacting intestinal barrier function? Br. J. Nutr. 2017, 117, 93–107. [Google Scholar] [CrossRef]
- Sudha, M.R.; Ahire, J.J.; Jayanthi, N.; Tripathi, A.; Nanal, S. Effect of multi-strain probiotic (UB0316) in weight management in overweight/obese adults: A 12-week double blind, randomised, placebo-controlled study. Benef. Microbes 2019, 10, 855–866. [Google Scholar] [CrossRef]
- Lin, C.L.; Hsu, Y.J.; Ho, H.H.; Chang, Y.C.; Kuo, Y.W.; Yeh, Y.T.; Lee, M.C. Bifidobacterium longum subsp. longum OLP-01 Supplementation during Endurance Running Training Improves Exercise Performance in Middle-and Long-Distance Runners: A Double-Blind Controlled Trial. Nutrients 2020, 12, 1972. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.-C.; Hsu, Y.-J.; Ho, H.; Kuo, Y.; Lin, W.-Y.; Tsai, S.-Y.; Chen, W.-L.; Lin, C.-L.; Huang, C.-C. Effectiveness of human-origin Lactobacillus plantarum PL-02 in improving muscle mass, exercise performance and anti-fatigue. Sci. Rep. 2021, 11, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.-C.; Hsu, Y.-J.; Ho, H.-H.; Hsieh, S.-H.; Kuo, Y.-W.; Sung, H.-C.; Huang, C.-C. Lactobacillus salivarius Subspecies salicinius SA-03 is a New Probiotic Capable of Enhancing Exercise Performance and Decreasing Fatigue. Microorganisms 2020, 8, 545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsu, Y.-J.; Chiu, C.-C.; Lee, M.-C.; Huang, W.-C. Combination of Treadmill Aerobic Exercise with Bifidobacterium longum OLP-01 Supplementation for Treatment of High-Fat Diet-Induced Obese Murine Model. Obes. Facts 2021, 14, 306–319. [Google Scholar] [CrossRef]
- Monda, V.; Villano, I.; Messina, A.; Valenzano, A.; Esposito, T.; Moscatelli, F.; Viggiano, A.; Cibelli, G.; Chieffi, S.; Monda, M.; et al. Exercise Modifies the Gut Microbiota with Positive Health Effects. Oxidative Med. Cell. Longev. 2017, 2017, 3831972. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-C.; Huang, W.-C.; Ng, X.E.; Lee, M.-C.; Hsu, Y.-J.; Huang, C.-C.; Wu, H.-H.; Yeh, C.-L.; Shirakawa, H.; Budijanto, S.; et al. Rice Bran Reduces Weight Gain and Modulates Lipid Metabolism in Rats with High-Energy-Diet-Induced Obesity. Nutrients 2019, 11, 2033. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.-C.; Hsu, M.-C.; Huang, W.-C.; Yang, H.-R.; Hou, C.-C. Triterpenoid-Rich Extract fromAntrodia camphorataImproves Physical Fatigue and Exercise Performance in Mice. Evid.-Based Complement. Altern. Med. 2012, 2012, 364741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeong, J.H.; Park, H.G.; Lee, Y.R.; Lee, W.L. Moderate exercise training is more effective than resveratrol supplementation for ameliorating lipid metabolic complication in skeletal muscle of high fat diet-induced obese mice. J. Exerc. Nutr. Biochem. 2015, 19, 131–137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, W.-C.; Hsu, Y.