The Antioxidant, Anti-Platelet and Anti-Coagulant Properties of Phenolic Compounds, Associated with Modulation of Hemostasis and Cardiovascular Disease, and Their Possible Effect on COVID-19
Abstract
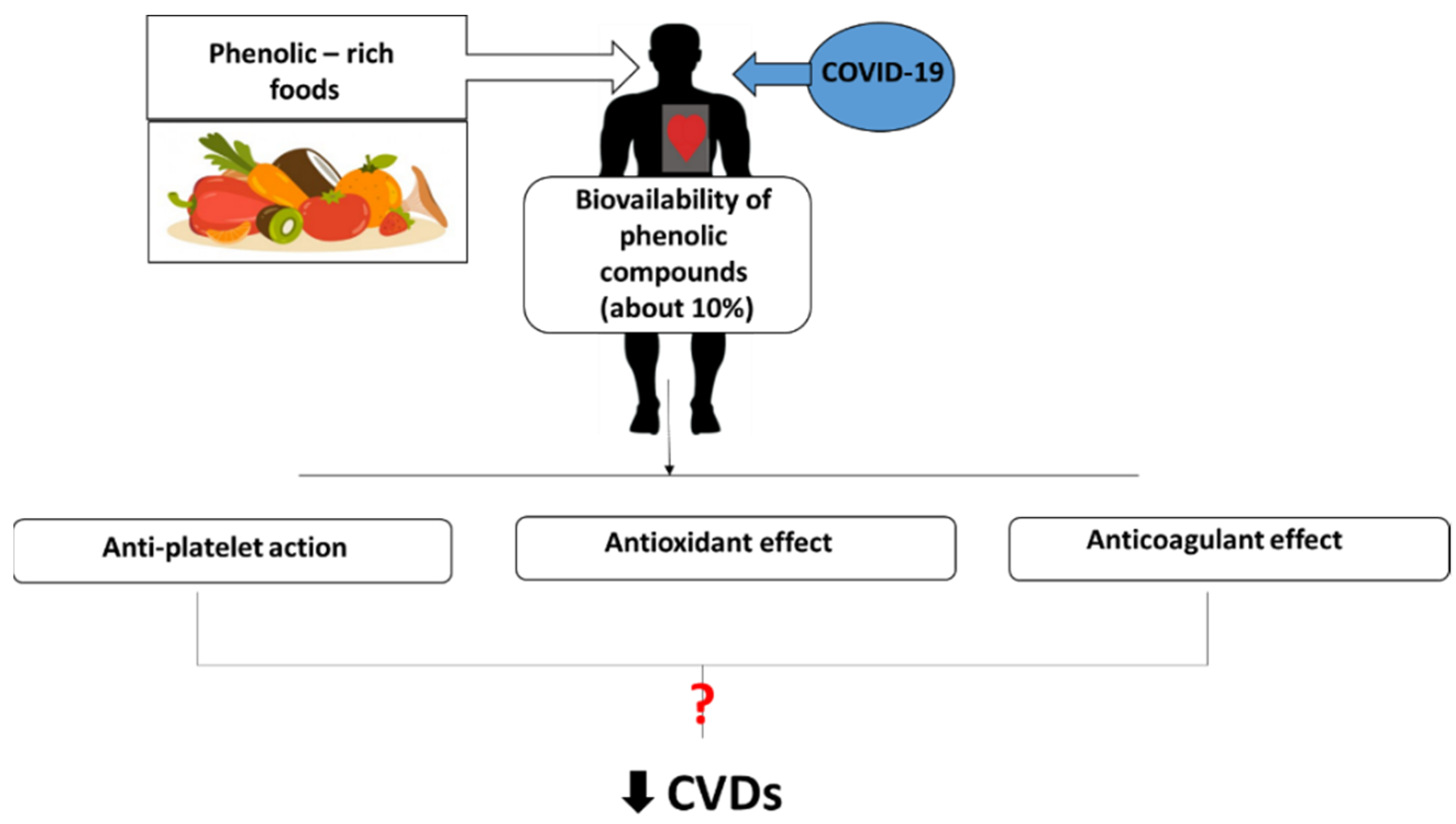
:1. Introduction
2. Antioxidant, Anti-Platelet and Anticoagulant Potential of Phenolic Compounds
2.1. Resveratrol
2.2. Curcumin
2.3. Quercetin
3. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ahmed, S.; Zimba, O.; Gasparyan, A.Y. Thrombosis in coronavirus disease 2019 (COVID-19) through the prism of Virchow’s triad. Clin. Rheumatol. 2020, 1, 2529–2543. [Google Scholar] [CrossRef] [PubMed]
- Amgalan, A.; Othman, M. Hemostatic laboratory derangements in COVID-19 with a focus on platelet count. Platelets 2020, 31, 740–745. [Google Scholar] [CrossRef] [PubMed]
- Baergen, R.N.; Heller, D.S. Placental pathology in COVID-19 positive mothers: Preliminary findings. Pediatr. Dev. Pathol. 2020, 23, 177–180. [Google Scholar] [CrossRef] [PubMed]
- Bao, C.; Tao, X.; Ciu, W.; Yi, B.; Pan, T.; Young, K.H.; Qian, W. SARS-CoV-2 induced thrombocytopenia as an important biomarker significantly correlated with abnormal coagulation function, increased intravascular blood clot risk and mortality in COVID-19 patients. Exp. Hematol. Oncol. 2020, 9, 1–8. [Google Scholar] [CrossRef]
- Connors, J.M.; Levy, J. COVID-19 and its implications for thrombosis and anticoagulation. Blood 2020, 135, 2033–2040. [Google Scholar] [CrossRef]
- Cui, S.; Chen, S.; Li, X.; Liu, S.; Wang, F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J. Thromb. Haemost. 2020, 18, 1421–1424. [Google Scholar] [CrossRef]
- Fox, S.E.; Akmatbekov, A.; Harbert, J.L.; Li, G.; Brown, J.Q.; Vander Heide, R.S. Pulmonary and cardiac pathology in Covid-19: The first autopsy series from New Orleans. Lancet Resp. Med. 2020, 11, 1–11. [Google Scholar]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [Green Version]
- Lax, S.F.; Skok, K.; Zechner, P.; Kessler, H.H.; Kaufmann, N.; Koelbinger, C.; Vander, K.; Bargfrieder, U.; Trauner, M. Pulmonary arterial thrombosis in COVID-19 with fatal outcome: Results from a prospective, single-center. Ann. Intern. Med. 2020, 1, 350–361. [Google Scholar] [CrossRef]
- Lin, J.; Yan, H.; Chen, H.; He, C.; Lin, C.; He, H.; Zhang, S.; Shi, S.; Lin, K. COVID-19 and coagulation dysfunction in adults: A systematic review and meta-analysis. J. Med. Virol. 2020, 1, 934–944. [Google Scholar] [CrossRef]
- Lippi, G.; Plebani, M.; Hanry, B.M. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: A meta-analysis. Clin. Chim. Acta 2020, 506, 145–148. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.; Wang, J. Dynamic changes in routine blood parameters of a severe COVID-19 case. Clin. Chim. Acta 2020, 508, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Manne, B.K.; Denorme, F.; Middleton, E.D.; Portier, I.; Rowley, J.W.; Stubben, C.J.; Petrey, A.C.; Tolley, N.D.; Guo, L.; Cody, M.J.; et al. Platelet gene expression and function in COVID-19 patients. Blood 2020, 1, 1317–1329. [Google Scholar] [CrossRef] [PubMed]
