Effect of Serum Spermidine on the Prognosis in Patients with Acute Myocardial Infarction: A Cohort Study
Abstract
:1. Introduction
2. Methods
2.1. Study Population
2.2. Measurement of Serum SPERMIDINE and Oxidative Stress
2.3. Follow-Up and Endpoints
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Endpoints
3.3. Restricted Cubic Splines
3.4. ROC Curves
3.5. Mediation Analysis
4. Discussion
4.1. Spermidine and MACE
4.2. Potential Mechanistic Links
4.3. Merits and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mendis, S.; Puska, P.; Norrving, B. Global Atlas on Cardiovascular Disease Prevention and Control; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Reiner, Ž. Hypertriglyceridaemia and risk of coronary artery disease. Nat. Rev. Cardiol. 2017, 14, 401–411. [Google Scholar] [CrossRef] [PubMed]
- Braunwald, E. The war against heart failure: The Lancet lecture. Lancet 2015, 385, 812–824. [Google Scholar] [CrossRef]
- de Freitas, I.A.; de Alcantara Lima, N.; da Silva, G.B., Jr.; de Castro, R.L., Jr.; Patel, P.; de Vasconcelos Lima, C.C.; da Costa Lino, D.O. Novel biomarkers in the prognosis of patients with atherosclerotic coronary artery disease. Rev. Port. Cardiol. Engl. Ed. 2020, 39, 667–672. [Google Scholar] [CrossRef] [PubMed]
- Madeo, F.; Eisenberg, T.; Pietrocola, F.; Kroemer, G. Spermidine in health and disease. Science 2018, 359, eaan2788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eisenberg, T.; Abdellatif, M.; Schroeder, S.; Primessnig, U.; Stekovic, S.; Pendl, T.; Harger, A.; Schipke, J.; Zimmermann, A.; Schmidt, A.; et al. Cardioprotection and lifespan extension by the natural polyamine spermidine. Nat. Med. 2016, 22, 1428–1438. [Google Scholar] [CrossRef]
