Bifidobacterium Species Colonization in Infancy: A Global Cross-Sectional Comparison by Population History of Breastfeeding
Abstract
:1. Introduction
2. Methods
2.1. Inclusion Criteria for Cohorts
2.2. 16S rRNA Gene Sequencing and Bif-TRFLP/BLIR
3. Results
3.1. Study Cohorts and Breastfeeding Patterns
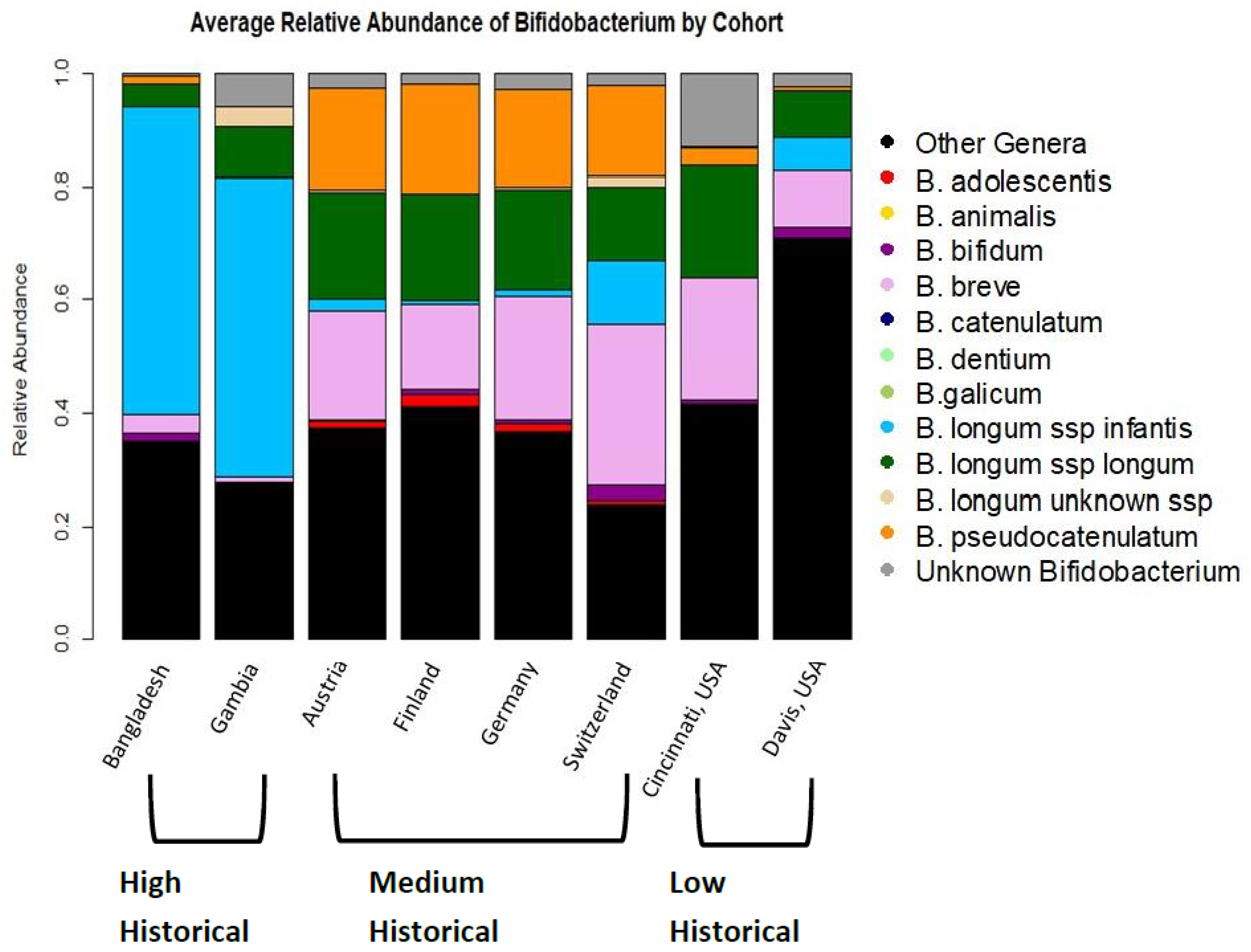
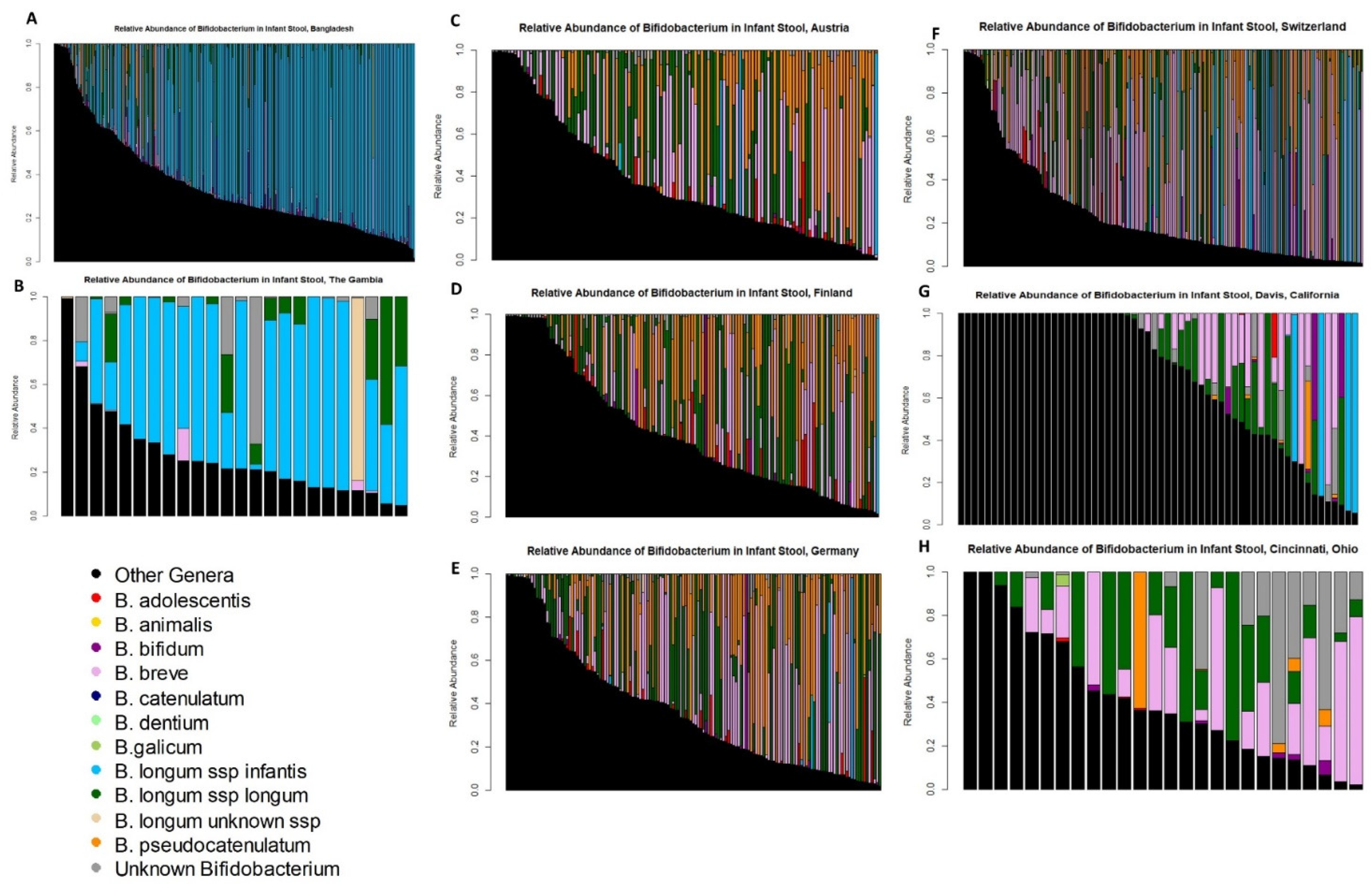
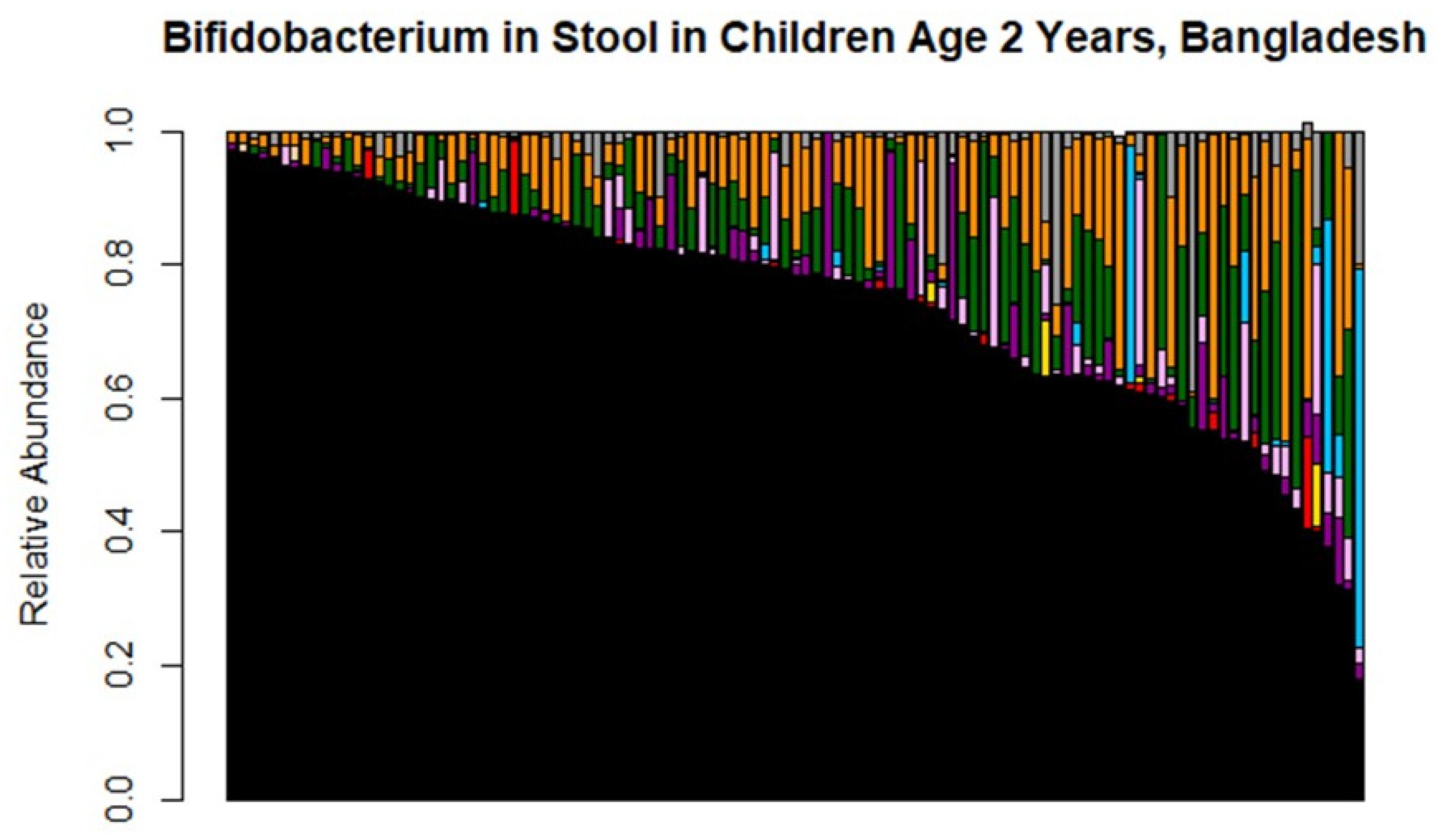
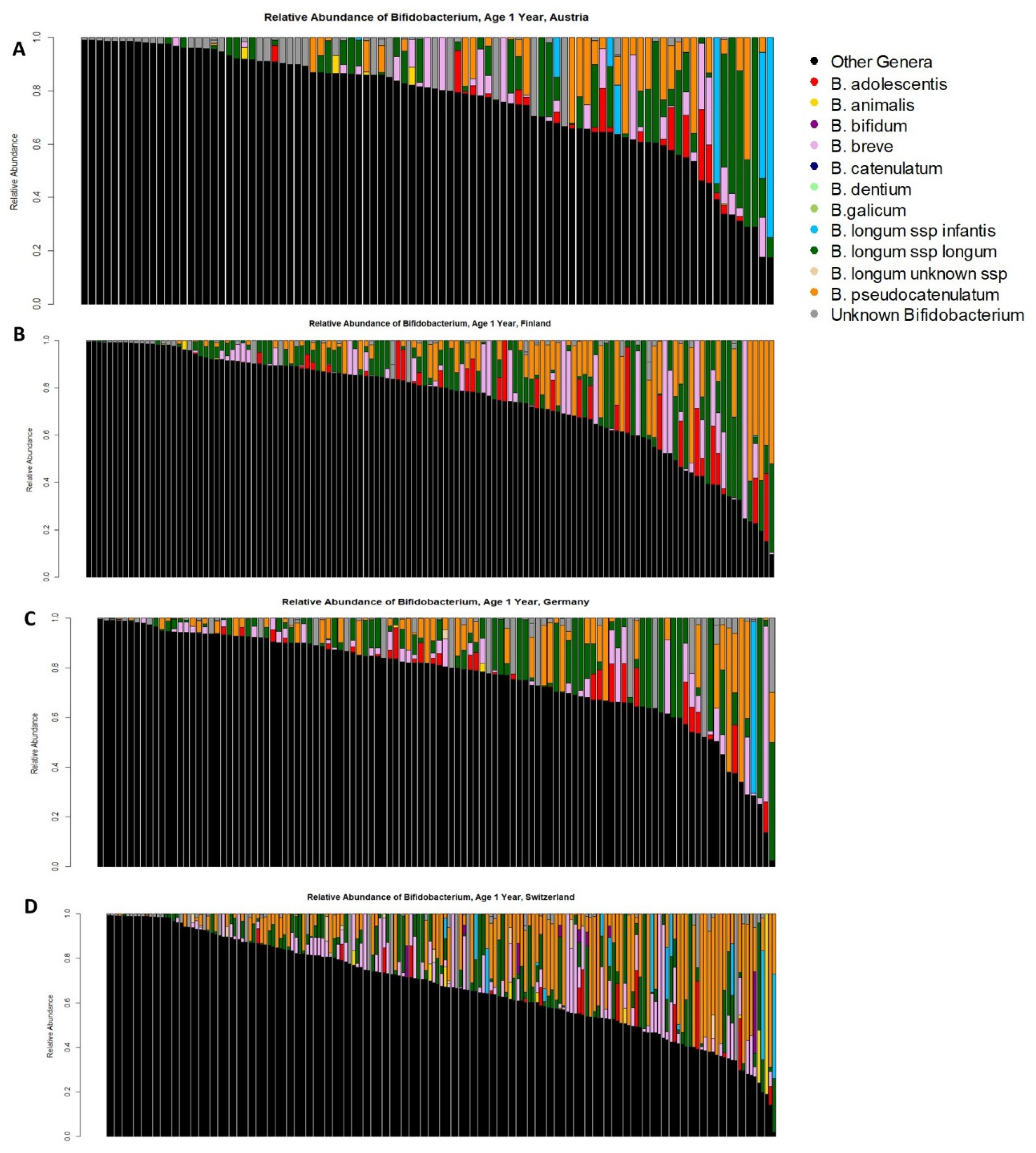
3.2. Bifidobacterium Species across Cohorts and Breastfeeding Patterns
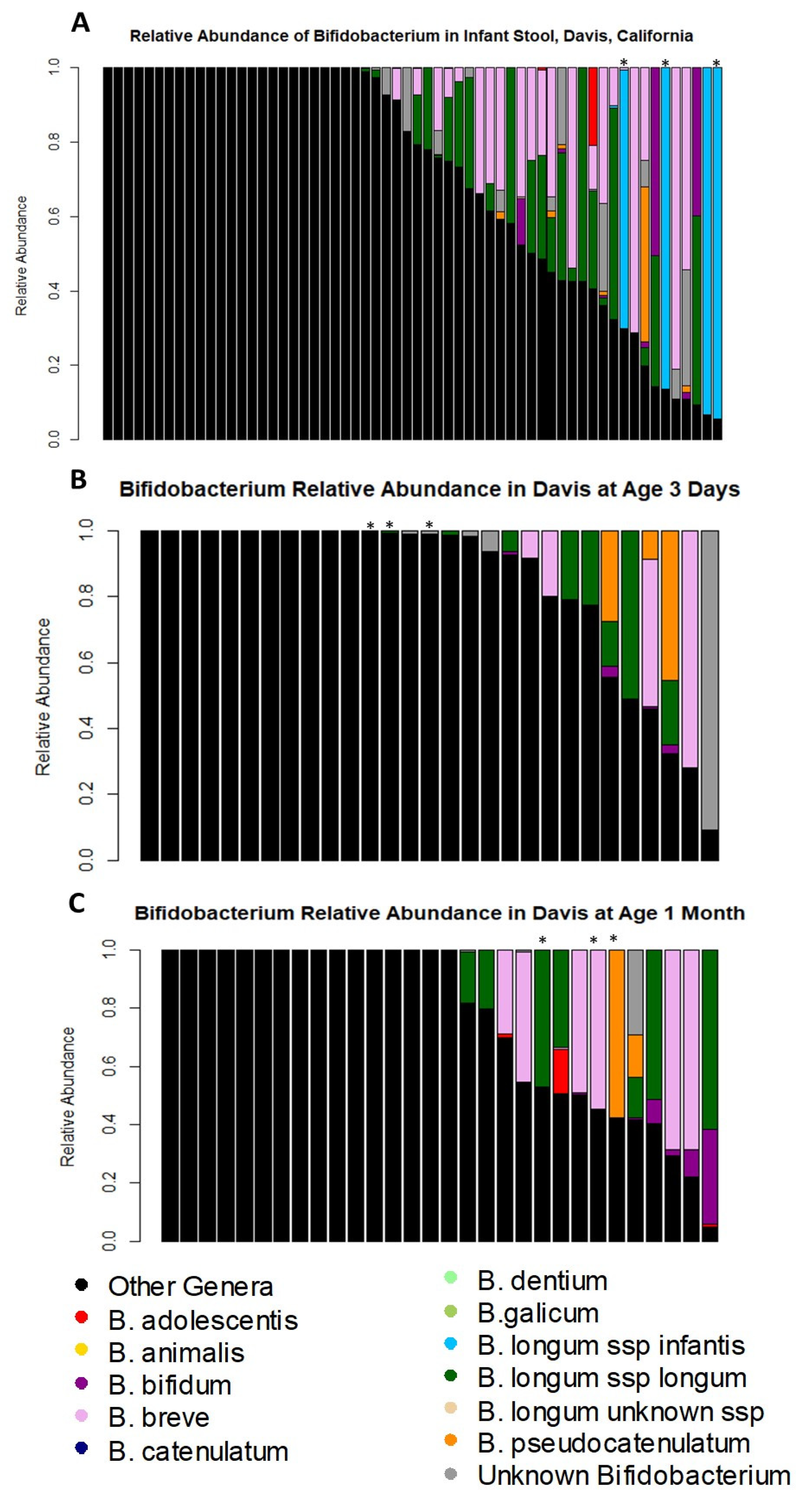
3.3. Timing of Bifidobacterium Species Colonization
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Seppo, A.E.; Bu, K.; Jumabaeva, M.; Thakar, J.; Choudhury, R.A.; Yonemitsu, C.; Bode, L.; Martina, C.A.; Allen, M.; Tamburini, S.; et al. Infant gut microbiome is enriched with Bifidobacterium longum ssp. infantis in Old Order Mennonites with traditional farming lifestyle. Allergy 2021, 76, 3489–3503. [Google Scholar] [CrossRef] [PubMed]
- Cukrowska, B.; Bierła, J.B.; Zakrzewska, M.; Klukowski, M.; Maciorkowska, E. The relationship between the infant gut microbiota and allergy. The role of Bifidobacterium breve and prebiotic oligosaccharides in the activation of anti-allergic mechanisms in early life. Nutrients 2020, 12, 946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huda, M.N.; Ahmad, S.M.; Alam, M.J.; Khanam, A.; Kalanetra, K.M.; Taft, D.H.; Raqib, R.; Underwood, M.A.; Mills, D.A.; Stephensen, C.B. Bifidobacterium abundance in early infancy and vaccine response at 2 years of age. Pediatrics 2019, 143, e20181489. [Google Scholar] [CrossRef] [Green Version]
- Taft, D.H.; Liu, J.; Maldonado-Gomez, M.X.; Akre, S.; Huda, M.N.; Ahmad, S.M.; Stephensen, C.B.; Mills, D.A. Bifidobacterial dominance of the gut in early life and acquisition of antimicrobial resistance. mSphere 2018, 3, e00441-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casaburi, G.; Frese, S.A. Colonization of breastfed infants by Bifidobacterium longum subsp. infantis EVC001 reduces virulence gene abundance. Hum. Microbiome J. 2018, 9, 7–10. [Google Scholar] [CrossRef]