-J.; Huang, C.-C.; Liu, H.-C.; Lee, M.-C. Exercise Training Combined with Bifidobacterium longum OLP-01 Supplementation Improves Exercise Physiological Adaption and Performance. Nutrients 2020, 12, 1145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chamberland, V.; Rioux, P. Not only students can express alcohol dehydrogenase: Goldfish can too! Adv. Physiol. Educ. 2010, 34, 222–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jang, H.R.; Park, H.-J.; Kang, D.; Chung, H.; Nam, M.H.; Lee, Y.; Park, J.-H.; Lee, H.-Y. A protective mechanism of probiotic Lactobacillus against hepatic steatosis via reducing host intestinal fatty acid absorption. Exp. Mol. Med. 2019, 51, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, Y.C.; Liu, J.R. Effect of Lactobacillus rhamnosus GG on energy metabolism, leptin resistance, and gut microbiota in mice with diet-induced obesity. Nutrients 2020, 12, 2557. [Google Scholar] [CrossRef] [PubMed]
- MacArthur, D.G.; Seto, J.T.; Chan, S.; Quinlan, K.G.; Raftery, J.M.; Turner, N.; North, K.N. An Actn3 knockout mouse provides mechanistic insights into the association between α-actinin-3 deficiency and human athletic performance. Hum. Mol. Genet. 2008, 17, 1076–1086. [Google Scholar] [CrossRef] [Green Version]
- Cunha, T.F.; Moreira, J.B.N.; Paixão, N.A.; Campos, J.C.; Monteiro, A.W.A.; Bacurau, A.V.N.; Bueno, C.R.; Ferreira, J.C.B.; Brum, P.C. Aerobic exercise training upregulates skeletal muscle calpain and ubiquitin-proteasome systems in healthy mice. J. Appl. Physiol. 2012, 112, 1839–1846. [Google Scholar] [CrossRef] [Green Version]
- Murray, B.; Rosenbloom, C. Fundamentals of glycogen metabolism for coaches and athletes. Nutr. Rev. 2018, 76, 243–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W.-Q.; Zhao, T.-T.; Gui, D.-K.; Gao, C.-L.; Gu, J.-L.; Gan, W.-J.; Huang, W.; Xu, Y.; Zhou, H.; Chen, W.-N.; et al. Sodium Butyrate Improves Liver Glycogen Metabolism in Type 2 Diabetes Mellitus. J. Agric. Food Chem. 2019, 67, 7694–7705. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Li, N.; Shi, J.; Li, H.; Yue, Y.; Jiao, W.; Wang, N.; Song, Y.; Huo, G.; Li, B. Lactobacillus acidophilus alleviates type 2 diabetes by regulating hepatic glucose, lipid metabolism and gut microbiota in mice. Food Funct. 2019, 10, 5804–5815. [Google Scholar] [CrossRef] [PubMed]
- Dent, P.; Lavoinne, A.; Nakielny, S.; Caudwell, F.B.; Watt, P.; Cohen, P. The molecular mechanism by which insulin stimulates glycogen synthesis in mammalian skeletal muscle. Nature 1990, 348, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.-J.; Wu, M.-F.; Lee, M.-C.; Huang, C.-C. Exercise training combined with Bifidobacterium longum OLP-01 treatment regulates insulin resistance and physical performance in db/db mice. Food Funct. 2021, 12, 7728–7740. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, I. Blood lactate. Sports Med. 1986, 3, 10–25. [Google Scholar] [CrossRef] [PubMed]
