Inverse Association of Plasma Vanadium Concentrations with Gestational Diabetes Mellitus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Laboratory Measurements
2.3. Statistical Analysis
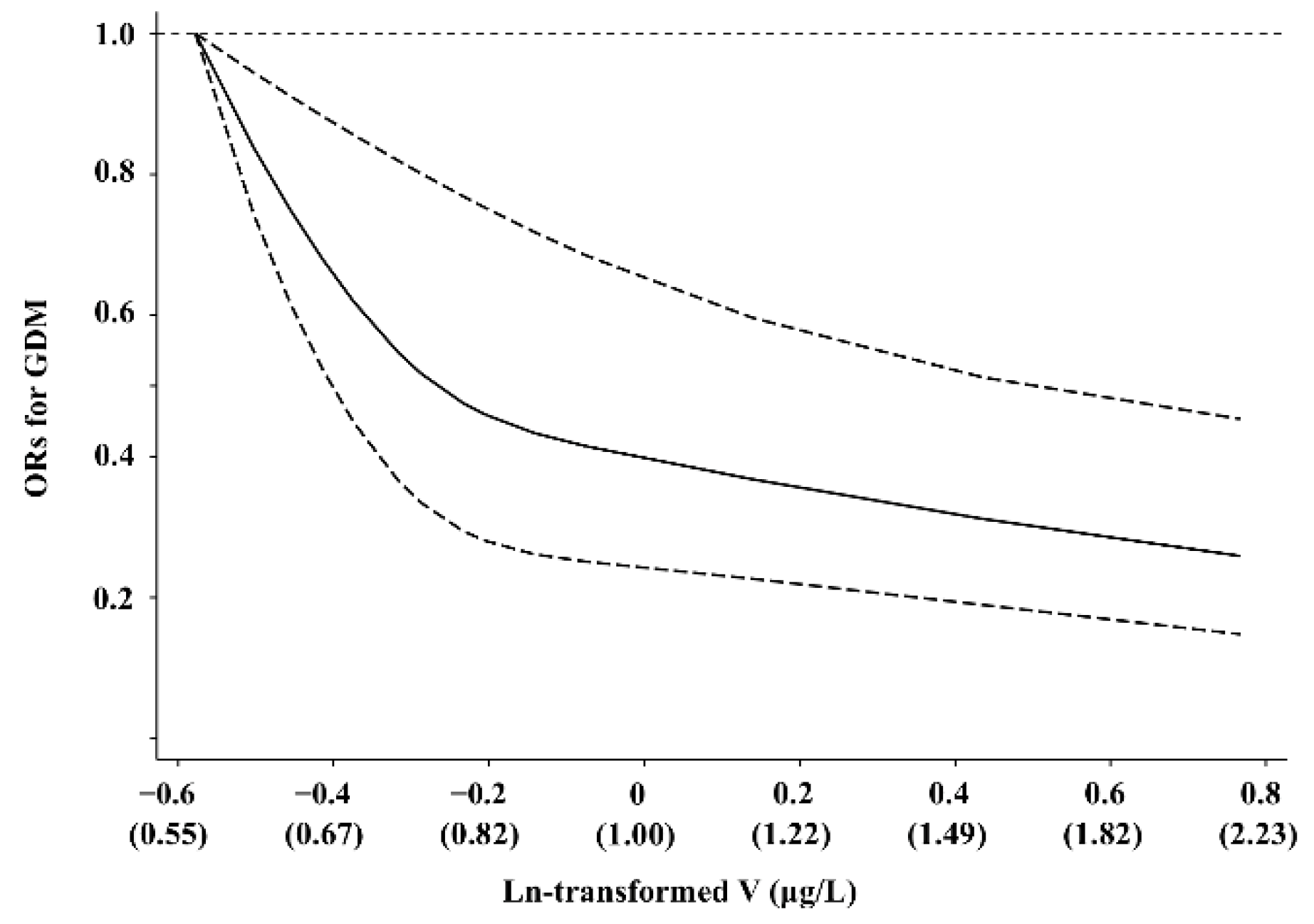
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- American Diabetes Association. Gestational diabetes mellitus. Diabetes Care 2004, 27 (Suppl. S1), S88–S90. [Google Scholar] [CrossRef] [Green Version]
- Farrar, D.; Simmonds, M.; Bryant, M.; Sheldon, T.A.; Tuffnell, D.; Golder, S.; Dunne, F.; Lawlor, D.A. Hyperglycaemia and risk of adverse perinatal outcomes: Systematic review and meta-analysis. BMJ 2016, 354, i4694. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reece, E.A.; Leguizamon, G.; Wiznitzer, A. Gestational diabetes: The need for a common ground. Lancet 2009, 373, 1789–1797. [Google Scholar] [CrossRef] [PubMed]
- Sellers, E.A.; Dean, H.J.; Shafer, L.A.; Martens, P.J.; Phillips-Beck, W.; Heaman, M.; Prior, H.J.; Dart, A.B.; McGavock, J.; Morris, M.; et al. Exposure to Gestational Diabetes Mellitus: Impact on the Development of Early-Onset Type 2 Diabetes in Canadian First Nations and Non-First Nations Offspring. Diabetes Care 2016, 39, 2240–2246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scibior, A.; Pietrzyk, L.; Plewa, Z.; Skiba, A. Vanadium: Risks and possible benefits in the light of a comprehensive overview of its pharmacotoxicological mechanisms and multi-applications with a summary of further research trends. J. Trace Elem. Med. Biol. 2020, 61, 126508. [Google Scholar] [CrossRef] [PubMed]
- Barceloux, D.G. Vanadium. J. Toxicol. Clin. Toxicol. 1999, 37, 265–278. [Google Scholar] [CrossRef]
- Mukherjee, B.; Patra, B.; Mahapatra, S.; Banerjee, P.; Tiwari, A.; Chatterjee, M. Vanadium—An element of atypical biological significance. Toxicol. Lett. 2004, 150, 135–143. [Google Scholar] [CrossRef]
- Heyliger, C.E.; Tahiliani, A.G.; McNeill, J.H. Effect of vanadate on elevated blood glucose and depressed cardiac performance of diabetic rats. Science 1985, 227, 1474–1477. [Google Scholar] [CrossRef]