- Salamanna, F.; Maglio, M.; Landini, M.P.; Fini, M. Platelet functions and activities as potential hematologic parameters related to coronavirus disease 2019 (COVID-19). Platelets 2020, 31, 627–632. [Google Scholar] [CrossRef] [PubMed]
- Srivastova, S.; Garg, I.; Bansal, A.; Kumar, B. COVID-19 infection and thrombosis. Clin. Chim. Acta 2020, 510, 344–346. [Google Scholar] [CrossRef] [PubMed]
- Violi, F.; Pastori, D.; Cangemi, R.; Pignatelli, P.; Loffredo, L. Hypercoagulation and anthithrombotic treatment in coronavirus 2019: A new challenge. Thromb. Haemost. 2020, 120, 949–956. [Google Scholar]
- Xu, P.; Zhou, Q.; Xu, J. Mechanism of thrombocytopenia in COVID-19 patients. Ann. Hematol. 2020, 99, 1205–1208. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Yang, Q.; Wang, Y.; Wu, Y.; Xu, J.; Yu, Y.; Shang, Y. Thrombocytopenia and its association with mortality in patients with COVID-19. J. Thromb. Haemost. 2020, 18, 1469–1472. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, K.; Zuo, P.; Liu, Y.; Zhang, M.; Xie, S.; Zhang, H.; Chen, X.; Liu, C. Early decrease in blood platelet count is associated with poor prognosis in COVID-19 patients—Indications for predictive, preventive, and personalized medical approach. EPMA J. 2020, 11, 139–145. [Google Scholar] [CrossRef]
- Zhu, J.; Li, B.; Zhang, J. Coagulation dysfunction is associated with severity of COVID-19: A meta-analysis. J. Med. Virol. 2020, 1, 962–972. [Google Scholar] [CrossRef]
- Ulanowska, M.; Olas, B. Modulation of hemostasis in COVID-19; blood platelets may be important pieces in the COVID-19 puzzle. Pathogens 2020, 10, 370. [Google Scholar] [CrossRef] [PubMed]
- Yamamato, J.; Ijiri, Y.; Tamura, Y.; Iwasaki, M.; Murakami, M.; Okada, Y. Revolution of antithrombotic fruits and vegetables: Great variation between varieties. Drug Discover. Therapeut. 2016, 10, 129–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, J.; Zhou, Y.; Li, S.; Zhang, P.; Zhou, T.; Xu, D.P.; Li, H.B. Effects and mechanisms of fruit and vegetable juice on cardiovascular diseases. Int. J. Mol. Sci. 2018, 18, 555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olas, B. The multifunctionality of berries toward blood platelets and the role of berry phenolics in cardiovascular disorder. Platelets 2017, 28, 540–549. [Google Scholar] [CrossRef]
- Olas, B. Anti-aggregatory potential of selected vegetables—Promising dietary components for the prevention and treatment of cardiovascular disease. Adv. Nutr. 2019, 1, 280–290. [Google Scholar] [CrossRef]
- El-Missiry, M.; Fekri, A.; Kesar, L.A.; Othman, A.I. Polyphenols are potential nutritional adjuvants for targeting COVID-19. Phytother. Res. 2021, 35, 2879–2889. [Google Scholar] [CrossRef]