- Kiechl, S.; Pechlaner, R.; Willeit, P.; Notdurfter, M.; Paulweber, B.; Willeit, K.; Werner, P.; Ruckenstuhl, C.; Iglseder, B.; Weger, S.; et al. Higher spermidine intake is linked to lower mortality: A prospective population-based study. Am. J. Clin. Nutr. 2018, 108, 371–380. [Google Scholar] [CrossRef]
- Soda, K.; Kano, Y.; Chiba, F. Food polyamine and cardiovascular disease—An epidemiological study. Glob. J. Health Sci. 2012, 4, 170–178. [Google Scholar] [CrossRef]
- Meana, C.; Rubín, J.M.; Bordallo, C.; Suarez, L.; Bordallo, J.; Sanchez, M. Correlation between endogenous polyamines in human cardiac tissues and clinical parameters in patients with heart failure. J. Cell. Mol. Med. 2016, 20, 302–312. [Google Scholar] [CrossRef]
- Steven, S.; Frenis, K.; Oelze, M.; Kalinovic, S.; Kuntic, M.; Bayo Jimenez, M.T.; Vujacic-Mirski, K.; Helmstädter, J.; Kröller-Schön, S.; Münzel, T.; et al. Vascular Inflammation and Oxidative Stress: Major Triggers for Cardiovascular Disease. Oxid. Med. Cell. Longev. 2019, 2019, 7092151. [Google Scholar] [CrossRef] [Green Version]
- Senoner, T.; Dichtl, W. Oxidative Stress in Cardiovascular Diseases: Still a Therapeutic Target? Nutrients 2019, 11, 2090. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Lee, S.E.; Kim, G.-D.; Park, H.R.; Park, Y.S. Hemeoxygenase-1 mediates an adaptive response to spermidine-induced cell death in human endothelial cells. Oxid. Med. Cell. Longev. 2013, 2013, 238734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chai, N.; Zhang, H.; Li, L.; Yu, X.; Liu, Y.; Lin, Y.; Wang, L.; Yan, J.; Nikolaevna, S.E.; Zhao, Y. Spermidine Prevents Heart Injury in Neonatal Rats Exposed to Intrauterine Hypoxia by Inhibiting Oxidative Stress and Mitochondrial Fragmentation. Oxid. Med. Cell. Longev. 2019, 2019, 5406468. [Google Scholar] [PubMed]