- Trevino, S.; Diaz, A.; Sanchez-Lara, E.; Sanchez-Gaytan, B.L.; Perez-Aguilar, J.M.; Gonzalez-Vergara, E. Vanadium in Biological Action: Chemical, Pharmacological Aspects, and Metabolic Implications in Diabetes Mellitus. Biol. Trace Elem. Res. 2019, 188, 68–98. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Sun, T.; Liu, J.; Shan, Z.; Jin, Y.; Chen, S.; Bao, W.; Hu, F.B.; Liu, L. Inverse association of plasma vanadium levels with newly diagnosed type 2 diabetes in a Chinese population. Am. J. Epidemiol. 2014, 180, 378–384. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Gao, D.; Zhang, G.; Zhang, X.; Li, Q.; Gao, Q.; Chen, R.; Xu, S.; Huang, L.; Zhang, Y.; et al. Exposure to multiple metals in early pregnancy and gestational diabetes mellitus: A prospective cohort study. Environ. Int. 2020, 135, 105370. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, T.; Zhang, Y.; Hu, Q.; Wang, X.; Chang, H.; Mao, J.H.; Snijders, A.M.; Xia, Y. Contribution of trace element exposure to gestational diabetes mellitus through disturbing the gut microbiome. Environ. Int. 2021, 153, 106520. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2018. Diabetes Care 2018, 41, S13–S27. [Google Scholar] [CrossRef] [Green Version]
- Li, P.; Yin, J.; Zhu, Y.; Li, S.; Chen, S.; Sun, T.; Shan, Z.; Wang, J.; Shang, Q.; Li, X.; et al. Association between plasma concentration of copper and gestational diabetes mellitus. Clin. Nutr. 2019, 38, 2922–2927. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, J.; Peng, S.; Wang, X.; Luo, L.; Liu, L.; Huang, Q.; Tian, M.; Zhang, X.; Shen, H. Multiple elements related to metabolic markers in the context of gestational diabetes mellitus in meconium. Environ. Int. 2018, 121, 1227–1234. [Google Scholar] [CrossRef]
- Lv, Y.; Xie, L.; Dong, C.; Yang, R.; Long, T.; Yang, H.; Chen, L.; Zhang, L.; Chen, X.; Luo, X.; et al. Co-exposure of serum calcium, selenium and vanadium is nonlinearly associated with increased risk of type 2 diabetes mellitus in a Chinese population. Chemosphere 2021, 263, 128021. [Google Scholar] [CrossRef]
- Brichard, S.M.; Bailey, C.J.; Henquin, J.C. Marked improvement of glucose homeostasis in diabetic ob/ob mice given oral vanadate. Diabetes 1990, 39, 1326–1332. [Google Scholar] [CrossRef]
- Wang, J.; Yuen, V.G.; McNeill, J.H. Effect of vanadium on insulin sensitivity and appetite. Metab. Clin. Exp. 2001, 50, 667–673. [Google Scholar] [CrossRef] [Green Version]