- Zahedipour, F.; Hosseini, S.A.; Sathyapalan, T.; Majeed, M.; Jamialahmadi, T.; Al-Rasadi, K.; Banach, M.; Sahebkar, A. Potential effects of curcumin in the treatment of COVID-19 infection. Phytother. Res. 2020, 34, 2911–2920. [Google Scholar] [CrossRef]
- Augusti, P.R.; Gricy, M.M.; Denardin, C.C.; Prazeres, I.D.; Serra, A.T.; Bronze, M.R.; Emanulli, T. Bioactivity, biovailability, and gut microbiota transformations of dietary phenolic compounds: Implications for COVID-19. J. Nutr. Biochem. 2021, 97, 108787. [Google Scholar] [CrossRef]
- Ghidoli, M.; Colombo, F.; Sandiorgio, S.; Landoni, M.; Giupponi, L.; Nielsen, E.; Pilu, R. Food containing bioactive flavonoids and other phenolic or sulfur phytochemicals with antiviral effect: Can we design a promising diet against COVID-19? Front. Nutr. 2021, 8, 303. [Google Scholar] [CrossRef]
- Giordo, R.; Zinellu, A.; Eid, A.H.; Pintus, G. Therapeutic potential of resveratrol in COVID-19-associated hemostatic disorders. Molecules 2021, 26, 856. [Google Scholar] [CrossRef]
- Biancatelli, R.M.L.C.; Berrill, M.; Catravas, J.D.; Marik, P.E. Quercetin and vitamin C: An experimental, synergistic therapy for the prevention and treatment of SARS-COV-2 related disease (COVID-19). Front. Immunol. 2020, 11, 1451. [Google Scholar] [CrossRef] [PubMed]
- Bastaminejad, S.; Bakhtiyari, S. Quercetin and its relative therapeutic potential against COVID-19: A retrospective review and prospestive pverview. Curr. Mol. Med. 2021, 21, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Derosa, G.; Maffioli, P.; D’Angelo, A.; Di Pierro, F. A role for quercetin in coronavirus disease 2019 (COVID-19). Phytother. Res. 2021, 35, 1230–1236. [Google Scholar] [CrossRef] [PubMed]
- Davella, R.; Gurrapu, S.; Mamidala, E. Phenolic compounds as promising drug candidates against COVID-19—An integrated molecular docking and dynamics simulation study. Mater. Today 2022, 51, 522–527. [Google Scholar] [CrossRef]
- Ter Ellen, B.M.; Kumar, N.D.; Bouma, E.M.; Troost, B.; van de Pol, D.P.I.; van der Ende-Metselaar, H.H.; Apperloo, L.; van Gosliga, D.; van den Berge, M.; Nawijn, M.C.; et al. Resveratrol and pterostilbene inhibit SARS-CoV-2 replication in air-liquid interface cultured human primary bronchial epithelial cells. Viruses 2021, 13, 1335. [Google Scholar] [CrossRef] [PubMed]
- Tietjen, Y.; Cassel, J.; Register, E.T.; Zhou, X.Y.; Messick, T.E.; Keeney, F.; Lu, L.D.; Beattie, K.D.; Rali, T.; Tebas, P.; et al. The natural stilbenoid (-)-hopeaphenol inhibits cellular entry of SARS-CoV-2 USA-WA1/2020, B.1.1.7, and B.1.351 variants. Antimicrob. Agents Chemother. 2021, 65, e00772-21. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Wei, J.; Huang, T.; Lei, L.; Shen, C.; Lai, J.; Yang, M.; Liu, L.; Yang, Y.; Lin, G.; et al. Resveratrol inhibits the repolication of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in cultured Vero cells. Phytother. Res. 2021, 3, 1127–1129. [Google Scholar] [CrossRef]