- Henrick, B.M.; Chew, S.; Casaburi, G.; Brown, H.K.; Frese, S.A.; Zhou, Y.; Underwood, M.A.; Smilowitz, J.T. Colonization by B. infantis EVC001 modulates enteric inflammation in exclusively breastfed infants. Pediatr. Res. 2019, 86, 749–757. [Google Scholar] [CrossRef] [PubMed]
- Henrick, B.M.; Rodriguez, L.; Lakshmikanth, T.; Pou, C.; Henckel, E.; Arzoomand, A.; Olin, A.; Wang, J.; Mikes, J.; Tan, Z.; et al. Bifidobacteria-mediated immune system imprinting early in life. Cell 2021, 184, 3884–3898.e11. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Ouwehand, A.C.; Isolauri, E.; Hashimoto, H.; Benno, Y.; Salminen, S. Comparison of mucosal adhesion and species identification of bifidobacteria isolated from healthy and allergic infants. FEMS Immunol. Med. Microbiol. 2001, 30, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Tannock, G.W.; Lawley, B.; Munro, K.; Gowri Pathmanathan, S.; Zhou, S.J.; Makrides, M.; Gibson, R.A.; Sullivan, T.; Prosser, C.G.; Lowry, D.; et al. Comparison of the compositions of the stool microbiotas of infants fed goat milk formula, cow milk-based formula, or breast milk. Appl. Environ. Microbiol. 2013, 79, 3040–3048. [Google Scholar] [CrossRef] [Green Version]
- Tannock, G.W.; Lee, P.S.; Wong, K.H.; Lawley, B. Why Don’t all infants have bifidobacteria in their stool? Front. Microbiol. 2016, 7, 834. [Google Scholar] [CrossRef] [Green Version]
- Blaser, M.J. The past and future biology of the human microbiome in an age of extinctions. Cell 2018, 172, 1173–1177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blaser, M.J. The theory of disappearing microbiota and the epidemics of chronic diseases. Nat. Rev. Immunol. 2017, 17, 461–463. [Google Scholar] [CrossRef] [PubMed]