- Sjödin, B.; Jacobs, I. Onset of Blood Lactate Accumulation and Marathon Running Performance. Endoscopy 1981, 2, 23–26. [Google Scholar] [CrossRef] [PubMed]
- Saey, D.; Michaud, A.; Couillard, A.; Côté, C.H.; Mador, M.J.; LeBlanc, P.; Maltais, F. Contractile fatigue, muscle morphometry, and blood lactate in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2005, 171, 1109–1115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joyner, M.J.; Coyle, E.F. Endurance exercise performance: The physiology of champions. J. Physiol. 2008, 586, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y. Application of serum CK and BUN determination in monitoring pre-competition training of badminton athletes. J. Huazhong Univ. Sci. Technol. 2007, 27, 114–116. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, L.C.; Bessa, A.; Freitas-Dias, R.; Luzes, R.; Werneck-De-Castro, J.P.S.; Bassini, A.; Cameron, L.-C. A sportomics strategy to analyze the ability of arginine to modulate both ammonia and lymphocyte levels in blood after high-intensity exercise. J. Int. Soc. Sports Nutr. 2012, 9, 30. [Google Scholar] [CrossRef] [Green Version]
- Huang, W.-C.; Chiu, W.-C.; Chuang, H.-L.; Tang, D.-W.; Lee, Z.-M.; Deh-Wei, T.; Chen, F.-A. Effect of Curcumin Supplementation on Physiological Fatigue and Physical Performance in Mice. Nutrients 2015, 7, 905–921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agha, M.; Agha, R. The rising prevalence of obesity: Part A: Impact on public health. Int. J. Surg. Oncol. 2017, 2, e17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luscombe-Marsh, N.; Noakes, M.; Wittert, G.; Keogh, J.; Foster, P.; Clifton, P.M. Carbohydrate-restricted diets high in either monounsaturated fat or protein are equally effective at promoting fat loss and improving blood lipids. Am. J. Clin. Nutr. 2005, 81, 762–772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saris, W.; Astrup, A.; Prentice, A.; Zunft, H.; Formiguera, X.; De Venne, W.V.-V.; Raben, A.; Poppitt, S.; Seppelt, B.; Johnston, S.; et al. Randomized controlled trial of changes in dietary carbohydrate/fat ratio and simple vs complex carbohydrates on body weight and blood lipids: The CARMEN study. Int. J. Obes. 2000, 24, 1310–1318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perdikari, A.; Leparc, G.G.; Balaz, M.; Pires, N.D.; Lidell, M.E.; Sun, W.; Fernandez-Albert, F.; Müller, S.; Akchiche, N.; Dong, H.; et al. BATLAS: Deconvoluting Brown Adipose Tissue. Cell Rep. 2018, 25, 784–797. [Google Scholar] [CrossRef] [Green Version]