- Eisenberg, T.; Knauer, H.; Schauer, A.; Büttner, S.; Ruckenstuhl, C.; Carmona-Gutierrez, D.; Ring, J.; Schroeder, S.; Magnes, C.; Antonacci, L.; et al. Induction of autophagy by spermidine promotes longevity. Nat. Cell Biol. 2009, 11, 1305–1314. [Google Scholar] [CrossRef] [PubMed]
- LaRocca, T.J.; Gioscia-Ryan, R.A.; Hearon, C.M.; Seals, D.R. The autophagy enhancer spermidine reverses arterial aging. Mech. Ageing Dev. 2013, 134, 314–320. [Google Scholar] [CrossRef] [Green Version]
- Thygesen, K.; Alpert, J.S.; White, H.D. Joint ESC/ACCF/AHA/WHF Task Force for the Redefinition of Myocardial Infarction. Universal definition of myocardial infarction. J. Am. Coll. Cardiol. 2007, 50, 2173–2195. [Google Scholar] [CrossRef] [Green Version]
- Draper, H.H.; Hadley, M. Malondialdehyde determination as index of lipid peroxidation. Methods Enzymol. 1990, 186, 421–431. [Google Scholar]
- Soda, K.; Kano, Y.; Sakuragi, M.; Takao, K.; Lefor, A.; Konishi, F. Long-term oral polyamine intake increases blood polyamine concentrations. J. Nutr. Sci. Vitaminol. 2009, 55, 361–366. [Google Scholar] [CrossRef] [Green Version]
- Handa, A.K.; Fatima, T.; Mattoo, A.K. Polyamines: Bio-Molecules with Diverse Functions in Plant and Human Health and Disease. Front. Chem. 2018, 6, 10. [Google Scholar] [CrossRef] [Green Version]
- Vargas, A.J.; Wertheim, B.C.; Gerner, E.W.; Thomson, C.A.; Rock, C.L.; Thompson, P.A. Dietary polyamine intake and risk of colorectal adenomatous polyps. Am. J. Clin. Nutr. 2012, 96, 133–141. [Google Scholar] [CrossRef] [Green Version]