- Sonnenburg, E.D.; Smits, S.A.; Tikhonov, M.; Higginbottom, S.K.; Wingreen, N.S.; Sonnenburg, J.L. Diet-induced extinctions in the gut microbiota compound over generations. Nature 2016, 529, 212–215. [Google Scholar] [CrossRef] [Green Version]
- Davis, E.C.; Wang, M.; Donovan, S.M. The role of early life nutrition in the establishment of gastrointestinal microbial composition and function. Gut Microbes 2017, 8, 143–171. [Google Scholar] [CrossRef] [PubMed]
- Logan, W.R. The intestinal flora of infants and young children. J. Pathol. Bacteriol. 1913, 18, 527–551. [Google Scholar] [CrossRef] [Green Version]
- Ward, R.E.; Niñonuevo, M.; Mills, D.A.; Lebrilla, C.B.; German, J.B. In vitro fermentability of human milk oligosaccharides by several strains of bifidobacteria. Mol. Nutr. Food Res. 2007, 51, 1398–1405. [Google Scholar] [CrossRef] [PubMed]
- LoCascio, R.G.; Ninonuevo, M.R.; Freeman, S.L.; Sela, D.A.; Grimm, R.; Lebrilla, C.B.; Mills, D.A.; German, J.B. Glycoprofiling of bifidobacterial consumption of human milk oligosaccharides demonstrates strain specific, preferential consumption of small chain glycans secreted in early human lactation. J. Agric. Food Chem. 2007, 55, 8914–8919. [Google Scholar] [CrossRef] [PubMed]
- Sela, D.A.; Chapman, J.; Adeuya, A.; Kim, J.H.; Chen, F.; Whitehead, T.R.; Lapidus, A.; Rokhsar, D.S.; Lebrilla, C.B.; German, J.B.; et al. The genome sequence of Bifidobacterium longum subsp. infantis reveals adaptations for milk utilization within the infant microbiome. Proc. Natl. Acad. Sci. USA 2008, 105, 18964. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Newburg, D.; Neubauer, S.; Jensen, R. Handbook of Milk Composition; Jensen, R.G., Ed.; Academic Press: San Diego, CA, USA, 1995; pp. 273–349. [Google Scholar]
- Petherick, A. Development: Mother’s milk: A rich opportunity. Nature 2010, 468, S5. [Google Scholar] [CrossRef]