- Hsieh, P.-S.; Ho, H.-H.; Hsieh, S.-H.; Kuo, Y.-W.; Tseng, H.-Y.; Kao, H.-F.; Wang, J.-Y. Lactobacillus salivarius AP-32 and Lactobacillus reuteri GL-104 decrease glycemic levels and attenuate diabetes-mediated liver and kidney injury in db/db mice. BMJ Open Diabetes Res. Care 2020, 8, e001028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazloom, K.; Siddiqi, I.; Covasa, M. Probiotics: How Effective Are They in the Fight against Obesity? Nutrients 2019, 11, 258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karimi, G.; Jamaluddin, R.; Mohtarrudin, N.; Ahmad, Z.; Khazaai, H.; Parvaneh, M. Single-species versus dual-species probiotic supplementation as an emerging therapeutic strategy for obesity. Nutr. Metab. Cardiovasc. Dis. 2017, 27, 910–918. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Strain ID | Species | Origin | Note |
|---|---|---|---|
| OLP-01 | Bifidobacterium longum subsp. longum | Human gut, high-intensity weightlifting athlete | Four strains were isolated from same Olympic athlete |
| PL-02 | Lactobacillus plantarum | ||
| SA-03 | Lactobacillus salivarius subsp. salicinius | ||
| LY-66 | Lactococcus lactis subsp. lactis | ||
| TYCA06 | Lactobacillus acidophilus | Human gut, healthy people | Bioflag Biotech Co., Ltd. (Tainan, Taiwan) |
| AP-32 | Lactobacillus salivarius subsp. salicinius | ||
| SLIM-02 | Bifidobacterium bifidum | ||
| LGG | Lactobacillus rhamnosus | Chr. Hansen (Hoersholm, Denmark) | |
| CS-773 | Lactobacillus casei | Human gut, healthy infant | Bioflag Biotech Co., Ltd. (Tainan, Taiwan) |
| MP108 | Lactobacillus rhamnosus | ||
| BLI-02 | Bifidobacterium longum subsp. infantis | Healthy human breast | Bioflag Biotech Co., Ltd. (Tainan, Taiwan) |
| bv-77 | Lactobacillus rhamnosus | ||
| CP-9 | Bifidobacterium animalis subsp. lactis | ||
| SY-66 | Streptococcus thermophilus | Fermented food product | Bioflag Biotech Co., Ltd. (Tainan, Taiwan) |
| LPL28 | Lactobacillus plantarum | Fermented food product, miso | |
| TWK-10 | Lactobacillus plantarum | Fermented food product, Taiwanese Kimchi | SYNBIO TECH INC. (Kaohsiung, Taiwan) |
| Treatment | EFP (g) | Perinephric Fat (g) | Mesenteric Fat (g) | BAT (g) |
|---|---|---|---|---|
| LFD | 0.34 ± 0.09 ### | 0.08 ± 0.01 ### | 0.68 ± 0.06 ### | 0.06 ± 0.01 ### |
| HFD | 3.06 ± 0.19 | 1.02 ± 0.13 | 1.3 ± 0.16 | 0.14 ± 0.03 |
| TWK10 + HFD | 1.23 ± 0.24 ### | 0.48 ± 0.27 ### | 0.98 ± 0.25 ## | 0.08 ± 0.02 ### |
| $ OLP-01 + HFD (Olympic strain) | 1.17 ± 0.40 ### | 0.49 ± 0.27 ### | 0.89 ± 0.14 ### | 0.09 ± 0.03 ## |
| SLIM-02 + HFD | 1.53 ± 0.57 ### | 0.58 ± 0.29 ## | 0.96 ± 0.08 ### | 0.08 ± 0.01 ### |
| LGG + HFD | 1.48 ± 0.38 ### | 0.57 ± 0.22 ## | 0.78 ± 0.14 ### | 0.09 ± 0.02 ## |
| $ PL-02 + HFD (Olympic strain) | 0.82 ± 0.26 ### | 0.33 ± 0.15 ### | 0.58 ± 0.18 ### | 0.09 ± 0.01## |
| $ SA-03 + HFD (Olympic strain) | 1.835 ± 0.58 ### | 0.86 ± 0.19 | 0.98 ± 0.21 ## | 0.11 ± 0.02 # |
| CP-9 + HFD | 1.69 ± 0.58 ### | 0.84 ± 0.3 | 0.93 ± 0.15 ### | 0.11 ± 0.01 |