- Vargas, A.J.; Ashbeck, E.L.; Wertheim, B.C.; Wallace, R.B.; Neuhouser, M.L.; Thomson, C.A.; Thompson, P.A. Dietary polyamine intake and colorectal cancer risk in postmenopausal women. Am. J. Clin. Nutr. 2015, 102, 411–419. [Google Scholar] [CrossRef] [Green Version]
- Pucciarelli, S.; Moreschini, B.; Micozzi, D.; De Fronzo, G.S.; Carpi, F.M.; Polzonetti, V.; Vincenzetti, S.; Mignini, F.; Napolioni, V. Spermidine and spermine are enriched in whole blood of nona/centenarians. Rejuvenation Res. 2012, 15, 590–595. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Doyle, K.M.; Tatlisumak, T. Polyamines in the brain: Distribution, biological interactions, and their potential therapeutic role in brain ischaemia. Curr. Med. Chem. 2007, 14, 1807–1813. [Google Scholar] [PubMed] [Green Version]
- Matsumoto, M.; Kitada, Y.; Naito, Y. Endothelial Function is improved by Inducing Microbial Polyamine Production in the Gut: A Randomized Placebo-Controlled Trial. Nutrients 2019, 11, 1188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, J.; Yan, J.Y.; Wang, Y.X.; Ling, Y.N.; Song, X.D.; Wang, S.Y.; Liu, H.Q.; Liu, Q.C.; Zhang, Y.; Yang, P.Z.; et al. Spermidine-enhanced autophagic flux improves cardiac dysfunction following myocardial infarction by targeting the AMPK/mTOR signalling pathway. Br. J. Pharmacol. 2019, 176, 3126–3142. [Google Scholar] [CrossRef]
- Singh, S.; Kumar, R.; Garg, G.; Singh, A.K.; Verma, A.K.; Bissoyi, A.; Rizvi, S.I. Spermidine, a caloric restriction mimetic, provides neuroprotection against normal and D-galactose-induced oxidative stress and apoptosis through activation of autophagy in male rats during aging. Biogerontology 2021, 22, 35–47. [Google Scholar] [CrossRef]