- Ballard, O.; Morrow, A.L. Human milk composition: Nutrients and bioactive factors. Pediatr. Clin. 2013, 60, 49–74. [Google Scholar]
- Bode, L. Human milk oligosaccharides: Every baby needs a sugar mama. Glycobiology 2012, 22, 1147–1162. [Google Scholar] [CrossRef] [Green Version]
- Sela, D.A.; Mills, D.A. Nursing our microbiota: Molecular linkages between bifidobacteria and milk oligosaccharides. Trends Microbiol. 2010, 18, 298–307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mattarelli, P.; Biavati, B. The Bifidobacteria and Related Organisms: Biology, Taxonomy, and Applications; Mattarelli, P., Biavati, B., Holzapfel, W.H., Wood, B.J.B., Eds.; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Milani, C.; Mancabelli, L.; Lugli, G.A.; Duranti, S.; Turroni, F.; Ferrario, C.; Mangifesta, M.; Viappiani, A.; Ferretti, P.; Gorfer, V.; et al. Exploring vertical transmission of bifidobacteria from mother to child. Appl. Environ. Microbiol. 2015, 81, 7078–7087. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davis, J.C.C.; Lewis, Z.T.; Krishnan, S.; Bernstein, R.M.; Moore, S.E.; Prentice, A.M.; Mills, D.A.; Lebrilla, C.B.; Zivkovic, A.M. Growth and morbidity of gambian infants are influenced by maternal milk oligosaccharides and infant gut microbiota. Sci. Rep. 2017, 7, 40466. [Google Scholar] [CrossRef] [PubMed]
- von Mutius, E.; Schmid, S.; the PASTURE Study Group. The PASTURE project: EU support for the improvement of knowledge about risk factors and preventive factors for atopy in Europe. Allergy 2006, 61, 407–413. [Google Scholar] [CrossRef]
- Depner, M.; PASTURE Study Group; Taft, D.H.; Kirjavainen, P.V.; Kalanetra, K.M.; Karvonen, A.M.; Peschel, S.; Schmausser-Hechfellner, E.; Roduit, C.; Frei, R.; et al. Maturation of the gut microbiome during the first year of life contributes to the protective farm effect on childhood asthma. Nat. Med. 2020, 26, 1766–1775. [Google Scholar] [CrossRef]