- Bahun, M.; Jukic, M.; Oblak, D.; Kranjc, L.; Bajc, G.; Butala, M.; Bozovicar, K.; Bratkovic, T.; Podlipnik, C.; Ulrih, N.P. Inhibition of the SARS-CoV-2 3CL pro main protease by plant polyphenols. Food Chem. 2022, 373, 131594. [Google Scholar] [CrossRef]
- Olas, B.; Wachowicz, B. Resveratrol, a phenolic antioxidant with effects on blood platelet functions. Platelets 2000, 2, 251–260. [Google Scholar] [CrossRef]
- Olas, B.; Nowak, P.; Wachowicz, B. Resveratrol protects against peroxynitrite—Induced thiol oxidation in blood platelets. Cell Mol. Biol. Lett. 2004, 9, 577–587. [Google Scholar]
- Olas, B.; Nowak, P.; Kolodziejczyk, J.; Wachowicz, B. The effects of antioxidants on peroxynitrite-induced changes in platelet proteins. Thromb. Res. 2004, 113, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Olas, B.; Nowak, P.; Ponczek, M.; Wachowicz, B. Resveratrol, a natural phenolic compound may reduce carbonylation of proteins induced by peroxynitrite in blood platelets. Gen. Physiol. Biophys. 2006, 25, 215–222. [Google Scholar] [PubMed]
- Olas, B.; Wachowicz, B.; Bald, E.; Glowacki, R. The protective effects of resveratrol against changes in blood platelet thiols induced by platinum compounds. J. Physiol. Pharm. 2004, 2, 467–476. [Google Scholar]
- Olas, B.; Zbikowska, H.M.; Wachowicz, B.; Krajewski, T.; Buczynski, A.; Magnuszewska, A. Inhibitory effect of resveratrol on free radical generation in blood platelets. Acta Biochim. Pol. 1999, 46, 961–966. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olas, B.; Nowak, P.; Kolodziejczyk, J.; Ponczek, M.; Wachowicz, B. Protective effects of resveratrol against oxidative/nitrative modifications of plasma proteins and lipids exposed to peroxynitrite. J. Nutr. Biochem. 2006, 17, 96–102. [Google Scholar] [CrossRef]
- Gligorijevic, N.; Radomirivic, M.; Rajkovic, A.; Nedic, O.; Velickovic, T.C. Fibrinogen increases resveratrol solubility and prevents it from oxidation. Foods 2020, 9, 780. [Google Scholar] [CrossRef]
- Shahidi, M.; Parkizkary, F.; Sharifi, R.; Ghotaslou, A.; Barati, M. Effects of resveratrol on coagulative, fibrinolytic, and inflammatory marker expression and secretion by endothelial cells (human umbilitical endothelial cells. Blood Coagul. Fibrinolysis 2020, 31, 207–212. [Google Scholar] [CrossRef]
- Kolodziejczyk, J.; Olas, B.; Saluk-Juszczak, J.; Wachowicz, B. Antioxidative properties of curcumin in the protection of blood platelets and plasma against peroxynitrite-induced oxidative stress. Platelets 2011, 22, 270–276. [Google Scholar] [CrossRef]
- Manikandan, P.; Sumitra, M.; Aishwarya, S.; Manohar, B.M.; Lokanadam, B.; Puvanakrishnan, R. Curcumin modulates free radical quenching in myocardial ischaemia in rats. Int. J. Biochem. Cell Biol. 2004, 36, 1967–1980. [Google Scholar] [CrossRef]
- Kim, D.C.; Ku, S.K.; Bae, J.S. Anticoagulant activities of curcumin and its derivative. MBM Rep. 2012, 45, 221–226. [Google Scholar] [CrossRef] [Green Version]