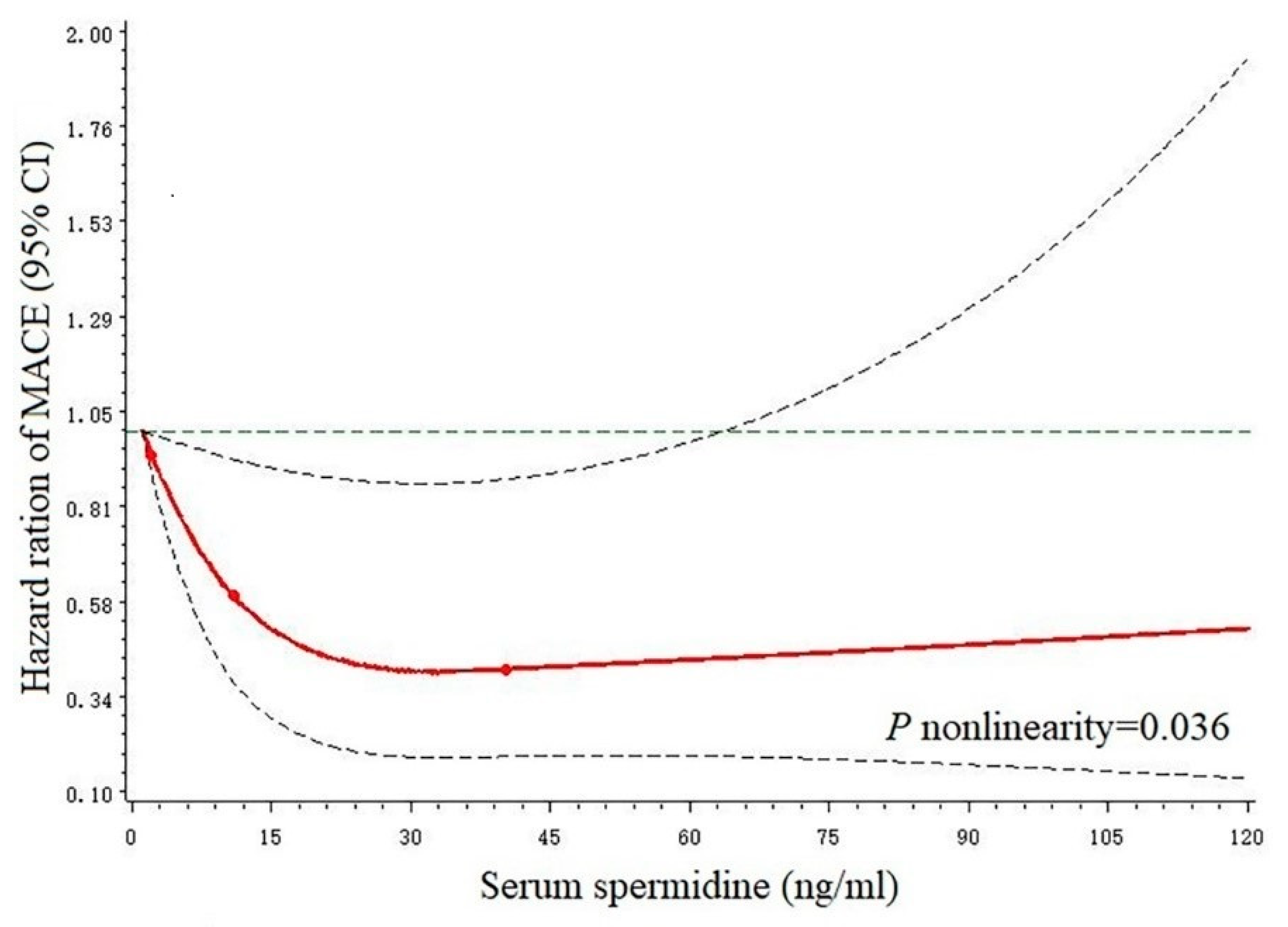
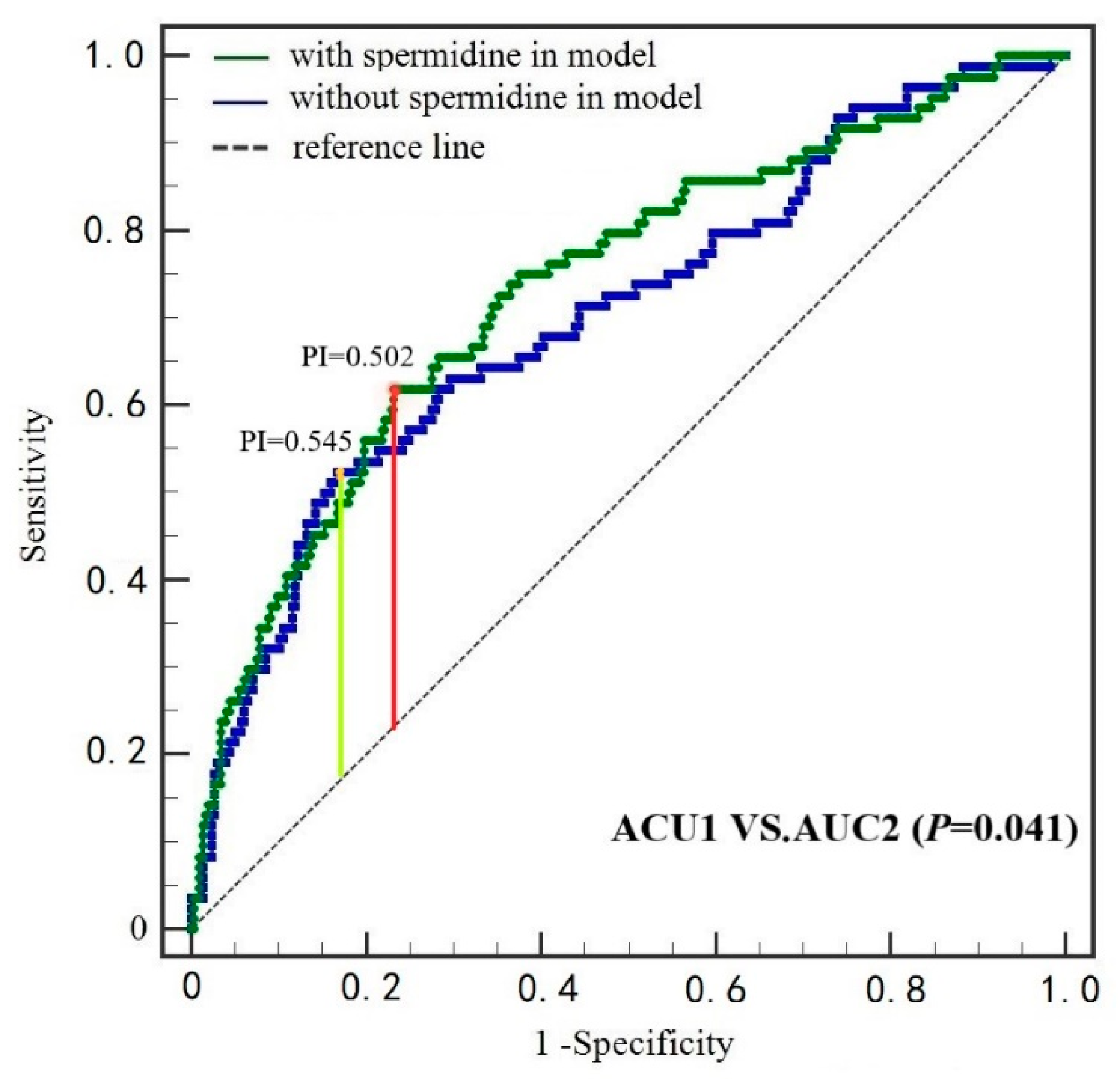
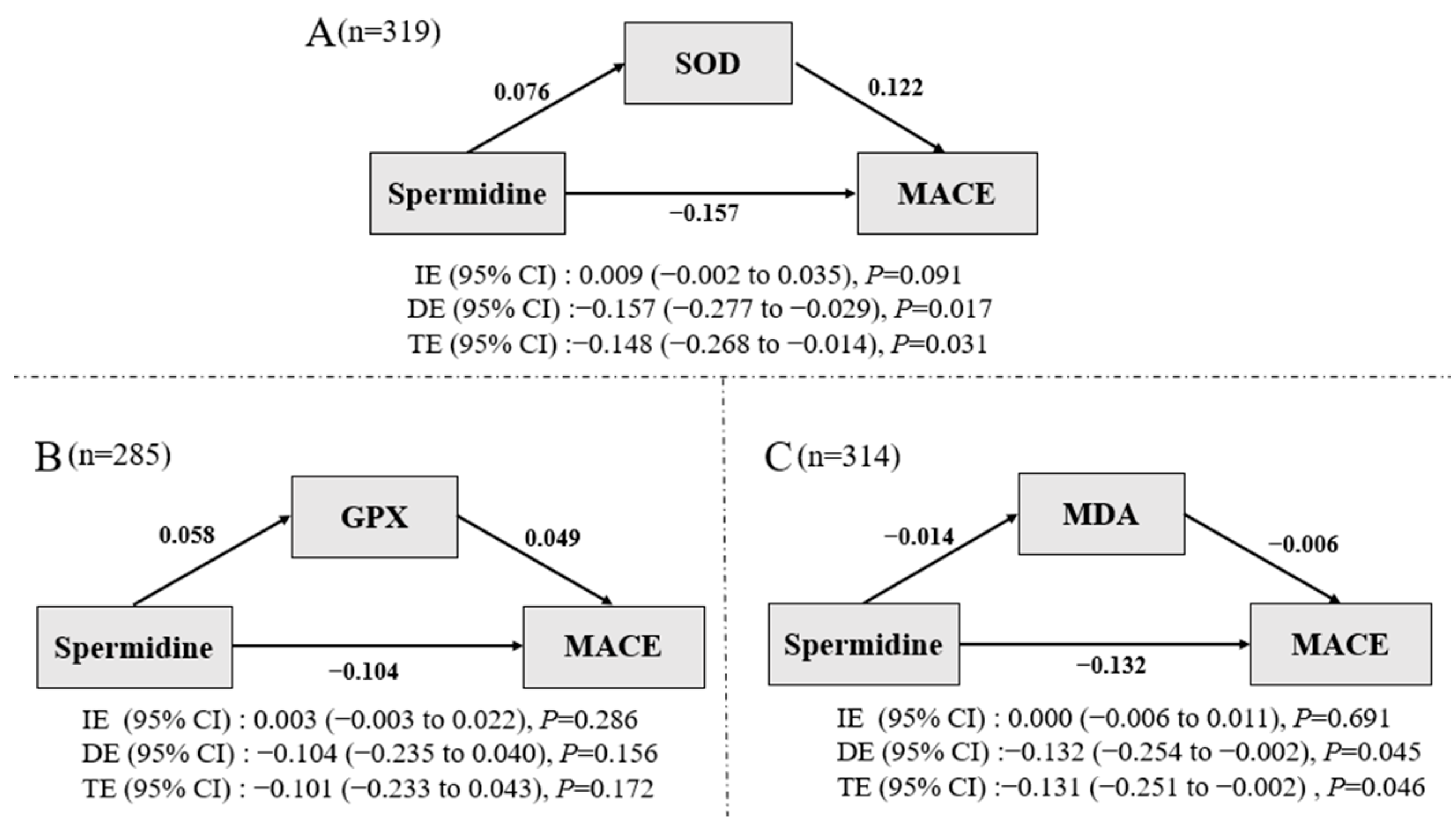
| Characteristics | Combined | T1 (≤7.59 ng/mL) | T2 (7.59~15.38 ng/mL) | T3 (≥15.38 ng/mL) | p-Value |
|---|---|---|---|---|---|
| Ages, years | 53.0 (62.0, 71.0) | 59 (51.0, 67.0) | 62.0 (54.0, 73.3) | 65.0 (55.8, 72.3) | 0.003 |
| Females, % | 102 (27.1) | 28 (22.4) | 44 (34.9) | 30 (23.8) | 0.050 |
| New AMI, % | 314 (83.3) | 103 (82.4) | 104 (82.5) | 107 (84.9) | 0.834 |
| Previous histories | |||||
| Stroke, % | 46 (12.2) | 13 (10.4) | 12 (9.5) | 21 (16.7) | 0.168 |
| Hypertension, % | 220 (58.4) | 64 (51.2) | 74 (58.7) | 82 (65.1) | 0.083 |
| Diabetes, % | 95 (25.2) | 29 (23.2) | 33 (26.2) | 33 (26.2) | 0.820 |
| Smoking, % | 227 (60.2) | 86 (68.8) | 67 (53.2) | 74 (58.7) | 0.037 |
| Systolic BP, mmHg | 130.0 (116.0, 145.5) | 129.0 (116.0, 143.5) | 129.5 (118.0, 144.3) | 130.5 (115.0, 150.0) | 0.771 |
| Diastolic BP, mmHg | 79.3 ± 14.2 | 79.7 ± 14.2 | 78.2 ± 14.0 | 80.1 ± 14.5 | 0.799 |
| Heart rate, beats/min | 77.0 (67.0, 87.0) | 78.0 (68.0, 88.0) | 76.0 (66.0, 86.0) | 76.0 (65.0, 88.0) | 0.782 |
| Creatinine, µmol/L | 70.6 (60.7, 84.9) | 70.0 (61.7, 87.1) | 69.0 (59.0, 83.0) | 73.2 (62.3, 86.3) | 0.354 |
| Total cholesterol, mmol/L | 4.6 (3.8, 5.2) | 4.60 (3.9, 5.3) | 4.6 (3.8, 5.1) | 4.3 (3.7, 5.2) | 0.648 |
| Low-density lipoprotein, mmol/L | 0.9 (0.8, 1.1) | 0.9 (0.8, 1.2) | 0.9 (0.8, 1.1) | 0.9 (0.8, 1.1) | 0.720 |
| High-density lipoprotein, mmol/L | 2.8 (2.2, 3.5) | 2.7 (2.2, 3.3) | 2.9 (2.1, 3.5) | 2.8 (2.2, 3.5) | 0.758 |
| Triglycerides, mmol/L | 1.5 (1.0, 2.4) | 1.5 (1.1, 3.0) | 1.5 (1.0, 2.2) | 1.4 (0.9, 2.1) | 0.040 |