- Smilowitz, J.T.; O’Sullivan, A.; Barile, D.; German, J.B.; Lönnerdal, B.; Slupsky, C.M. The human milk metabolome reveals diverse oligosaccharide profiles. J. Nutr. 2013, 143, 1709–1718. [Google Scholar] [CrossRef] [Green Version]
- Lewis, Z.T.; Totten, S.M.; Smilowitz, J.T.; Popovic, M.; Parker, E.; Lemay, D.G.; Van Tassell, M.L.; Miller, M.J.; Jin, Y.-S.; German, J.B.; et al. Maternal fucosyltransferase 2 status affects the gut bifidobacterial communities of breastfed infants. Microbiome 2015, 3, 13. [Google Scholar] [CrossRef] [Green Version]
- Morrow, A.L.; Staat, M.A.; DeFranco, E.A.; McNeal, M.M.; Cline, A.R.; Conrey, S.C.; Schlaudecker, E.P.; Piasecki, A.M.; Burke, R.M.; Niu, L.; et al. Pediatric respiratory and enteric virus acquisition and immunogenesis in US mothers and children aged 0–2: PREVAIL cohort study. JMIR Res. Protoc. 2021, 10, e22222. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Gonzalez Peña, A.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [Green Version]
- Callahan, B.J.; Mcmurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [Green Version]
- Lewis, Z.T.; Bokulich, N.A.; Kalanetra, K.M.; Ruiz-Moyano, S.; Underwood, M.A.; Mills, D.A. Use of bifidobacterial specific terminal restriction fragment length polymorphisms to complement next generation sequence profiling of infant gut communities. Anaerobe 2012, 19, 62–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- The R Project for Statistical Computing. Available online: https://www.R-project.org/ (accessed on 22 February 2022).
- Ferry, B. Breastfeeding; International Statistical Institute World Fertility Survey: Voorburg, The Netherlands; London, UK, 1981. [Google Scholar]
- Greiner, T. Breastfeeding in Bangladesh: A review of the literature. Bangladesh J. Nutr. 1997, 10. [Google Scholar] [CrossRef]
