Influences of Vitamin B12 Supplementation on Cognition and Homocysteine in Patients with Vitamin B12 Deficiency and Cognitive Impairment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics, Approval, and Consent to Participate in This Research
2.2. Blood Tests
2.3. Scoring of Brain Atrophy by Z-Score of Voxel-Based Specific Regional Analysis System for Alzheimer’s Disease (VSRAD)
2.4. Statistical Analysis
3. Results
3.1. Vitamin B12 Deficit and Hyperhomocysteinemia
3.2. Hippocampal Atrophy and Hyperhomocysteinemia
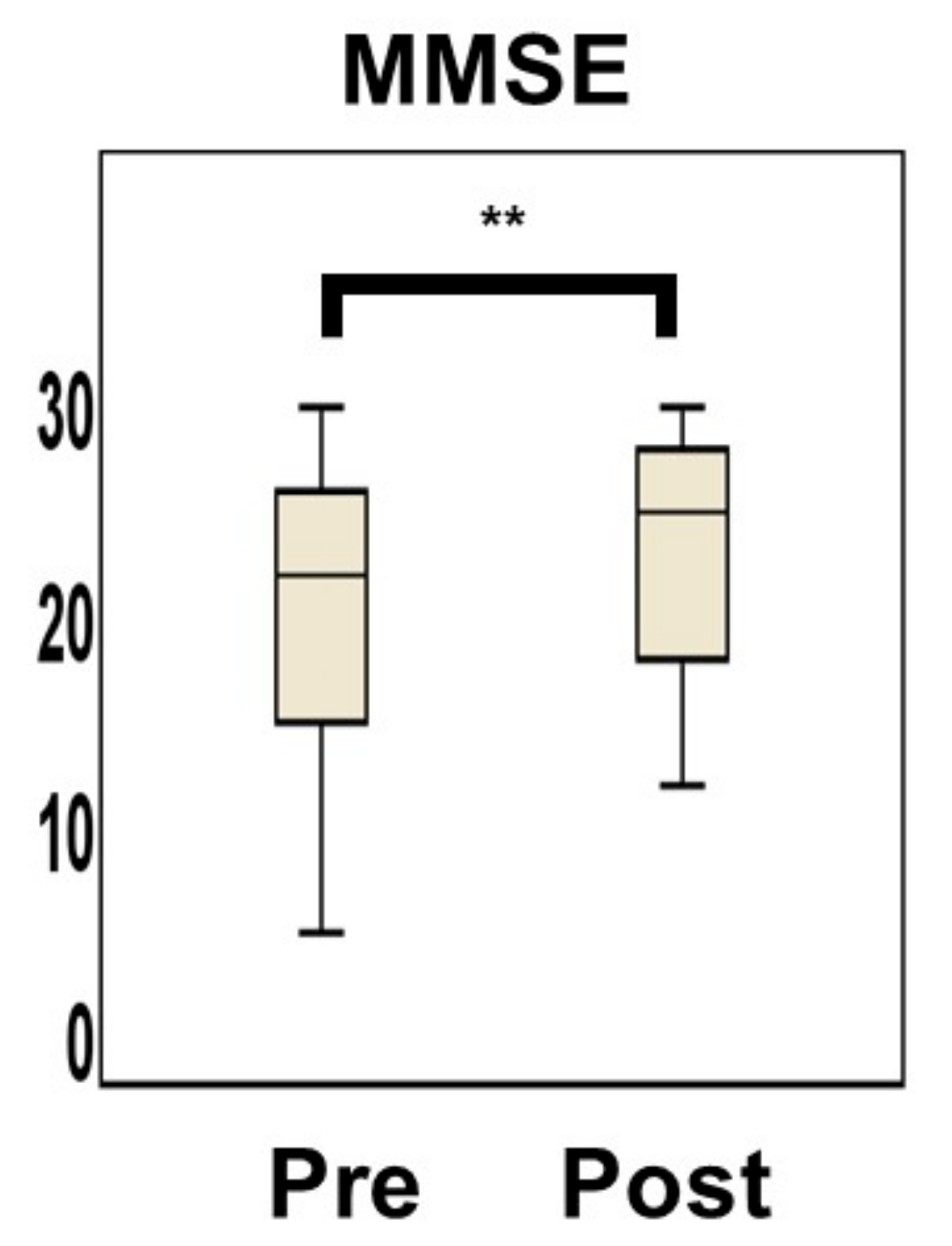
3.3. Changes in Homocysteine Levels and MMSE Score by Vitamin B12 Supplementation
3.4. Vitamin B12 Deficiency and MCV
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Van der Lee, S.J.; Wolters, F.J.; Ikram, M.K.; Hofman, A.; Ikram, M.A.; Amin, N.; van Duijn, C.M. The effect of APOE and other common genetic variants on the onset of Alzheimer’s disease and dementia: A community-based cohort study. Lancet Neurol. 2018, 17, 434–444. [Google Scholar] [CrossRef]
- Hama, Y.; Hamano, T.; Shirafuji, N.; Hayashi, K.; Ueno, A.; Enomoto, S.; Nagata, M.; Kimura, H.; Matsunaga, A.; Ikawa, M.; et al. Influences of folate supplementation on homocysteine and cognition in patients with folate deficiency and cognitive impairment. Nutrients 2020, 12, 3138. [Google Scholar] [CrossRef] [PubMed]
- Blasko, I.; Hinterberger, M.; Kemmler, G.; Jungwirth, S.; Krampla, W.; Leitha, T.; Heinz Tragl, K.; Fischer, P. Conversion from mild cognitive impairment to dementia: Influence of folic acid and vitamin B12 use in the VITA cohort. J. Nutr. Health Aging 2012, 16, 687–694. [Google Scholar] [CrossRef] [PubMed]
- White, L.; Petrovitch, H.; Ross, G.W.; Masaki, K.H.; Abbott, R.D.; Teng, E.L.; Rodriguez, B.L.; Blanchette, P.L.; Havlik, R.J.; Wergowske, G.; et al. Prevalence of dementia in older Japanese-American men in Hawaii: The Honolulu-Asia Aging Study. JAMA 1996, 276, 955–960. [Google Scholar] [CrossRef] [PubMed]
- Weytingh, M.D.; Bossuyt, P.M.; van Crevel, H. Reversible dementia: More than 10% or less than 1%? A quantitative review. J. Neurol. 1995, 242, 466–471. [Google Scholar] [CrossRef] [PubMed]
- Shirafuji, N.; Hamano, T.; Yen, S.H.; Kanaan, N.M.; Yoshida, H.; Hayashi, K.; Ikawa, M.; Yamamura, O.; Kuriyama, M.; Nakamoto, Y. Homocysteine increases tau phosphorylation, truncation and oligomerization. Int. J. Mol. Sci. 2018, 19, 891. [Google Scholar] [CrossRef] [Green Version]
- Ducloux, D.; Chalopin, J.M. Homocysteine in cerebral macroangiopathy and microangiopathy. Lancet 1999, 354, 1029–1030. [Google Scholar] [CrossRef]
- Seshadri, S.; Beiser, A.; Selhub, J.; Jacques, P.F.; Rosenberg, I.H.; D’Agostino, R.B.; Wilson, P.W.; Wolf, P.A. Plasma homocysteine as a risk factor for dementia and Alzheimer’s disease. N. Engl. J. Med. 2002, 346, 476–483. [Google Scholar] [CrossRef]
