Depression, Is It Treatable in Adults Utilising Dietary Interventions? A Systematic Review of Randomised Controlled Trials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Source/Literature Search
2.2. Eligibility Criteria
2.2.1. Type of Participants
2.2.2. Type of Intervention
2.2.3. Type of Studies
2.2.4. Type of Outcomes
2.3. Risk of Bias
2.4. Data Extraction
3. Results
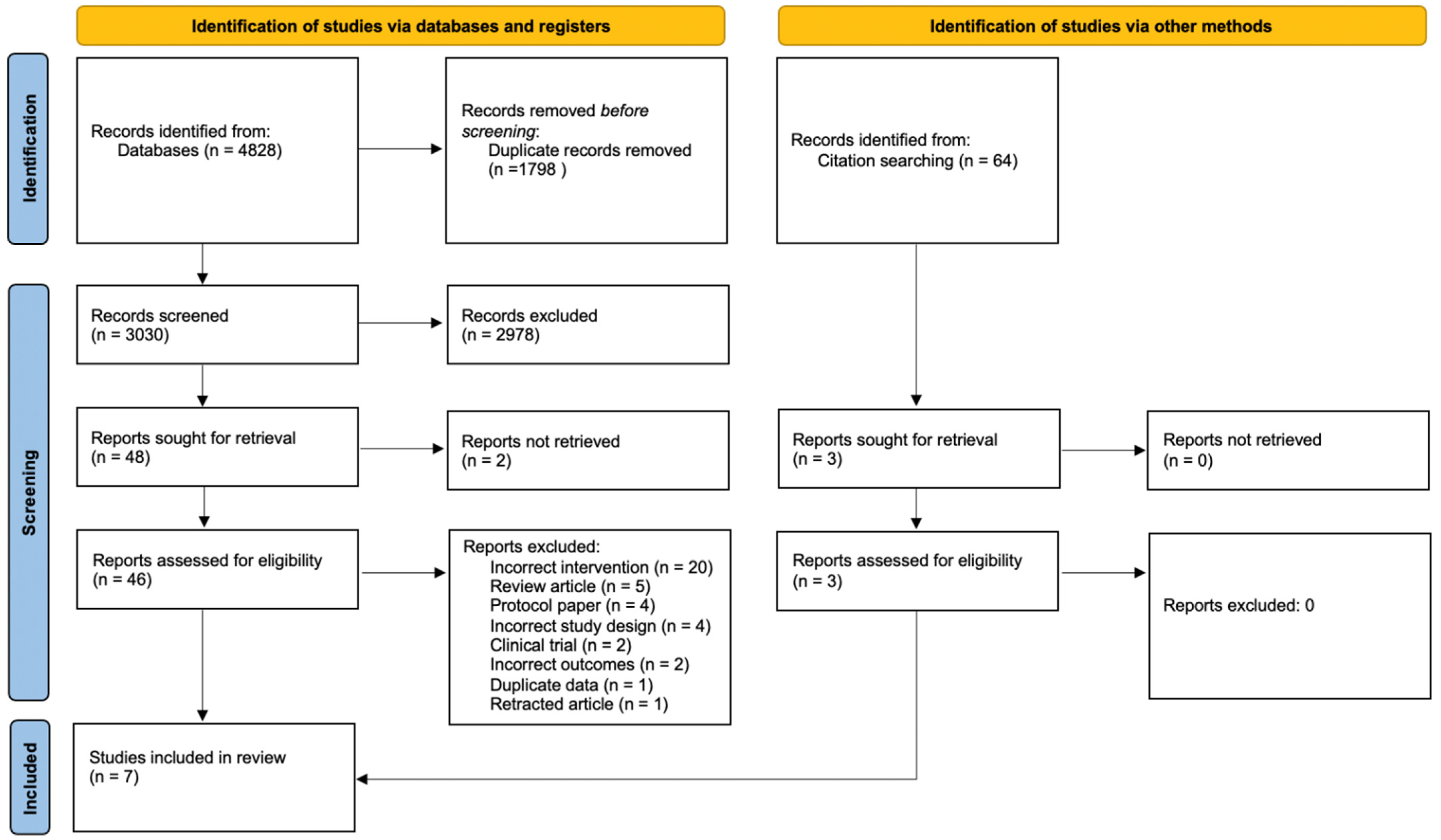
3.1. Study Selection
3.2. Quality Assessment
3.3. Study Characteristics
3.3.1. Location and Sample Size
3.3.2. Population Characteristics
3.3.3. Study Aims and Primary Outcomes
3.4. Intervention Description
3.4.1. Session Details and Follow-Up Duration
3.4.2. Intervention Style and Programme Components
3.4.3. Depression Outcome Measures
3.5. Results for Depression
3.6. Quality Rating
4. Discussion
4.1. Strengths and Limitations
4.2. Future Research Implications
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fact Sheet: Depression. Available online: https://www.who.int/news-room/fact-sheets/detail/depression (accessed on 16 August 2021).