| Fasting glucose, mmol/L | 6.1 (5.2, 7.8) | 6.1 (5.3, 7.8) | 6.0 (5.2, 7.5) | 6.2 (5.2, 8.5) | 0.881 |
| Cardiac Troponins I, μg/L | 9.2 (1.28, 34.9) | 9.7 (1.3, 35.5) | 6.4 (1.0, 27.6) | 11.6 (1.4, 37.0) | 0.284 |
| ST-segment elevation, % | 187 (49.6) | 71 (56.8) | 54 (42.9) | 62 (49.2) | 0.087 |
| Killip II/III class, % | 38 (10.1) | 11 (8.8) | 11 (8.7) | 16 (12.7) | 0.489 |
| PCI, % | 273 (72.4) | 94 (75.2) | 96 (76.2) | 83 (65.9) | 0.130 |
| Medical treatment | |||||
| Aspirin, % | 364 (96.6) | 120 (96.0) | 121 (96.0) | 123 (97.6) | 0.723 |
| Statin, % | 363 (96.3) | 118 (94.4) | 122 (96.8) | 123 (97.6) | 0.373 |
| Clopidogrel, % | 261 (69.2) | 94 (75.2) | 82 (65.1) | 85 (67.5) | 0.192 |
| Beta-blocker, % | 245 (65.0) | 78 (62.4) | 83 (65.9) | 84 (66.7) | 0.753 |
| Ticagrelor, % | 112 (29.7) | 28 (22.4) | 43 (34.1) | 41 (32.5) | 0.088 |
| ACEI/ARB, % | 228 (60.5) | 71 (56.8) | 70 (55.6) | 87 (69.0) | 0.054 |
| SOD a, ng/mL | 53.7 (43.5, 66.0) | 53.8 (40.3, 65.1) | 53.7 (45.0, 66.8) | 53.6 (43.6, 65.8) | 0.630 |
| GPX b, ng/mL | 78.3 (33.9, 154.6) | 70.9 (30.9, 139.5) | 88.6 (39.5, 170.9) | 69.7 (35.6, 194.4) | 0.433 |
| MDA c, μg/mL | 16.2 (10.4, 20.1) | 15.4 (9.1, 18.6) | 16.3 (11.1, 20.2) | 16.6 (11.0, 21.0) | 0.188 |
| Characteristic | HR (95% CI) | p-Value |
|---|---|---|
| Ages, years | 1.034 (1.016–1.051) | <0.001 |
| Females, % | 2.168 (1.405–3.346) | <0.001 |
| New AMI, % | 1.049 (0.600–1.837) | 0.866 |
| Previous histories | ||
| Stroke, % | 1.311 (0.712–2.417) | 0.385 |
| Hypertension, % | 0.872 (0.568–1.341) | 0.534 |
| Diabetes, % | 1.295 (0.811–2.068) | 0.279 |
| Smoking, % | 0.535 (0.349–0.822) | 0.004 |
| Systolic BP, mmHg | 1.001 (0.991–1.011) | 0.888 |
| Diastolic BP, mmHg | 0.994 (0.979–1.010) | 0.473 |