- Van de Lagemaat, E.E.; de Groot, L.C.; van den Heuvel, E.G. Vitamin B12 in Relation to Oxidative Stress: A Systematic Review. Nutrients 2019, 11, 482. [Google Scholar] [CrossRef] [Green Version]
- Ho, P.I.; Collins, S.C.; Dhitavat, S.; Ortiz, D.; Ashline, D.; Rogers, E.; Shea, T.B. Homocysteine potentiates beta-amyloid neurotoxicity: Role of oxidative stress. J. Neurochem. 2001, 78, 249–253. [Google Scholar] [CrossRef]
- Clarke, R.; Smith, A.D.; Jobst, K.A.; Refsum, H.; Sutton, L.; Ueland, P.M. Folate, vitamin B12, and serum total homocysteine levels in confirmed Alzheimer disease. Arch. Neurol. 1998, 55, 1449–1455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miwa, K.; Tanaka, M.; Okazaki, S.; Yagita, Y.; Sakaguchi, M.; Mochizuki, H.; Kitagawa, K. Increased total homocysteine levels predict the risk of incident dementia independent of cerebral small-vessel diseases and vascular risk factors. J. Alzheimers Dis. 2016, 49, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Hooshmand, B.; Polvikoski, T.; Kivipelto, M.; Tanskanen, M.; Myllykangas, L.; Erkinjuntti, T.; Mäkelä, M.; Oinas, M.; Paetau, A.; Scheltens, P.; et al. Plasma homocysteine, Alzheimer and cerebrovascular pathology: A population-based autopsy study. Brain 2013, 136, 2707–2716. [Google Scholar] [CrossRef] [Green Version]
- Roher, A.E.; Tyas, S.L.; Maarouf, C.L.; Daugs, I.D.; Kokjohn, T.A.; Emmerling, M.R.; Garami, Z.; Belohlavek, M.; Sabbagh, M.N.; Sue, L.I.; et al. Intracranial atherosclerosis as a contributing factor to Alzheimer’s disease dementia. Alzheimers Dement. 2011, 7, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Quadri, P.; Fragiacomo, C.; Pezzati, R.; Zanda, E.; Forloni, G.; Tettamanti, M.; Lucca, U. Homocysteine, folate, and vitamin B-12 in mild cognitive impairment, Alzheimer’s disease, vascular dementia. Am. J. Clin. Nutr. 2004, 80, 114–122. [Google Scholar] [PubMed]
- Nilsson, K.; Gustafson, L.; Hultberg, B. Elevated plasma homocysteine level in vascular dementia reflects the vascular disease process. Dement. Geriatr. Cogn. Dis. Extra 2013, 3, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Mattson, M.P.; Shea, T.B. Folate and homocysteine metabolism in neural plasticity and neurodegenerative disorders. Trends. Neurosci. 2003, 26, 137–146. [Google Scholar] [CrossRef]
- Rodriguez-Oroz, M.C.; Lage, P.M.; Sanchez-Mut, J.; Lamet, I.; Pagonabarraga, J.; Toledo, J.B.; García-Garcia, D.; Clavero, P.; Samaranch, L.; Irurzun, C.; et al. Homocysteine and cognitive impairment in Parkinson’s disease: A biochemical, neuroimaging, and genetic study. Mov. Disord. 2009, 24, 1437–1444. [Google Scholar] [CrossRef]