- Boden, G.; Chen, X.; Ruiz, J.; van Rossum, G.D.; Turco, S. Effects of vanadyl sulfate on carbohydrate and lipid metabolism in patients with non-insulin-dependent diabetes mellitus. Metab. Clin. Exp. 1996, 45, 1130–1135. [Google Scholar] [CrossRef]
- Molero, J.C.; Perez, C.; Martinez, C.; Villar, M.; Andres, A.; Fermin, Y.; Carrascosa, J.M. Activation of MAP kinase by insulin and vanadate in adipocytes from young and old rats. Mol. Cell. Endocrinol. 2002, 189, 77–84. [Google Scholar] [CrossRef]
- Lu, B.; Ennis, D.; Lai, R.; Bogdanovic, E.; Nikolov, R.; Salamon, L.; Fantus, C.; Le-Tien, H.; Fantus, I.G. Enhanced sensitivity of insulin-resistant adipocytes to vanadate is associated with oxidative stress and decreased reduction of vanadate (+5) to vanadyl (+4). J. Biol. Chem. 2001, 276, 35589–35598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Elberg, G.; Sekar, N.; bin He, Z.; Shechter, Y. Antilipolytic actions of vanadate and insulin in rat adipocytes mediated by distinctly different mechanisms. Endocrinology 1997, 138, 2274–2279. [Google Scholar] [CrossRef] [PubMed]
- Marita, A.R.; Anilkumar, K.L. Effect of vanadate on glycogen synthesis in dexamethasone-treated 3T3 adipocytes: Evidence for a novel insulin sensitizing action. Diabetes Obes. Metab. 2001, 3, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Donthi, R.V.; Huisamen, B.; Lochner, A. Effect of vanadate and insulin on glucose transport in isolated adult rat cardiomyocytes. Cardiovasc. Drugs Ther. 2000, 14, 463–470. [Google Scholar] [CrossRef]
- Seale, A.P.; de Jesus, L.A.; Park, M.C.; Kim, Y.S. Vanadium and insulin increase adiponectin production in 3T3-L1 adipocytes. Pharmacol. Res. 2006, 54, 30–38. [Google Scholar] [CrossRef]
- Han, E.S.; Krauss, R.M.; Xu, F.; Sridhar, S.B.; Ferrara, A.; Quesenberry, C.P.; Hedderson, M.M. Prepregnancy Adverse Lipid Profile and Subsequent Risk of Gestational Diabetes. J. Clin. Endocrinol. Metab. 2016, 101, 2721–2727. [Google Scholar] [CrossRef] [Green Version]
- Cohen, N.; Halberstam, M.; Shlimovich, P.; Chang, C.J.; Shamoon, H.; Rossetti, L. Oral vanadyl sulfate improves hepatic and peripheral insulin sensitivity in patients with non-insulin-dependent diabetes mellitus. J. Clin. Investig. 1995, 95, 2501–2509. [Google Scholar] [CrossRef] [Green Version]
- Halberstam, M.; Cohen, N.; Shlimovich, P.; Rossetti, L.; Shamoon, H. Oral vanadyl sulfate improves insulin sensitivity in NIDDM but not in obese nondiabetic subjects. Diabetes 1996, 45, 659–666. [Google Scholar] [CrossRef]