| Heart rate, beats/min | 1.008 (0.996–1.020) | 0.210 |
| Creatinine, µmol/L | 1.000 (0.999–1.000) | 0.718 |
| Total cholesterol, mmol/L | 0.993 (0.963–1.024) | 0.649 |
| Low-density lipoprotein, mmol/L | 0.965 (0.781–1.193) | 0.745 |
| High-density lipoprotein, mmol/L | 0.956 (0.800–1.142 | 0.618 |
| Triglycerides, mmol/L | 0.892 (0.768–1.035) | 0.132 |
| Fasting glucose, mmol/L | 1.036 (0.966–1.111) | 0.328 |
| Cardiac Troponins I, μg/L | 1.000 (0.999–1.001) | 0.528 |
| ST-segment elevation, % | 1.176 (0.766–1.805) | 0.460 |
| Killip II/III class, % | 2.313 (1.323–4.044) | 0.003 |
| PCI, % | 0.831 (0.523–1.320) | 0.432 |
| Medical treatment | ||
| Aspirin, % | 0.408 (0.178–0.937) | 0.034 |
| Statin, % | 0.309 (0.149–0.641) | 0.002 |
| Clopidogrel, % | 1.241 (0.768–2.005) | 0.377 |
| Beta-blocker, % | 0.591 (0.385–0.908) | 0.016 |
| Ticagrelor, % | 0.572 (0.336–0.975) | 0.040 |
| ACEI/ARB, % | 0.814 (0.529–1.251) | 0.348 |
| Outcomes | T1 (≤7.59 ng/mL) | T2 (7.59~15.38 ng/mL) | T3 (≥15.38 ng/mL) |
|---|---|---|---|
| No. of recurrent AMI | 23 | 13 | 11 |
| Incidence rate (per 1000 person months) | 14.1 | 8.0 | 6.8 |
| HR (95% CI) | |||
| Model 1 | Ref. | 0.570 (0.289–1.125) | 0.479 (0.233–0.982) |
| Model 2 | Ref. | 0.441 (0.215–0.907) | 0.450 (0.213–0.948) |
| No. of strokes | 5 | 2 | 3 |
| Incidence rate (per 1000 person months) | 2.9 | 1.2 | 1.8 |
| HR (95% CI) | |||
| Model 1 | Ref. | 0.416 (0.081–2.146) | 0.652 (0.156–2.731) |
| Model 2 | Ref. | 0.184 (0.030–1.131) | 0.293 (0.057–1.495) |
| No. of CVD deaths | 6 | 5 | 5 |
| Incidence rate (per 1000 person months) | 3.5 | 2.9 | 3.0 |
| HR (95% CI) | |||
| Model 1 | Ref. | 0.839 (0.256–2.750) | 0.853 (0.260–2.798) |