- Selhub, J.; Jacques, P.F.; Bostom, A.G.; D’Agostino, R.B.; Wilson, P.W.; Belanger, A.J.; O’Leary, D.H.; Wolf, P.A.; Schaefer, E.J.; Rosenberg, I.H. Association between plasma homocysteine concentrations and extracranial carotid-artery stenosis. N. Engl. J. Med. 1995, 332, 286–291. [Google Scholar] [CrossRef]
- Bostom, A.G.; Rosenberg, I.H.; Silbershatz, H.; Jacques, P.F.; Selhub, J.; D’Agostino, R.B.; Wilson, P.W.; Wolf, P.A. Nonfasting plasma total homocysteine levels and stroke incidence in elderly persons: The Framingham Study. Ann. Intern. Med. 1999, 131, 352–355. [Google Scholar] [CrossRef]
- Ma, F.; Wu, T.; Zhao, J.; Ji, L.; Song, A.; Zhang, M.; Huang, G. Plasma homocysteine and serum folate and vitamin b(12) levels in mild cognitive impairment and Alzheimer’s disease: A case-control study. Nutrients 2017, 9, 725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wald, D.S.; Kasturiratne, A.; Simmonds, M. Serum homocysteine and dementia: Meta-analysis of eight cohort studies including 8669 participants. Alzheimers Dement. 2011, 7, 412–417. [Google Scholar] [CrossRef] [PubMed]
- Douaud, G.; Refsum, H.; de Jager, C.A.; Jacoby, R.; Nichols, T.E.; Smith, S.M.; Smith, A.D. Preventing Alzheimer’s disease-related gray matter atrophy by B-vitamin treatment. Proc. Natl. Acad. Sci. USA 2013, 110, 9523–9528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jatoi, S.; Hafeez, A.; Riaz, S.U.; Ali, A.; Ghauri, M.I.; Zehra, M. Low vitamin B12 levels: An underestimated cause of minimal cognitive impairment and dementia. Cureus 2020, 12, e6976. [Google Scholar] [CrossRef] [Green Version]
- Ford, A.H.; Flicker, L.; Alfonso, H.; Thomas, J.; Clarnette, R.; Martins, R.; Almeida, O.P. Vitamins B(12), B(6), and folic acid for cognition in older men. Neurology 2010, 75, 1540–1547. [Google Scholar] [CrossRef] [Green Version]
- McCaddon, A.; Miller, J.W. Assessing the association between homocysteine and cognition: Reflections on Bradford Hill, meta-analyses, and causality. Nutr. Rev. 2015, 73, 723–735. [Google Scholar] [CrossRef]
- Ford, A.H.; Almeida, O.P. Effect of vitamin B supplementation on cognitive function in the elderly: A systematic review and meta-Analysis. Drugs Aging 2019, 36, 419–434. [Google Scholar] [CrossRef]
- De Koning, E.J.; van der Zwaluw, N.L.; van Wijngaarden, J.P.; Sohl, E.; Brouwer-Brolsma, E.M.; van Marwijk, H.W.; Enneman, A.W.; Swart, K.M.; van Dijk, S.C.; Ham, A.C.; et al. Effects of two-year vitamin B(12) and folic acid supplementation on depressive symptoms and quality of life in older adults with elevated homocysteine concentrations: Additional results from the B-PROOF study, an RCT. Nutrients 2016, 8, 748. [Google Scholar] [CrossRef]