- Mosawy, S.; Jackson, D.E.; Woodman, O.L.; Linden, M.D. Treatment with quercetin and 3′,4′-dihydroxyflavonol inhibits platelet function and reduces thrombus formation in vivo. J. Thromb. Thrombolysis 2013, 36, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Mosawy, S.; Jackson, D.E.; Woodman, O.L.; Linden, M.D. Inhibition of platelet-mediated arterial thrombosis and platelet granule exocytosis by 3′,4′-dihydroxyflavonol and quercetin. Platelets 2013, 24, 594–604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mosawy, S.; Jackson, D.E.; Woodman, O.L.; Linden, M.D. The flavonols querectin and 3′,4′-dihydroxyflavonol reduce platelet function and delay thrombus formation in a model of type 1 diabetes. Diab. Vasc. Dis. Res. 2014, 11, 174–181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Navarro-Nunez, L.; Lozano, M.L.; Martinez, C.; Vincente, V.; Rivera, J. Effect of quercetin on platelet spreading on collagen and fibrinogen and on multiple platelet kinases. Fitoterapia 2010, 8, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Deuster, P. Comparison of quercetin and dihydroquercetin: Antioxidant—Independent actions on erythrocyte and platelet membrane. Chem. Biol. Interact. 2009, 182, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Kim, K.J.; Kim, S. Comparative effect of quercetin and quercetin-3-O-beta-d-glucoside on fibrin polymers, blood clots, and in rodent models. J. Biochem. Mol. Toxicol. 2016, 30, 548–558. [Google Scholar] [CrossRef] [PubMed]
- Marumo, M.; Ekawa, K.; Wakabayashi, I. Resveratrol inhibits Ca2+ signal and aggregation of platelets. Environ. Health Prev. Med. 2020, 25, 70. [Google Scholar] [CrossRef]
- Jang, J.Y.; Min, J.H.; Wang, S.B.; Chae, Y.H.; Baek, J.Y.; Kim, M.; Ryu, J.-S.; Chang, T.-S. Resveratrol inhibits collagen-induced platelet stimulation through suppressing NADPH oxidase and oxidative inactivation of SH2 domain-containing protein tyrosine phosphatase-2. Free Radic. Biol. Med. 2015, 89, 842–851. [Google Scholar] [CrossRef]
- Huang, W.-C.; Liu, J.-C.; Hsia, C.-W.; Fong, T.-H.; Hsia, C.-H.; Tran, O.-T.; Velusamy, M.; Yang, C.-H.; Sheu, J.-R. Ptherostilbene, a dimethylether analogue of resveratrol, possess high potency in the prevention of platelet activation in humans and the reduction of vascular thrombosis in mice. J. Agric. Food Chem. 2021, 69, 4697–4707. [Google Scholar] [CrossRef]
- Ravishankar, D.; Albadawi, D.A.I.; Chaggar, V.; Patra, P.H.; Williams, H.F.; Salamah, M.; Vaiyapuri, R.; Dash, P.R.; Patel, K.; Watson, K.A.; et al. Isorhapontigenin, a resveratrol analogue selectively inhibits ADP-stimulated platelet activation. Eur. J. Pharmacol. 2019, 862, 172627. [Google Scholar] [CrossRef]
- Keihanian, F.; Saeidina, A.; Bagheri, R.K.; Johnston, T.P.; Sahebkar, A. Curcumin, hemostasis, thrombosis, and coagulation. J. Cell. Physiol. 2018, 233, 4497–4511. [Google Scholar] [CrossRef] [PubMed]
- Tabeshpour, J.; Hashemzai, M.; Sahebkar, A. The regulatory role of curcumin on platelet functions. J. Cell. Biochem. 2018, 119, 8713–8722. [Google Scholar] [CrossRef] [PubMed]