- Schofield, D.; Cunich, M.; Shrestha, R.; Tanton, R.; Veerman, L.; Kelly, S.; Passey, M. Indirect costs of depression and other mental and behavioural disorders for Australia from 2015 to 2030. BJPsych Open 2019, 5, 1–7. [Google Scholar] [CrossRef] [PubMed]
- National Mental Health Reform 2011–12: The Challenges. Available online: https://www1.health.gov.au/internet/publications/publishing.nsf/Content/nmhr11–12~nmhr11–12-challenges (accessed on 16 August 2021).
- Depression: Impact. Available online: https://www.who.int/health-topics/depression#tab=tab_2 (accessed on 16 August 2021).
- Gin, S.M.; Mann, J.J. Depression. Lancet 2018, 392, 2299. [Google Scholar] [CrossRef]
- Low Dog, T. The role of nutrition in mental health. Altern. Ther. Health Med. 2010, 16, 42–46. [Google Scholar] [PubMed]
- Casacalenda, N.; Perry, J.C.; Looper, K. Remission in Major Depressive Disorder: A Comparison of Pharmacotherapy, Psychotherapy, and Control Conditions. Am. J. Psychiatry 2002, 159, 1354–1360. [Google Scholar] [CrossRef] [Green Version]
- Machmutow, K.; Meister, R.; Jansen, A.; Kriston, L.; Watzke, B.; Härter, M.C.; Liebherz, S.; Liebherz, S. Comparative effectiveness of continuation and maintenance treatments for persistent depressive disorder in adults. Cochrane Libr. 2019, 2019, CD012855. [Google Scholar] [CrossRef]
- Adan, R.A.H.; van der Beek, E.M.; Buitelaar, J.K.; Cryan, J.F.; Hebebrand, J.; Higgs, S.; Schellekens, H.; Dickson, S.L. Nutritional psychiatry: Towards improving mental health by what you eat. Eur. Neuropsychopharmacol. 2019, 29, 1321–1332. [Google Scholar] [CrossRef]
- Lassale, C.; Batty, G.D.; Baghdadli, A.; Jacka, F.; Sánchez-Villegas, A.; Kivimäki, M.; Akbaraly, T. Healthy dietary indices and risk of depressive outcomes: A systematic review and meta-analysis of observational studies. Mol. Psychiatry 2018, 24, 965–986. [Google Scholar] [CrossRef] [Green Version]
- Molendijk, M.; Molero, P.; Ortuño Sánchez-Pedreño, F.; Van der Does, W.; Angel Martínez-González, M. Diet quality and depression risk: A systematic review and dose-response meta-analysis of prospective studies. J. Affect. Disord. 2018, 226, 346–354. [Google Scholar] [CrossRef] [Green Version]
- Wu, P.-Y.; Lin, M.-Y.; Tsai, P.-S. Alternate healthy eating index and risk of depression: A meta-analysis and systemematic review. Nutr. Neurosci. 2020, 23, 101–109. [Google Scholar] [CrossRef]
- Marx, W.; Lane, M.; Hockey, M.; Aslam, H.; Berk, M.; Walder, K.; Borsini, A.; Firth, J.; Pariante, C.M.; Berding, K.; et al. Diet and depression: Exploring the biological mechanisms of action. Mol. Psychiatry 2021, 26, 134–150. [Google Scholar] [CrossRef]
- Jang, S.-H.; Woo, Y.S.; Lee, S.-Y.; Bahk, W.-M. The Brain-Gut-Microbiome Axis in Psychiatry. Int. J. Mol. Sci. 2020, 21, 7122. [Google Scholar] [CrossRef] [PubMed]
- Jacka, F.N. Nutritional Psychiatry: Where to Next? EBioMedicine 2017, 17, 24–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lai, J.S.; Hiles, S.; Bisquera, A.; Hure, A.J.; McEvoy, M.; Attia, J. A systematic review and meta-analysis of dietary patterns and depression in community-dwelling adults. Am. J. Clin. Nutr. 2014, 99, 181–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Psaltopoulou, T.; Sergentanis, T.N.; Panagiotakos, D.B.; Sergentanis, I.N.; Kosti, R.; Scarmeas, N. Mediterranean diet, stroke, cognitive impairment, and depression: A meta-analysis. Ann. Neurol. 2013, 74, 580–591. [Google Scholar] [CrossRef]