- Tokumitsu, K.; Yasui-Furukori, N.; Takeuchi, J.; Yachimori, K.; Sugawara, N.; Terayama, Y.; Tanaka, N.; Naraoka, T.; Shimoda, K. The combination of MMSE with VSRAD and eZIS has greater accuracy for discriminating mild cognitive impairment from early Alzheimer’s disease than MMSE alone. PLoS ONE 2021, 16, e0247427. [Google Scholar] [CrossRef]
- Hirata, Y.; Matsuda, H.; Nemoto, K.; Ohnishi, T.; Hirao, K.; Yamashita, F.; Asada, T.; Iwabuchi, S.; Samejima, H. Voxel-based morphometry to discriminate early Alzheimer’s disease from controls. Neurosci. Lett. 2005, 382, 269–274. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Berry, R.J. Lack of historical evidence to support folic acid exacerbation of the neuropathy caused by vitamin B12 deficiency. Am. J. Clin. Nutr. 2019, 110, 554–561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyake, K.; Akimoto, T.; Kusakabe, M.; Sato, W.; Yamada, A.; Yamawaki, H.; Kodaka, Y.; Shinpuku, M.; Nagoya, H.; Shindo, T.; et al. Water-soluble vitamin deficiencies in complicated peptic ulcer patients soon after ulcer onset in Japan. J. Nutr. Sci. Vitaminol. 2013, 59, 503–508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sashindran, V.K.; Aggarwal, V.; Khera, A. Prevalence of vitamin B12 deficiency in elderly population (>60 years) presenting with dementia to outpatient department. Med. J. Armed Forces India 2022, 78, 94–98. [Google Scholar] [CrossRef]
- Clarke, R.; Grimley, E.J.; Schneede, J.; Nexo, E.; Bates, C.; Flechter, A.; Prentice, A.; Johnston, C.; Ueland, P.M.; Refsum, H.; et al. Vitamin B12 and folate deficiency in later life. Age Ageing 2004, 33, 4e41. [Google Scholar]
- Mooijaart, S.P.; Gussekloo, J.; Frölich, M.; Jolles, J.; Stott, D.J.; Westendorp, R.G.; de Craen, A.J. Homocysteine, vitamin B-12, and folic acid and the risk of cognitive decline in old age: The leiden 85-plus study. Am. J. Clin. Nutr. 2005, 82, 866–871. [Google Scholar] [CrossRef]
- Madsen, S.K.; Rajagopalan, P.; Joshi, S.H.; Toga, A.W.; Thompson, P.M. Higher homocysteine associated with thinner cortical gray matter in 803 participants from the Alzheimer’s disease neuroimaging initiative. Neurobiol. Aging 2015, 36 (Suppl. 1), S203–S210. [Google Scholar] [CrossRef] [Green Version]
- Firbank, M.J.; Narayan, S.K.; Saxby, B.K.; Ford, G.A.; O’Brien, J.T. Homocysteine is associated with hippocampal and white matter atrophy in older subjects with mild hypertension. Int. Psychogeriatr. 2010, 22, 804–811. [Google Scholar] [CrossRef] [Green Version]
- Gallucci, M.; Zanardo, A.; Bendini, M.; Di Paola, F.; Boldrini, P.; Grossi, E. Serum folate, homocysteine, brain atrophy, and auto-CM system: The Treviso Dementia (TREDEM) study. J. Alzheimers Dis. 2014, 38, 581–587. [Google Scholar] [CrossRef]