- Madhyastha, R.; Madhyastha, H.; Nakajima, Y.; Omura, S.; Maruyama, M. Curcumin facilitates fibrinolysis and cellular migration during wound healing by modulating urokinase plasminogen activator expression. Pathophysiol. Haemost. Thromb. 2010, 37, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.C.; Zhao, L.X.; Lou, H.X. Curcumin alters the pharmacokinetics of warfarin and clopidogrel in Wistar rats but has not effect on anticoagulation or antiplatelet aggregation. Planta Med. 2013, 79, 971–977. [Google Scholar] [CrossRef] [PubMed]
- Rukoyatkina, N.; Shpakova, V.; Bogoutdinova, A.; Kharazova, A.; Mindukshev, I.; Gambaryan, S. Curcumin by activation of adenosine A2A receptor stimulates protein kinase a and potentiates inhibitory effect of cangrelor on platelets. Biochem. Biophys. Res. Commun. 2022, 586, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Chapman, K.; Scorgie, F.E.; Ariyarajah, A.; Stephens, T.; Enjeti, A.K.; Lincz, L.F. The effect of tetrahydrocurcumin compared to curcuminoids on human platelet aggregation and blood coagulation in vitro. Thromb. Res. 2019, 179, 28–30. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Ma, J.; Zhang, C.; Chen, B.; Zhang, X.; Yu, X.; Shui, H.; He, Q.; Ua, F. Tetrahydrocurcumin downregulates MAPKs/cPLA2 signaling and attenuates platelet thromboxane A2 generation, granule secretion, and thrombus growth. Thromb. Haemost. 2021, 1, 1–7. [Google Scholar] [CrossRef]
- Heidari, Z.; Mohammadi, M.; Sahebkar, A. Possible mechanisms and special clinical considerations of curcumin supplementation in patients with COVID-19. Adv. Exp. Med. Biol. 2021, 1308, 127–136. [Google Scholar]
- Anand David, A.V.; Arulmoni, R.; Parasuraman, S. Overviews of biological importance of quercetin: A bioactive flavonoid. Pharmacogn. Rev. 2016, 10, 84–89. [Google Scholar]
- Zhang, J.; Yu, W.J.; Yang, N.; Sun, L. Interaction between resveratrol and thrombin and its biological implication. Int. J. Food Sci. Nutr. 2011, 62, 814–820. [Google Scholar] [CrossRef]
- Patel, R.V.; Mistry, B.M.; Shinde, S.K.; Syed, R.; Singh, V.; Shin, H.S. Therapeutic potential of quercetin as cardiovascular agent. Eur. J. Med. Chem. 2018, 155, 889–904. [Google Scholar] [CrossRef] [PubMed]
- Baldissarelli, J.; Santi, A.; Schmatz, R.; Zanini, D.; Cardoso, A.M.; Abadalla, F.H.; Thome, G.R.; Murussi, C.; Polachini, C.R.N.; Delengare, D.P.; et al. Quercetin changes purinergic enzyme activities and oxidative profile in platelets of rats with hypothyroidism. Biomed. Pharmacother. 2016, 84, 1849–1857. [Google Scholar] [CrossRef] [PubMed]
- Applova, L.; Karlikova, I.; Warncke, P.; Macakova, K.; Hrubsa, M.; Macahcek, M.; Tvrdy, V.; Fischer, D.; Mladenka, P. 4-methylcatechol, a flavonoid metabolite with potent antiplatelet effects. Mol. Nutr. Food Res. 2019, 63, 1900261. [Google Scholar] [CrossRef] [PubMed]