- Tunali, S.; Yanardag, R. Effect of vanadyl sulfate on the status of lipid parameters and on stomach and spleen tissues of streptozotocin-induced diabetic rats. Pharmacol. Res. 2006, 53, 271–277. [Google Scholar] [CrossRef]
- Seo, M.S.; Kim, J.H.; Kim, H.J.; Chang, K.C.; Park, S.W. Honokiol activates the LKB1-AMPK signaling pathway and attenuates the lipid accumulation in hepatocytes. Toxicol. Appl. Pharmacol. 2015, 284, 113–124. [Google Scholar] [CrossRef]
- Zhang, L.; Huang, Y.; Liu, F.; Zhang, F.; Ding, W. Vanadium(IV)-chlorodipicolinate inhibits 3T3-L1 preadipocyte adipogenesis by activating LKB1/AMPK signaling pathway. J. Inorg. Biochem. 2016, 162, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Liu, F.; Zhang, F.; Liu, P.; Xu, T.; Ding, W. Vanadium(IV)-chlorodipicolinate alleviates hepatic lipid accumulation by inducing autophagy via the LKB1/AMPK signaling pathway in vitro and in vivo. J. Inorg. Biochem. 2018, 183, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Ahmadian, M.; Suh, J.M.; Hah, N.; Liddle, C.; Atkins, A.R.; Downes, M.; Evans, R.M. PPARgamma signaling and metabolism: The good, the bad and the future. Nat. Med. 2013, 19, 557–566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Variables | GDM (n = 252) | Controls (n = 252) | p |
|---|---|---|---|
| Age (years) | 30.05 ± 3.76 | 29.56 ± 3.74 | 0.161 |
| Parity, n (%) | 1.000 | ||
| 1 | 203 (80.56) | 203 (80.56) | |
| ≥2 | 49 (19.44) | 49 (19.44) | |
| Gestational age at blood sample collection (wk) | 28.49 ± 2.85 | 28.45 ± 3.09 | 0.903 |
| Pre-pregnancy BMI (kg/m2) | 22.22 ± 3.18 | 20.89 ± 2.80 | <0.001 |
| Family history of diabetes, n (%) | 65 (25.79) | 35 (13.89) | 0.001 |
| Alcohol drinking, n (%) | 12 (4.76) | 12 (4.76) | 1.000 |
| Smoking, n (%) | 4 (1.59) | 6 (2.38) | 0.523 |
| FPG (mmol/L) | 5.24 (5.06–5.45) | 4.70 (4.57–4.90) | <0.001 |
| OGTT-1h (mmol/L) | 9.60 (8.52–10.94) | 7.55 (6.50–8.49) | <0.001 |
| OGTT-2h (mmol/L) | 8.62 (7.51–9.47) | 6.96 (6.17–7.72) | <0.001 |
| FPI (μU/mL) | 10.36 (7.71–14.16) | 8.27 (5.96–10.50) | <0.001 |
| HOMA-IR | 2.44 (1.75–3.33) | 1.74 (1.25–2.28) | <0.001 |
| TC (mmol/L) | 5.49 (4.78–6.28) | 5.36 4.71–6.05) | 0.185 |
| TG (mmol/L) | 2.59 (2.00–3.18) | 2.27 (1.74–3.04) | 0.002 |