- Wald, D.S.; Kasturiratne, A.; Simmonds, M. Effect of folic acid, with or without other B vitamins, on cognitive decline: Meta-analysis of randomized trials. Am. J. Med. 2010, 123, 522.e522–527.e522. [Google Scholar] [CrossRef]
- Li, J.G.; Chu, J.; Barrero, C.; Merali, S.; Praticò, D. Homocysteine exacerbates β-amyloid pathology, tau pathology, and cognitive deficit in a mouse model of Alzheimer disease with plaques and tangles. Ann. Neurol. 2014, 75, 851–863. [Google Scholar] [CrossRef]
- Viswanathan, A.; Raj, S.; Greenberg, S.M.; Stampfer, M.; Campbell, S.; Hyman, B.T.; Irizarry, M.C. Plasma Abeta, homocysteine, and cognition: The vitamin intervention for stroke prevention (VISP) trial. Neurology 2009, 72, 268–272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lefèvre-Arbogast, S.; Féart, C.; Dartigues, J.F.; Helmer, C.; Letenneur, L.; Samieri, C. Dietary B vitamins and a 10-year risk of dementia in older persons. Nutrients 2016, 8, 761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Jager, C.A.; Oulhaj, A.; Jacoby, R.; Refsum, H.; Smith, A.D. Cognitive and clinical outcomes of homocysteine-lowering B-vitamin treatment in mild cognitive impairment: A randomized controlled trial. Int. J. Geriatr. Psychiatry 2012, 27, 592–600. [Google Scholar] [CrossRef] [PubMed]
- Huijts, M.; van Oostenbrugge, R.J.; Rouhl, R.P.; Menheere, P.; Duits, A. Effects of vitamin B12 supplementation on cognition, depression, and fatigue in patients with lacunar stroke. Int. Psychogeriatr. 2013, 25, 508–510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwok, T.; Lee, J.; Lam, L.; Woo, J. Vitamin B(12) supplementation did not improve cognition but reduced delirium in patients with vitamin B(12) deficiency. Arch. Gerontol. Geriatr. 2008, 46, 273–282. [Google Scholar] [CrossRef]
- West, E.D.; Ellis, F.R. The electroencephalogram in veganism, vegetarianism, vitamin B12 deficiency, and in controls. J. Neurol. Neurosurg. Psychiatry 1966, 29, 391–397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Velayudhan, L.; Ryu, S.H.; Raczek, M.; Philpot, M.; Lindesay, J.; Critchfield, M.; Livingston, G. Review of brief cognitive tests for patients with suspected dementia. Int. Psychogeriatr. 2014, 26, 1247–1262. [Google Scholar] [CrossRef] [Green Version]
- Seltzer, B.; Zolnouni, P.; Nunez, M.; Goldman, R.; Kumar, D.; Ieni, J.; Richardson, S. Donepezil “402” Study Group. Efficacy of Donepezil in Early-Stage Alzheimer Disease: A Randomized Placebo-Controlled Trial. Arch. Neurol. 2004, 61, 1852–1856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, A.D.; Smith, S.M.; de Jager, C.A.; Whitbread, P.; Johnston, C.; Agacinski, G.; Oulhaj, A.; Bradley, K.M.; Jacoby, R.; Refsum, H. Homocysteine-lowering by B vitamins slows the rate of accelerated brain atrophy in mild cognitive impairment: A randomized controlled trial. PLoS ONE 2010, 5, e12244. [Google Scholar] [CrossRef]