- Akter, S.; Rahman, M.M. Duration of breastfeeding and its correlates in Bangladesh. J. Health Popul. Nutr. 2010, 28, 595–601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whitehead, R. Nutritional requirements: Infant feeding practices and the development of malnutrition in rural Gambia. Food Nutr. Bull. 1979, 1, 1–6. [Google Scholar] [CrossRef]
- UNICEF. The Gambia Multiple Indicator Cluster Survey 2010, Final Report; UNICEF: New York, NY, USA, 2011. [Google Scholar]
- Thorvaldsen, G. Was there a European breastfeeding pattern? Hist. Fam. 2008, 13, 283–295. [Google Scholar] [CrossRef]
- Kersting, M.; Wember, T.; Goddemeier, T.; Koester, H.; Wennemann, J.; Schöch, G. Breast feeding studies 1981–1983 in 1500 mothers in Dortmund and Haltern. III. Rates of breast feeding and duration of breast feeding in the first half year. Mon. Kinderheilkd. 1987, 135, 314–319. [Google Scholar]
- Helsing, E. Supporting breastfeeding: What governments and health workers can do—European experiences. Int. J. Gynecol. Obstet. 1990, 31, 69–76. [Google Scholar] [CrossRef]
- Verronen, P. Imetys on muotia (breast-feeding is popular). Suom Lääkäril 1984, 39, 1078–1079. [Google Scholar]
- Brunn, S. Grass-roots support for breast-feeding. World Health Forum 1986, 7, 65–68. [Google Scholar]
- Tönz, O.; Schwaninger, U.; Holzherr, E.; Schafroth, M. Infant nutrition in Switzerland 1978. A prospective study on the nutritional habits during the first 6 months of life. I. Natural nutrition: Breast feeding. Schweiz. Med. Wochenschr. 1980, 110, 937–947. [Google Scholar] [PubMed]
- Bürger, B.; Schindler, K.; Tripolt, T.; Stüger, H.; Wagner, K.-H.; Weber, A.; Wolf-Spitzer, A. Breastfeeding prevalence in Austria according to the WHO IYCF Indicators—The SUKIE-study. Nutrients 2021, 13, 2096. [Google Scholar] [CrossRef] [PubMed]
- Hasunen, K. Imeväisikäisten Ruokinta Suomessa Vuonna; Ministry of Social Affairs and Health: Helsinki, Finland, 2005.