- Firth, J.; Marx, W.; Dash, S.; Carney, R.; Teasdale, S.B.; Solmi, M.; Stubbs, B.; Schuch, F.B.; Carvalho, A.F.; Jacka, F.; et al. The Effects of Dietary Improvement on Symptoms of Depression and Anxiety: A Meta-Analysis of Randomized Controlled Trials. Psychosom. Med. 2019, 81, 265–280. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Monteiro, C.A.; Moubarac, J.C.; Cannon, G.; Ng, S.W.; Popkin, B. Ultra-processed products are becoming dominant in the global food system. Obes. Rev. 2013, 14, 21–28. [Google Scholar] [CrossRef]
- Busch, A.M.; Whited, M.C.; Appelhans, B.M.; Schneider, K.L.; Waring, M.E.; DeBiasse, M.A.; Oleski, J.L.; Crawford, S.L.; Pagoto, S.L. Reliable change in depression during behavioral weight loss treatment among women with major depression. Obesity 2013, 21, E211–E218. [Google Scholar] [CrossRef] [Green Version]
- Snaith, P. What Do Depression Rating Scales Measure? Br. J. Psychiatry 1993, 163, 293–298. [Google Scholar] [CrossRef]
- Nezu, A.M.; Ronan, G.F.; Meadows, E.A. Measures of depression, depressive symptomatology, and depressive mood. In Practitioner’s Guide to Empirically Based Measures of Depression, 2000th ed.; Kluwer Academic/Plenum Publishers: New York, NY, USA, 2000; Chapter 4. [Google Scholar]
- Giglio, M.G.; Huttenhower, C.; Gevers, D.; Petrosino, J.F.; Lobos, E.A.; Madupu, R.; Mitreva, M.; Versalovic, J.; Wollam, A.M.; Abolude, O.O.; et al. A framework for human microbiome research. Nature 2012, 486, 215–221. [Google Scholar] [CrossRef] [Green Version]
- Crutzen, R. Adding effect sizes to a systematic review on interventions for promoting physical activity among European teenagers. Int. J. Behav. Nutr. Phys. Act. 2010, 7, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Assaf, A.R.P.; Beresford, S.A.A.P.; Risica, P.M.D.R.D.; Aragaki, A.; Brunner, R.L.P.; Bowen, D.J.P.; Naughton, M.P.; Rosal, M.C.P.; Snetselaar, L.P.; Wenger, N.M.D. Low-Fat Dietary Pattern Intervention and Health-Related Quality of Life: The Women’s Health Initiative Randomized Controlled Dietary Modification Trial. J. Acad. Nutr. Diet. 2016, 116, 259–271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Francis, H.M.; Stevenson, R.J.; Chambers, J.R.; Gupta, D.; Newey, B.; Lim, C.K. A brief diet intervention can reduce symptoms of depression in young adults – A randomised controlled trial. PLoS ONE 2019, 14, e0222768. [Google Scholar] [CrossRef] [PubMed]
- Jacka, F.N.; O’Neil, A.; Opie, R.; Itsiopoulos, C.; Cotton, S.; Mohebbi, M.; Castle, D.; Dash, S.; Mihalopoulos, C.; Chatterton, M.L.; et al. A randomised controlled trial of dietary improvement for adults with major depression (the ‘SMILES’ trial). BMC Med. 2017, 15, 23. [Google Scholar] [CrossRef] [Green Version]
- Kontogianni, M.D.; Vijayakumar, A.; Rooney, C.; Noad, R.L.; Appleton, K.M.; McCarthy, D.; Donnelly, M.; Young, I.S.; McKinley, M.C.; McKeown, P.P.; et al. A High Polyphenol Diet Improves Psychological Well-Being: The Polyphenol Intervention Trial (PPhIT). Nutrients 2020, 12, 2445. [Google Scholar] [CrossRef]
- Lindseth, G.; Helland, B.; Caspers, J. The Effects of Dietary Tryptophan on Affective Disorders. Arch. Psychiatr. Nurs. 2015, 29, 102–107. [Google Scholar] [CrossRef] [Green Version]
- McMillan, L.; Owen, L.; Kras, M.; Scholey, A. Behavioural effects of a 10-day Mediterranean diet. Results from a pilot study evaluating mood and cognitive performance. Appetite 2011, 56, 143–147. [Google Scholar] [CrossRef]