| bv-77 + HFD | 0.92 ± 0.34 ### | 0.47 ± 0.24 ### | 0.76 ± 0.13 ### | 0.09 ± 0.01 ### |
| AP-32 + HFD | 1.46 ± 0.5 ### | 0.66 ± 0.26 # | 0.96 ± 0.28 ### | 0.1 ± 0.03 # |
| BLI-02 + HFD | 0.98 ± 0.48 ### | 0.39 ± 0.24 ### | 0.785 ± 0.23 ### | 0.09 ± 0.03 ## |
| LPL28 + HFD | 1.31 ± 0.2 ### | 0.68 ± 0.33 # | 0.87 ± 0.23 ### | 0.1 ± 0.02 # |
| MP108 + HFD | 1.89 ± 0.33 ### | 0.81 ± 0.24 | 0.97 ± 0.19 ## | 0.1 ± 0.01 # |
| TYCA06 + HFD | 0.7 ± 0.47 ### | 0.43 ± 0.26 ### | 0.77 ± 0.11 ### | 0.09 ± 0.02 ## |
| $ LY-66 + HFD (Olympic strain) | 1.15 ± 0.17 ### | 0.46 ± 0.12 ### | 0.85 ± 0.06 ### | 0.1 ± 0.02 # |
| SY-66 + HFD | 1.4 ± 0.35 ### | 0.65 ± 0.12 # | 0.92 ± 0.09 ### | 0.1 ± 0.02 # |
| CS-773 + HFD | 0.92 ± 0.39 ### | 0.5 ± 0.26 ### | 0.76 ± 0.19 ### | 0.1 ± 0.02 # |
| Treatment | GOT (U/L) | GPT (U/L) | ALB (mg/dL) | TC (mg/dL) | TG (mg/dL) | HDL (mg/dL) | LDL (mg/dL) | BUN (mg/dL) | Crea (mg/dL) | UA (mg/dL) | TP (mg/dL) | CPK (U/L) | Glu (mg/dL) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LFD | 71.12 ± 3.87 ### | 38.5 ± 5.07 ## | 3.15 ± 0.12 | 150.87 ± 11.43 ### | 57.25 ± 8.97 ### | 89.26 ± 7.07 ### | 17.47 ± 2.86 ### | 14.62 ± 2.52 | 0.36 ± 0.018 | 1.32 ± 0.27 | 5.31 ± 0.23 | 371 ± 38.3 ## | 218.12 ± 41.6 |
| HFD | 118 ± 6.16 | 50.87 ± 5.64 | 3.13 ± 0.07 | 261.5 ± 18.05 | 93 ± 11.25 | 103.56 ± 4.61 | 74.63 ± 7.3 | 14.6 ± 3.04 | 0.36 ± 0.01 | 1.47 ± 0.5 | 5.4 ± 0.18 | 491.75 ± 90.06 | 259.37 ± 43.16 |
| $ OLP-01+ HFD | 102.62 ± 6.61 ### | 40.75 ± 7.24 # | 3.12 ± 0.16 | 201.12 ± 12.59 ### | 63.87 ± 11.17 ### | 96.83 ± 8.6 # | 49.85 ± 6.56 ### | 14.13 ± 1.68 | 0.37 ± 0.02 | 1.37 ± 0.3 | 5.36 ± 0.37 | 490.62 ± 64.67 | 244.87 ± 29.3 |
| $ PL-02 + HFD | 99.62 ± 7.63 ### | 38.75 ± 9.79 ## | 3.06 ± 0.17 | 183.12 ± 6.95 ### | 65.87 ± 15.27 ### | 95.75 ± 4.5 # | 48.81 ± 3.99 ### | 14.27 ± 2.64 | 0.36 ± 0.02 | 1.31 ± 0.2 | 5.37 ± 0.19 | 475.87 ± 70.22 | 248.87 ± 60.17 |
| $ LY-66 + HFD | 97.75 ± 8.34 ### | 39.5 ± 9.79 ## | 3.22 ± 0.18 | 203.5 ± 15.44 ### | 66.25 ± 12.15 ### | 105.57 ± 6.07 | 48.85 ± 5.32 ### | 14.03 ± 1.13 | 0.36 ± 0.02 | 1.33 ± 0.11 | 5.53 ± 0.17 | 462.87 ± 92.29 | 243.87 ± 29.42 |
| CS-773 + HFD | 102.12 ± 5.35 ### | 37 ± 7.67 ### | 3.14 ± 0.06 | 203.37 ± 15.28 ### | 68.75 ± 12.54 ### | 96.86 ± 5.28 # | 46.77 ± 3.87 ### | 13.57 ± 1.98 | 0.36 ± 0.03 | 1.4 ± 0.24 | 5.5 ± 0.2 | 455.25 ± 83.35 | 241.87 ± 60.76 |
| TYCA06 + HFD | 101 ± 7.25 ### | 41.25 ± 5.89 # | 3.1 ± 0.18 | 190.5 ± 12.43 ### | 70.12 ± 13.68 ### | 98.73 ± 9.38 | 45.57 ± 3.07 ### | 14.28 ± 2.1 | 0.36 ± 0.01 | 1.47 ± 0.42 | 5.33 ± 0.20 | 461.5 ± 56.33 | 241.5 ± 52.24 |
| LGG + HFD | 105.5 ± 4.07 ## | 35.62 ± 5.68 ### | 3.13 ± 0.06 | 202.87 ± 13.19 ### | 62.87 ± 9.59 ### | 98.23 ± 4.74 | 54.91 ± 6.56 ### | 14.56 ± 2.57 | 0.36 ± 0.01 | 1.43 ± 0.25 | 5.31 ± 0.21 | 483.25 ± 55.59 | 256.87 ± 30.16 |