| LDL cholesterol (mmol/L) | 3.24 (2.51–3.99) | 3.02 (2.36–3.72) | 0.047 |
| HDL cholesterol (mmol/L) | 1.34 (1.17–1.56) | 1.38 (1.13–1.64) | 0.512 |
| V (μg/L) | 0.73 (0.63–0.89) | 0.80 (0.70–1.11) | <0.001 |
| Tertiles of Plasma V Concentration | p-Trend | Per SD Increment of ln-Transformed Plasma V | |||
|---|---|---|---|---|---|
| Tertile 1 (<0.68 μg/L) | Tertile 2 (0.68–0.97 μg/L) | Tertile 3 (≥0.97 μg/L) | |||
| No. of cases/controls | 129/84 | 65/84 | 58/84 | ||
| Crude model | 1 | 0.48 (0.30–0.76) | 0.40 (0.25–0.65) | 0.001 | 0.72 (0.61–0.86) |
| Model 1 a | 1 | 0.51 (0.32–0.80) | 0.41 (0.25–0.67) | 0.002 | 0.70 (0.58–0.85) |
| Model 2 b | 1 | 0.46 (0.28–0.78) | 0.35 (0.20–0.61) | 0.002 | 0.68 (0.56–0.84) |
| Variables | Unadjusted | Adjusted a | ||
|---|---|---|---|---|
| r | p | r | p | |
| FPG (mmol/L) | −0.15 | 0.001 | −0.05 | 0.294 |
| OGTT-1h (mmol/L) | −0.15 | 0.001 | −0.10 | 0.040 |
| OGTT-2h (mmol/L) | −0.17 | 0.001 | −0.09 | 0.043 |
| FPI (μU/mL) | −0.09 | 0.048 | −0.04 | 0.396 |
| HOMA-IR | −0.09 | 0.036 | −0.03 | 0.482 |
| TC (mmol/L) | −0.05 | 0.291 | −0.09 | 0.046 |
| TG (mmol/L) | −0.05 | 0.246 | −0.10 | 0.030 |
| LDL cholesterol (mmol/L) | −0.11 | 0.016 | −0.14 | 0.002 |
| HDL cholesterol (mmol/L) | 0.06 | 0.195 | 0.09 | 0.051 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Zhu, Y.; Yin, J.; Li, B.; Li, P.; Cao, B.; Wang, Q.; Xu, J.; Liu, L. Inverse Association of Plasma Vanadium Concentrations with Gestational Diabetes Mellitus. Nutrients 2022, 14, 1415. https://doi.org/10.3390/nu14071415
Li X, Zhu Y, Yin J, Li B, Li P, Cao B, Wang Q, Xu J, Liu L. Inverse Association of Plasma Vanadium Concentrations with Gestational Diabetes Mellitus. Nutrients. 2022; 14(7):1415. https://doi.org/10.3390/nu14071415
Chicago/Turabian StyleLi, Xiaoqin, Yalun Zhu, Jiawei Yin, Ben Li, Peiyun Li, Benfeng Cao, Qiang Wang, Jian Xu, and Liegang Liu. 2022. "Inverse Association of Plasma Vanadium Concentrations with Gestational Diabetes Mellitus" Nutrients 14, no. 7: 1415. https://doi.org/10.3390/nu14071415
APA StyleLi, X., Zhu, Y., Yin, J., Li, B., Li, P., Cao, B., Wang, Q., Xu, J., & Liu, L. (2022). Inverse Association of Plasma Vanadium Concentrations with Gestational Diabetes Mellitus. Nutrients, 14(7), 1415. https://doi.org/10.3390/nu14071415