- Park, M.; Choi, J.; Lee, H.-J. Flavonoid-Rich Orange Juice Intake and Altered Gut Microbiome in Young Adults with Depressive Symptom: A Randomized Controlled Study. Nutrients 2020, 12, 1815. [Google Scholar] [CrossRef]
- Jacka, F.N.; Kremer, P.J.; Berk, M.; de Silva-Sanigorski, A.M.; Moodie, M.; Leslie, E.R.; Pasco, J.A.; Swinburn, B.A. A Prospective Study of Diet Quality and Mental Health in Adolescents. PLoS ONE 2011, 6, e24805. [Google Scholar] [CrossRef]
- Cezary, C.; Tomasz, P.; Jan, C.; Michał, F.; Paulina, K.; Janusz, B. Tryptophan Intake and Metabolism in Older Adults with Mood Disorders. Nutrients 2020, 12, 3183. [Google Scholar] [CrossRef]
- Bayes, J.; Schloss, J.; Sibbritt, D. Effects of Polyphenols in a Mediterranean Diet on Symptoms of Depression: A Systematic Literature Review. Adv. Nutr. 2020, 11, 602–615. [Google Scholar] [CrossRef] [PubMed]
- Godos, J.; Castellano, S.; Ray, S.; Grosso, G.; Galvano, F. Dietary Polyphenol Intake and Depression: Results from the Mediterranean Healthy Eating, Lifestyle and Aging (MEAL) Study. Molecules 2018, 23, 999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tiwari, U.; Cummins, E. Factors influencing levels of phytochemicals in selected fruit and vegetables during pre- and post-harvest food processing operations. Food Res. Int. 2013, 50, 497–506. [Google Scholar] [CrossRef]
- Hoffman, R.; Gerber, M. Constituents and physiological effects of Mediterranean plant foods. In The Mediterranean Diet: Health and Science, 1st ed.; Wiley-Blackwell: Chichester, UK, 2012; Chapter 2. [Google Scholar]
- Saffel-Shrier, S.; Johnson, M.A.; Francis, S.L. Position of the Academy of Nutrition and Dietetics and the Society for Nutrition Education and Behavior: Food and Nutrition Programs for Community-Residing Older Adults. J. Acad. Nutr. Diet. 2019, 119, 1188–1204. [Google Scholar] [CrossRef] [Green Version]
- Holmes, A.L.; Sanderson, B.; Maisiak, R.; Brown, A.; Bittner, V. Dietitian Services Are Associated with Improved Patient Outcomes and the MEDFICTS Dietary Assessment Questionnaire Is a Suitable Outcome Measure in Cardiac Rehabilitation. J. Am. Diet. Assoc. 2005, 105, 1533–1540. [Google Scholar] [CrossRef]
- Sánchez-Villegas, A.; Henríquez-Sánchez, P.; Ruiz-Canela, M.; Lahortiga, F.; Molero, P.; Toledo, E.; Martínez-González, M.A. A longitudinal analysis of diet quality scores and the risk of incident depression in the SUN Project. BMC Med. 2015, 13, 197. [Google Scholar] [CrossRef] [Green Version]
- Trabulsi, J.; Schoeller, D.A. Evaluation of dietary assessment instruments against doubly labeled water, a biomarker of habitual energy intake. Am. J. Physiol. Endocrinol. Metab. 2001, 281, E891–E899. [Google Scholar] [CrossRef] [Green Version]
- Barnaba, L.; Intorre, F.; Azzini, E.; Ciarapica, D.; Venneria, E.; Foddai, M.S.; Maiani, F.; Raguzzini, A.; Polito, A. Evaluation of adherence to Mediterranean diet and association with clinical and biological markers in an Italian population. Nutrition 2020, 77, 110813. [Google Scholar] [CrossRef]
- McMartin, S.E.; Jacka, F.N.; Colman, I. The association between fruit and vegetable consumption and mental health disorders: Evidence from five waves of a national survey of Canadians. Prev. Med. 2013, 56, 225–230. [Google Scholar] [CrossRef]