- Kohlhuber, M.; Rebhan, B.; Schwegler, U.; Koletzko, B.; Fromme, H. Breastfeeding rates and duration in Germany: A Bavarian cohort study. Br. J. Nutr. 2008, 99, 1127–1132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dratva, J.; Gross, K.; Späth, A.; Zemp, E. SWIFS-Swiss Infant Feeding Study: A National Study on Infant Feeding and Health in the Child′s First Year: Executive Summary; Swiss Tropical and Public Health Institute: Basel, Switzerland, 2014. [Google Scholar]
- Wolf, J.H. Low breastfeeding rates and public health in the United States. Am. J. Public Health 2003, 93, 2000–2010. [Google Scholar] [CrossRef]
- Ryan, A.S.; Rush, D.; Krieger, F.W.; Lewandowski, G.E. Recent declines in breast-feeding in the United States, 1984 through 1989. Pediatrics 1991, 88, 719–727. [Google Scholar] [CrossRef]
- Ryan, A.S. The resurgence of breastfeeding in the United States. Pediatrics 1997, 99, e12. [Google Scholar] [CrossRef] [Green Version]
- Callen, J.; Pinelli, J. Incidence and duration of breastfeeding for term infants in Canada, United States, Europe, and Australia: A literature review. Birth 2004, 31, 285–292. [Google Scholar] [CrossRef]
- Rupnicki, S. Breastfeeding Report Card United States, 2020; CDC: Atlanta, GA, USA, 2020. [Google Scholar]
- U.S. Department of Health & Human Services. Breastfeeding Report Card. National Immunization Survey. Available online: http://www.cdc.gov/breastfeeding/data/report_card2.htm (accessed on 15 April 2009).
- Duranti, S.; Lugli, G.A.; Mancabelli, L.; Armanini, F.; Turroni, F.; James, K.; Ferretti, P.; Gorfer, V.; Ferrario, C.; Milani, C.; et al. Maternal inheritance of bifidobacterial communities and bifidophages in infants through vertical transmission. Microbiome 2017, 5, 66. [Google Scholar] [CrossRef]
- Asakuma, S.; Hatakeyama, E.; Urashima, T.; Yoshida, E.; Katayama, T.; Yamamoto, K.; Kumagai, H.; Ashida, H.; Hirose, J.; Kitaoka, M. Physiology of consumption of human milk oligosaccharides by infant gut-associated bifidobacteria. J. Biol. Chem. 2011, 286, 34583–34592. [Google Scholar] [CrossRef] [Green Version]
- Lefebvre, G.; Shevlyakova, M.; Charpagne, A.; Marquis, J.; Vogel, M.; Kirsten, T.; Kiess, W.; Austin, S.; Sprenger, N.; Binia, A. Time of lactation and maternal fucosyltransferase genetic polymorphisms determine the variability in human milk oligosaccharides. Front. Nutr. 2020, 7, 574459. [Google Scholar] [CrossRef]
- O’Leary, J. Do Old Order Mennonites Hold the Key to Understanding Food Allergies? Available online: https://www.urmc.rochester.edu/news/story/do-old-order-mennonites-hold-the-key-to-understanding-food-allergies (accessed on 25 August 2021).
- Poupard, J.A.; Husain, I.; Norris, R.F. Biology of the bifidobacteria. Bacteriol. Rev. 1973, 37, 136–165. [Google Scholar] [CrossRef] [PubMed]
- Placek, P.J.; Taffel, S.M. Trends in cesarean section rates for the United States, 1970–1978. Public Health Rep. 1980, 95, 540–548. [Google Scholar] [PubMed]
- Schuchat, A. Group B streptococcus. Lancet 1999, 353, 51–56. [Google Scholar] [CrossRef]
- Hassan, M.Z.; Monjur, M.R.; Biswas, M.A.A.J.; Chowdhury, F.; Kafi, M.A.H.; Braithwaite, J.; Jaffe, A.; Homaira, N. Antibiotic use for acute respiratory infections among under-5 children in Bangladesh: A population-based survey. BMJ Glob. Health 2021, 6, e004010. [Google Scholar] [CrossRef] [PubMed]
- Akay, H.K.; Tokman, H.B.; Hatipoglu, N.; Hatipoglu, H.; Siraneci, R.; Demirci, M.; Borsa, B.A.; Yuksel, P.; Karakullukcu, A.; Kangaba, A.A.; et al. The relationship between bifidobacteria and allergic asthma and/or allergic dermatitis: A prospective study of 0–3 years-old children in Turkey. Anaerobe 2014, 28, 98–103. [Google Scholar] [CrossRef] [PubMed]
| Country of Origin | Published | Study | Enrollment Based on Intent to Breastfeed? | Total Number Infants in Cohort | Number of Infants with Stool Samples | Number of Breastfed Infants at Time of Sample Collection |
|---|---|---|---|---|---|---|
| Austria | Partially | PASTURE | No | 207 | 181 | 122 |
| Bangladesh | Yes | Efficacy of Newborn Vitamin A Supplementation in Improving Immune Function (clinicaltrials.gov NTC01583972) | No | 306 | 274 | 274 |
| Finland | Partially | PASTURE | No | 171 | 153 | 135 |
| Gambia | Yes | Sub-study in The Early Nutrition and Immune Development (ENID) Trial, ISRCTN49285450 | Yes | 33 | 24 | 24 |
| Germany | Partially | PASTURE | No | 237 | 198 | 149 |
| Switzerland | Partially | PASTURE | No | 231 | 227 | 189 |