- Clarke, R.; Bennett, D.; Parish, S.; Lewington, S.; Skeaff, M.; Eussen, S.J.; Lewerin, C.; Stott, D.J.; Armitage, J.; Hankey, G.J.; et al. Effects of homocysteine lowering with B vitamins on cognitive aging: Meta-analysis of 11 trials with cognitive data on 22,000 individuals. Am. J. Clin. Nutr. 2014, 100, 657–666. [Google Scholar] [CrossRef] [PubMed] [Green Version]







| Age (Mean ± SD) | 80.1 ± 8.2 |
|---|---|
| Range | 49–91 |
| Male sex, n (%) | 22 (56.4) |
| Education (Year) (Median (IQR)) | 12 (8–12) |
| Range | 6–16 |
| MMSE (Median (IQR)) | 22 (15–26) |
| Range | 5–30 |
| Vitamin B12 (Median (IQR)), pmol/L | 142 (122–154) |
| Range | 55–171 |
| Normal Range | (172–674) [33] |
| Folate (Median (IQR)), ng/mL | 6.8 (5.0–8.4) |
| Range | 3.8–53.0 |
| Normal Range | (3.6–12.9) [2] |
| Hcy(Median (IQR)), nmol/mL | 16.7 (12.0–27.7) |
| Range | 8.2–96.0 |
| Normal range | (3.7–13.5) [2] |
| MCV (Median (IQR)), fL | 97.0 (91.0–97.9) |
| Range | 81.2–108.0 |
| Normal range | (83.6–98.2) [2] |
| MRI hippocampal atrophy | |
| Z-score (Median (IQR)) | 1.7 (1.2–2.6) |
| Range | 0.8–3.1 |
| Cutoff | 1.35 [29] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ueno, A.; Hamano, T.; Enomoto, S.; Shirafuji, N.; Nagata, M.; Kimura, H.; Ikawa, M.; Yamamura, O.; Yamanaka, D.; Ito, T.; et al. Influences of Vitamin B12 Supplementation on Cognition and Homocysteine in Patients with Vitamin B12 Deficiency and Cognitive Impairment. Nutrients 2022, 14, 1494. https://doi.org/10.3390/nu14071494
Ueno A, Hamano T, Enomoto S, Shirafuji N, Nagata M, Kimura H, Ikawa M, Yamamura O, Yamanaka D, Ito T, et al. Influences of Vitamin B12 Supplementation on Cognition and Homocysteine in Patients with Vitamin B12 Deficiency and Cognitive Impairment. Nutrients. 2022; 14(7):1494. https://doi.org/10.3390/nu14071494
Chicago/Turabian StyleUeno, Asako, Tadanori Hamano, Soichi Enomoto, Norimichi Shirafuji, Miwako Nagata, Hirohiko Kimura, Masamichi Ikawa, Osamu Yamamura, Daiki Yamanaka, Tatsuhiko Ito, and et al. 2022. "Influences of Vitamin B12 Supplementation on Cognition and Homocysteine in Patients with Vitamin B12 Deficiency and Cognitive Impairment" Nutrients 14, no. 7: 1494. https://doi.org/10.3390/nu14071494
APA StyleUeno, A., Hamano, T., Enomoto, S., Shirafuji, N., Nagata, M., Kimura, H., Ikawa, M., Yamamura, O., Yamanaka, D., Ito, T., Kimura, Y., Kuriyama, M., & Nakamoto, Y. (2022). Influences of Vitamin B12 Supplementation on Cognition and Homocysteine in Patients with Vitamin B12 Deficiency and Cognitive Impairment. Nutrients, 14(7), 1494. https://doi.org/10.3390/nu14071494