| Serum Metabolites | LFD | HFD | LGG + HFD | $ OLP-01 + HFD | $ PL-02 + HFD | $ LY-66 + HFD |
|---|---|---|---|---|---|---|
| Lactate (nmol/L)_ before swimming (A) | 3.81 ± 0.39 | 3.85 ± 0.59 | 3.85 ± 0.25 | 3.82 ± 0.32 | 3.86 ± 0.16 | 3.82 ± 0.20 |
| Lactate (nmol/L)_10 min after swimming (B) | 5.85 ± 0.44 ### | 7.06 ± 0.65 | 6.31 ± 0.60 # | 5.53 ± 0.59 ### | 5.20 ± 0.67 ### | 5.11 ± 0.62 ### |
| Lactate (nmol/L)_rest after 20 min of swimming (C) | 5.10 ± 0.23 ### | 6.10 ± 0.34 | 5.34 ± 0.30 ### | 4.81 ± 0.41 ### | 4.44 ± 0.44 ### | 4.42 ± 0.52 ### |
| Lactate production rate = (B/A) | 1.54 ± 0.07 ### | 1.85 ± 0.12 | 1.64 ± 07 ### | 1.45 ± 0.04 ### | 1.34 ± 0.13 ### | 1.33 ± 0.10 ### |
| Lactate clearance rate = (B − C)/B | 0.13 ± 0.04 | 0.13 ± 0.05 | 0.15 ± 0.04 | 0.13 ± 0.03 | 0.14 ± 0.05 | 0.13 ± 0.05 |
| Glucose (mg/dL; 10 min after swimming) | 130 ± 19 | 176 ± 11 | 175 ± 16 | 173 ± 14 | 178 ± 18 | 174 ± 12 |
| Ammonia (NH3) (umol/L; 10 min after swimming) Decline rate of NH3 (%; compared to HFD) | 137 ± 9 ### | 165 ± 7 | 131 ± 10 ### | 117 ± 9 ### | 114 ± 7 ### | 122 ± 8 ### |
| 16.84% ### | - | 20.33% ### | 29.14% ### | 31.03% ### | 20.33% ### | |
| Creatine kinase (CK) (U/L; 10 min after swimming) Decline rate of CK (%; compared to HFD) | 1345 ± 179 ### | 1641 ± 163 | 1334 ± 172 ### | 1140 ± 100 ### | 921 ± 133 ### | 902 ± 162 ### |
| 18.05% ### | - | 18.71% ### | 30.51% ### | 43.88% ### | 45.05% ### | |
| BUN (mg/dL; 10 min after swimming) Decline rate of BUN (%; compared to HFD) | 24.6 ± 1.2 ### | 27.6 ± 1.2 | 24.3 ± 0.9 ### | 22.2 ± 0.8 ### | 22.0 ± 1.1 ### | 21.7 ± 0.7 ### |
| 10.92% ### | - | 11.92% ### | 19.54% ### | 20.35% ### | 21.31% ### |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, W.-Y.; Kuo, Y.-W.; Lin, J.-H.; Lin, C.-H.; Chen, J.-F.; Tsai, S.-Y.; Lee, M.-C.; Hsu, Y.-J.; Huang, C.-C.; Tsou, Y.-A.; et al. Probiotic Strains Isolated from an Olympic Woman’s Weightlifting Gold Medalist Increase Weight Loss and Exercise Performance in a Mouse Model. Nutrients 2022, 14, 1270. https://doi.org/10.3390/nu14061270
Lin W-Y, Kuo Y-W, Lin J-H, Lin C-H, Chen J-F, Tsai S-Y, Lee M-C, Hsu Y-J, Huang C-C, Tsou Y-A, et al. Probiotic Strains Isolated from an Olympic Woman’s Weightlifting Gold Medalist Increase Weight Loss and Exercise Performance in a Mouse Model. Nutrients. 2022; 14(6):1270. https://doi.org/10.3390/nu14061270
Chicago/Turabian StyleLin, Wen-Yang, Yi-Wei Kuo, Jia-Hung Lin, Chi-Huei Lin, Jui-Fen Chen, Shin-Yu Tsai, Mon-Chien Lee, Yi-Ju Hsu, Chi-Chang Huang, Yung-An Tsou, and et al. 2022. "Probiotic Strains Isolated from an Olympic Woman’s Weightlifting Gold Medalist Increase Weight Loss and Exercise Performance in a Mouse Model" Nutrients 14, no. 6: 1270. https://doi.org/10.3390/nu14061270