- Santor, D.A.; Gregus, M.; Welch, A. FOCUS ARTICLE: Eight Decades of Measurement in Depression. Measurement 2006, 4, 135–155. [Google Scholar] [CrossRef]
- Fried, E.I.; Nesse, R.M. Depression sum-scores don’t add up: Why analyzing specific depression symptoms is essential. BMC Med. 2015, 13, 72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fried, E.I.; Nesse, R.M.; Zivin, K.; Guille, C.; Sen, S. Depression is more than the sum score of its parts: Individual DSM symptoms have different risk factors. Psychol. Med. 2014, 44, 2067–2076. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antony, M.M.; Barlow, D.H. Handbook of Assessment and Treatment Planning for Psychological Disorders, 2nd ed.; Guilford Publications: New York, NY, USA, 2010. [Google Scholar]
- Salk, R.H.; Hyde, J.S.; Abramson, L.Y. Gender Differences in Depression in Representative National Samples: Meta-Analyses of Diagnoses and Symptoms. Psychol. Bull. 2017, 143, 783–822. [Google Scholar] [CrossRef] [PubMed]
- Akincigil, A.; Olfson, M.; Siegel, M.; Zurlo, K.A.; Walkup, J.T.; Crystal, S. Racial and Ethnic Disparities in Depression Care in Community-Dwelling Elderly in the United States. Am. J. Public Health 2012, 102, 319–328. [Google Scholar] [CrossRef]
- Schuch, F.B.; Vancampfort, D.; Firth, J.; Rosenbaum, S.; Ward, P.B.; Silva, E.S.; Hallgren, M.; Ponce De Leon, A.; Dunn, A.L.; Deslandes, A.C.; et al. Physical Activity and Incident Depression: A Meta-Analysis of Prospective Cohort Studies. Am. J. Psychiatry 2018, 175, 631–648. [Google Scholar] [CrossRef] [PubMed]
- Robinson, R.L.; Grabner, M.; Palli, S.R.; Faries, D.; Stephenson, J.J. Covariates of depression and high utilizers of healthcare: Impact on resource use and costs. J. Psychosom. Res. 2016, 85, 35–43. [Google Scholar] [CrossRef] [Green Version]
- Munafò, M.R.; Araya, R. Cigarette smoking and depression: A question of causation. Br. J. Psychiatry 2018, 196, 425–426. [Google Scholar] [CrossRef] [Green Version]
- Staudacher, H.M.; Irving, P.M.; Lomer, M.C.E.; Whelan, K. The challenges of control groups, placebos and blinding in clinical trials of dietary interventions. Proc. Nutr. Soc. 2017, 76, 203–212. [Google Scholar] [CrossRef] [Green Version]
- Cumming, G. The New Statistics: Why and How. Psychol. Sci. 2014, 25, 7–29. [Google Scholar] [CrossRef]
- Vasishth, S.; Mertzen, D.; Jäger, L.A.; Gelman, A. The statistical significance filter leads to overoptimistic expectations of replicability. J. Mem. Lang. 2018, 103, 151–175. [Google Scholar] [CrossRef]
- van der Pols, J.C. Nutrition and mental health: Bidirectional associations and multidimensional measures. Public Health Nutr. 2018, 21, 829–830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McDowell, I. Measuring Health: A Guide to Rating Scales and Questionnaires, 3rd ed.; Oxford University Press: New York, NY, USA, 2006. [Google Scholar]
| Author and Year | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9 | Q10 | Overall Rating |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Assaf et al. (2016) [26] | U | Y | U | N | U | N | Y | Y | Y | Y | Neutral |
| Francis et al. (2019) [27] | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | Positive |
| Jacka et al. (2017) [28] | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Positive |
| Kontogianni et al. (2020) [29] | Y | Y | Y | Y | U | Y | Y | U | Y | Y | Positive |
| Lindseth et al. (2015) [30] | Y | U | N | U | Y | U | Y | N | U | Y | Neutral |