- Ferenczyova, K.; Kolocayova, K.; Bartekova, M. Potential implictions of quercetin and its derivative in cardioprotection. Int. J. Mol. Sci. 2020, 26, 1585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Phenolic Compound with Antiviral Potential | Main Source | Food Concentration | Effectiveness against Modulation of Hemostasis and CVDs | References |
|---|---|---|---|---|
| Resveratrol (3,4′,5—trihydroxystilbene) | Grapes, peanuts, pine, mulberry, black currant, strawberries, raspberries, apples | Grapes: 50–400 μg/g fresh weight; Red wine: 0.1–7 mg/L; White wine: 0.04 mg/L; Grape juice: 0.05 mg/L | Antioxidant activity: inhibition of lipid peroxidation, nitration and oxidation of blood platelet and plasma proteins (in vitro); Anti-platelet, anti-coagulant and antifibrinolytic activity: inhibition of ROS production, inhibition of activity of various enzymes, including COX (in vitro and in vivo) | [39,40,41,42,43,44,45,46,47] |
| Curcumin (1,7-bis-(4-hydroxy-3-metoxyphenylo)-1,6 heptadiene 3,5-dion) | Turmeric | 12.5–50 μg/mL (in vitro) 3 000 mg/100 g (in vivo) 0.1–50 μM (in vitro) 100 mg/kg (in vivo) | Antioxidant activity: inhibition of lipid peroxidation, nitration and oxidation of blood platelet and plasma proteins (in vitro and in vivo); Anticoagulant potential: prolongation prothrombin time and inhibition the generation of thrombin and activity of coagulation factor Xa (in vitro and in vivo) | [48,49,50] |
| Quercetin (3,3′,4′,5,6-pentahydroxyflavone) | Capers, buckwheat, onions, black tea, red wine, apples | Capers (raw) 234 mg/100 g; Buckwheat: 184–535 mg/100 g; Onions: 120 mg/100 g; Black tea: 10–20 mg/g | Antioxidant activity: inhibition of LDL oxidation and the protective effect on NO. function under oxidative stress (in vitro and in vivo), Anti-platelet, anti-coagulant and antifibrinolytic (in vitro and in vivo) | [51,52,53,54,55,56] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olas, B. The Antioxidant, Anti-Platelet and Anti-Coagulant Properties of Phenolic Compounds, Associated with Modulation of Hemostasis and Cardiovascular Disease, and Their Possible Effect on COVID-19. Nutrients 2022, 14, 1390. https://doi.org/10.3390/nu14071390
Olas B. The Antioxidant, Anti-Platelet and Anti-Coagulant Properties of Phenolic Compounds, Associated with Modulation of Hemostasis and Cardiovascular Disease, and Their Possible Effect on COVID-19. Nutrients. 2022; 14(7):1390. https://doi.org/10.3390/nu14071390
Chicago/Turabian StyleOlas, Beata. 2022. "The Antioxidant, Anti-Platelet and Anti-Coagulant Properties of Phenolic Compounds, Associated with Modulation of Hemostasis and Cardiovascular Disease, and Their Possible Effect on COVID-19" Nutrients 14, no. 7: 1390. https://doi.org/10.3390/nu14071390
APA StyleOlas, B. (2022). The Antioxidant, Anti-Platelet and Anti-Coagulant Properties of Phenolic Compounds, Associated with Modulation of Hemostasis and Cardiovascular Disease, and Their Possible Effect on COVID-19. Nutrients, 14(7), 1390. https://doi.org/10.3390/nu14071390