| Model 2 | Ref. | 0.845 (0.208–3.439) | 0.616 (0.150–2.521) |
| No. of all-cause mortality | 9 | 7 | 9 |
| Incidence rate (per 1000 person months) | 5.3 | 4.2 | 5.5 |
| HR (95% CI) | |||
| Model 1 | Ref. | 0.780 (0.290–2.094) | 1.031 (0.409–2.599) |
| Model 2 | Ref. | 0.822 (0.271–2.498) | 0.832 (0.285–2.427) |
| No. of MACE | 37 | 23 | 24 |
| Incidence rate (per 1000 person months) | 23.9 | 14.8 | 15.6 |
| HR (95% CI) | |||
| Model 1 | Ref. | 0.620 (0.368–1.043) | 0.656 (0.392–1.096) |
| Model 2 | Ref. | 0.516 (0.298–0.893) | 0.566 (0.329–0.974) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Z.; Jiao, Y.; Zhang, J.; Xu, Q.; Xu, J.; Li, R.; Yuan, W.; Guo, H.; Sun, Z.; Zheng, L. Effect of Serum Spermidine on the Prognosis in Patients with Acute Myocardial Infarction: A Cohort Study. Nutrients 2022, 14, 1394. https://doi.org/10.3390/nu14071394
Yu Z, Jiao Y, Zhang J, Xu Q, Xu J, Li R, Yuan W, Guo H, Sun Z, Zheng L. Effect of Serum Spermidine on the Prognosis in Patients with Acute Myocardial Infarction: A Cohort Study. Nutrients. 2022; 14(7):1394. https://doi.org/10.3390/nu14071394
Chicago/Turabian StyleYu, Zhecong, Yundi Jiao, Jin Zhang, Qianyi Xu, Jiahui Xu, Ruixue Li, Wei Yuan, Hui Guo, Zhaoqing Sun, and Liqiang Zheng. 2022. "Effect of Serum Spermidine on the Prognosis in Patients with Acute Myocardial Infarction: A Cohort Study" Nutrients 14, no. 7: 1394. https://doi.org/10.3390/nu14071394
APA StyleYu, Z., Jiao, Y., Zhang, J., Xu, Q., Xu, J., Li, R., Yuan, W., Guo, H., Sun, Z., & Zheng, L. (2022). Effect of Serum Spermidine on the Prognosis in Patients with Acute Myocardial Infarction: A Cohort Study. Nutrients, 14(7), 1394. https://doi.org/10.3390/nu14071394