| McMillan et al. (2011) [31] | U | N | Y | Y | Y | Y | Y | N | Y | N | Neutral |
| Park et al. (2020) [32] | Y | Y | Y | Y | U | Y | Y | N | Y | Y | Positive |
| Reference, Country | Population, Eligibility Criteria | Sample Size | Intervention | Depression and Diet Measures | Primary Statistical Outcomes |
|---|---|---|---|---|---|
| Assaf et al. (2016); USA [26] | Women aged 50–79; Baseline fat intake 32% of total calories | Intervention DM, n = 19,541 Comparison CG, n = 29,294 given no dietary instruction | Reduced fat, healthy diet intervention. Total dietary fat 20% of energy with individual goals set based on height. Fruit and vegetables 5 servings/day. Grains 6 servings/day. Group education sessions delivered by trained nutritionists. | RAND 36-Item Health Survey Subscale; Dietary Compliance—FFQ | RAND 36 DM mean score change (−0.05) significantly greater than mean score change CG (−0.12) Mean difference (0.07 [95%CI 0.02 to 0.12; p = 0.009]) |
| Francis et al. (2019); Australia [27] | Individuals aged 17–35; with a score of 7 or more on the DASS-21 and greater than 57 on the DFS with antidepressant use greater than 2 weeks if relevant | Intervention DC, n = 38 Comparison HD, n = 38; given no dietary instruction | Diet based on AGTHE and Mediterranean diet (decreased refined carbohydrate, fatty or processed meats and soft drinks). Education delivered by a qualified dietitian: face to face contact at baseline and day 21; phone contact at days 7, 14 and 3 months. | CESD-R; DASS-21; Dietary Compliance—Diet Compliance Score Questionnaire | CESD-R (DC/HD—day 21) significantly lower than HD at day 21 (F[1.75] = 7.792, p = 0.007, Cohen’s d = 0.65); when age, gender, physical activity and baseline BMI controlled for, signficance remained (F[1.71] = 7.091, p = 0.010; |
| Jacka et al. (2017); Australia [28] | Individuals aged >18 years that meet DSM-IV diagnostic criteria, have a score of 18+ on MADRS and a score of <75 on a dietary screening tool | Intervention DSG, n = 31 Dietary Counselling Comparison SSCG n = 25; Non-dietary counselling | Improved diet quality with recommended servings specified for 12 key food types. Seven 1-h individual dietary support sessions—weekly for first four weeks, and then fortnightly for six weeks; delivered by a clinical dietitian | MADRS; Secondary Measures—HADS and POMS; dietary compliance—ModiMedDiet via 7-day food diaries | MADRS—T(60.7) = 4.38, p < 0.001; Cohen’s d = 1.16 (95% CI −1.73, −0.59); After controlling for covariates t(58.7) = 4.40, p < 0.001; mean (±SE) |
| Kontogianni et al. (2020); UK [29] | Individuals aged 40–65 years with documented grade I or II hypertension | Intervention HPD group, n = 50 Comparison LPD group, n = 49 Continued with ‘washout’ diet | High antioxidant diet: 4 week ‘washout’ period with <2 fruit and vegetable portions daily plus exclusion of berries and dark chocolate. An 8 week period with consumption of 6 portions of fruit and vegetable (including a portion of berries) and 50 g of dark chocolate daily. Group education baseline and week 4. Qualifications of professionals delivering intervention not stated. | PANAS; BDI-II (21-item scale); DASS-21 (21-item scale); dietary compliance—4-day food diary at weeks 4 and 12 | BDI-II;—significant between-group difference (p = 0.01) for depressive symptoms;—significant within-group (HPD) difference 3.4 (p < 0.001) for depressive symptoms; PANAS—significant within-group (HPD) for PA 2.2 (p = 0.03). |