| Davis, CA, USA | Partially | UC Davis Lactation Cohort | Yes | 95 | 60 | 60 |
| Cincinnati, OH, USA | No | PREVAIL | No | 245 | 45 | 26 |
| Cohort Location | Ever Breastfed (Median Duration) | Historic Breastfeeding Pattern | Any Bifidobacterium | B. adolescentis | B. animalis | B. bifidum | B. breve | B. longum subsp. infantis | B. longum subsp. longum | B. longum subsp. Unknown | B. pseudocatenulatum |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Bangladesh | 100% (>2 years) | High | 100% | 5.8% | 2.6% | 32.5% | 40.1% | 83.6% | 26.3% | 1.1% | 8.4% |
| Gambia | 100% (Unknown) | High | 100% | 0% | 0% | 0% | 25% | 91.7% | 54.2% | 12.5% | 0% |
| Austria | 91.8% (6.8 months) | Medium | 100% | 23.7% | 0.82% | 6.6% | 55.7% | 4.1% | 61.5% | 4.9% | 52.4% |
| Finland | 99.3% (8 months) | Medium | 100% | 15.5% | 0% | 8.8% | 40.0% | 0.74% | 56.3% | 3.7% | 51.1% |
| Germany | 91.1% (7.4 months) | Medium | 100% | 18.1% | 0.67% | 7.4% | 61.1% | 4.0% | 61.7% | 4.0% | 57.0% |
| Switzerland | 97.3% (8 months) | Medium | 100% | 4.8% | 0% | 11.1% | 58.7% | 14.8% | 41.8% | 6.3% | 49.7% |
| Davis, CA, USA | 100% (9.3 months) | Low | 65% | 8.3% | 0% | 13.3% | 36.7% | 8.3% | 36.7% | 1.7% | 15% |
| Cincinnati, OH, USA | 86.5% (3.1 months) | Low | 97% | 11.5% | 0% | 19.2% | 61.5% | 0% | 69.2% | 0% | 19.2% |
| Cohort Breast-Feeding Pattern | B. adolescentis Odds Ratio (95% CI, p-Value) | B. animalis Odds Ratio (95% CI, p-Value) | B. bifidum Odds Ratio (95% CI, p-Value) | B. breve Odds Ratio (95% CI, p-Value) | B. longum Subspecies infantis Odds Ratio (95% CI, p-Value) | B. longum Subspecies longum Odds Ratio (95% CI, p-Value) | B. longum Unknown Subspecies Odds Ratio (95% CI, p-Value) | B. pseudocatenulatum Odds Ratio (95% CI, p-Value) |
|---|---|---|---|---|---|---|---|---|
| Medium | 4.1 (1.6–11, p = 0.0041) | NA | 0.22 (0.16–0.31, p < 0.0001) | 2.0 (1.3–3.1, p = 0.0019) | 0.010 (0.0037–0.029, p < 0.0001) | 2.2 (0.98–5.0, p = 0.055) | 2.8 (0.92–8.2, p = 0.069) | 12 (10–13, p < 0.0001) |
| Low | 2.4 (1.0–5.6, p = 0.041) | NA | 0.40 (0.28–0.58, p < 0.0001) | 1.4 (0.70–2.9, p = 0.32) | 0.0084 (0.0024–0.029, p < 0.0001) | 1.8 (0.57–5.7, p = 0.31) | 0.63 (0.16–2.4, p = 0.51) | 2.1 (1.8–2.5, p < 0.0001) |
| Country | Breast Fed at 1 Year (European Countries) or 2 Years (Bangladesh) | B. longum Subspecies infantis Detected | B. longum Subspecies infantis Not Detected | Percentage of Infants Colonized |
|---|---|---|---|---|
| Austria (p = 0.003) | Yes | 5 | 14 | 36% |
| No | 2 | 70 | 2.8% | |
| Finland (p = 0.65) | Yes | 1 | 37 | 2.6% |
| No | 0 | 91 | 0% | |
| Germany (p = 1) | Yes | 1 | 18 | 5.3% |
| No | 2 | 89 | 2.2% | |
| Switzerland (p < 0.0001) | Yes | 10 | 24 | 29% |
| No | 6 | 135 | 4.3% | |
| Bangladesh (p = 0.35) | Yes | 13 | 51 | 20.3% |
| No | 5 | 40 | 11.1% |
| Cohort Location | Any Bifidobacterium | B. adolescentis | B. animalis | B. bifidum | B. breve | B. longum subsp. infantis | B. longum subsp. longum | B. longum Unknown Subspecies | B. pseudocatenulatum |
|---|---|---|---|---|---|---|---|---|---|
| Austria | 100% | 23% | 5.5% | 1.1% | 35% | 7.7% | 53% | 0% | 36% |
| Finland | 100% | 25% | 2.3% | 0% | 38% | 0.8% | 70% | 0% | 47% |
| Germany | 100% | 27% | 3.6% | 0% | 44% | 2.7% | 67% | 0.9% | 52% |
| Switzerland | 100% | 9.1% | 6.8% | 4.6% | 51% | 9.1% | 62% | 7.4% | 61% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taft, D.H.; Lewis, Z.T.; Nguyen, N.; Ho, S.; Masarweh, C.; Dunne-Castagna, V.; Tancredi, D.J.; Huda, M.N.; Stephensen, C.B.; Hinde, K.; et al. Bifidobacterium Species Colonization in Infancy: A Global Cross-Sectional Comparison by Population History of Breastfeeding. Nutrients 2022, 14, 1423. https://doi.org/10.3390/nu14071423
Taft DH, Lewis ZT, Nguyen N, Ho S, Masarweh C, Dunne-Castagna V, Tancredi DJ, Huda MN, Stephensen CB, Hinde K, et al. Bifidobacterium Species Colonization in Infancy: A Global Cross-Sectional Comparison by Population History of Breastfeeding. Nutrients. 2022; 14(7):1423. https://doi.org/10.3390/nu14071423
Chicago/Turabian StyleTaft, Diana H., Zachery T. Lewis, Nhu Nguyen, Steve Ho, Chad Masarweh, Vanessa Dunne-Castagna, Daniel J. Tancredi, M. Nazmul Huda, Charles B. Stephensen, Katie Hinde, and et al. 2022. "Bifidobacterium Species Colonization in Infancy: A Global Cross-Sectional Comparison by Population History of Breastfeeding" Nutrients 14, no. 7: 1423. https://doi.org/10.3390/nu14071423