| Lindseth et al. (2015); USA [30] | University students aged > 18 years | Intervention HTD, n = 25 Comparison LTD, n = 25 within subject, crossover, double blinded study | High tryptophan diet: 4 days of meals that met EER and US RDA (5% variance). Caffeine limited to 100 mg/d. LTD phase—5 mg/kg body weight/d of tryptophan; HTD phase—10 mg/kg body weight/day of tryptophan. All meals provided in dining room, 2 week washout period between phases. Intervention conducted by dietitian, nurse and psychologist. | Zung’s SDS; PANAS direct observation of meal consumption | Within-subject analysis found low levels of tryptophan intake associated with increased rate of depression (paired t = 2.2, p = 0.02) |
| McMillan et al. (2011); Australia [31] | Females aged 19–30 years | Intervention DC n = 12 Comparison NC n = 13; Continued usual daily diet | Healthy diet intervention. Increased fruit, vegetables, fatty fish, nuts, seeds, low-fat natural dairy and wholegrain cereals; excluded red meat, refined sugars and flour, pre-packaged and processed foods, caffeinated products, soft drinks and condiments. | POMS; Bond-Lader VAS; dietary compliance—daily food diary | No significant change for depression; mean (±SD); Pre 21.92 ± 7.23; Post 18.83 ± 5.44 |
| Park et al. (2020); Korea [32] | Individuals aged between 20–30 years with a CES-D score ≥ 21 | Intervention FR n = 20; FL n = 20 Comparison n = 40; Pre-treatment sample | High flavonoid whole food intervention. Participants continued usual exercise and diet, limited HF and high-sucrose foods, fruits, juice, tea, jams and alcohol. 30–60 min before breakfast and dinner consumed 190 mL juice that naturally provided high (FR 157.9 mg/100 g) or low (FL 28.4 mg/100 g) level of flavonoids. | CES-D; dietary compliance—24 h dietary recall and FFQ | CES-D scores decreased to <20 points. Multiple regression analysis showed CES-D in FR decreased significantly (p < 0.0001) compared to FL (p < 0.001) post intervention |
| Author and Year | RAND36 | BDI-II | Bond and Lader VAS | CES-D/CESD-R | DASS-21 | HADS | MADRS | PANAS | POMS/POMS-A | Zung’s SDS |
|---|---|---|---|---|---|---|---|---|---|---|
| Assaf et al. (2016) [26] | √ | |||||||||
| Francis et al. (2019) [27] | √ | X | X | |||||||
| Jacka et al. (2017) [28] | X | √ | X | |||||||
| Kontogianni et al. (2020) [29] | √ | X+ | X | |||||||
| Lindseth et al. (2015) [30] | X | √ | ||||||||
| McMillan et al. (2011) [31] | √ | √ | ||||||||
| Park et al. (2020) [32] | √ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
O’Neill, S.; Minehan, M.; Knight-Agarwal, C.R.; Turner, M. Depression, Is It Treatable in Adults Utilising Dietary Interventions? A Systematic Review of Randomised Controlled Trials. Nutrients 2022, 14, 1398. https://doi.org/10.3390/nu14071398
O’Neill S, Minehan M, Knight-Agarwal CR, Turner M. Depression, Is It Treatable in Adults Utilising Dietary Interventions? A Systematic Review of Randomised Controlled Trials. Nutrients. 2022; 14(7):1398. https://doi.org/10.3390/nu14071398
Chicago/Turabian StyleO’Neill, Simone, Michelle Minehan, Catherine R. Knight-Agarwal, and Murray Turner. 2022. "Depression, Is It Treatable in Adults Utilising Dietary Interventions? A Systematic Review of Randomised Controlled Trials" Nutrients 14, no. 7: 1398. https://doi.org/10.3390/nu14071398







