Sodium Benzoate—Harmfulness and Potential Use in Therapies for Disorders Related to the Nervous System: A Review
Abstract
:1. Introduction
2. The Harmfulness of Sodium Benzoate
2.1. The Effect of Sodium Benzoate on the Oxidative Stress and Inflammation
2.2. Effect of Sodium Benzoate on the Embryos
2.3. Effect of Sodium Benzoate on Hormone Levels
2.4. Effect of Sodium Benzoate on Liver and Kidney Function
2.5. Sodium Benzoate and Children’s Hyperactivity
2.6. Sodium Benzoate—Irritating Effect on the Gastric Mucosa
2.7. Sodium Benzoate with Vitamin C
2.8. Effects of Sodium Benzoate on Memory and Anxiety Processes
3. Beneficial Properties of Sodium Benzoate
3.1. Effects of Sodium Benzoate on Oxidative Stress and Inflammation
3.2. Sodium Benzoate in Major Depressive Disorder and Anxiety
3.2.1. Pathogenesis of Major Depressive Disorder
3.2.2. Animal Models Studies
3.2.3. Human Studies
3.2.4. Effects of Sodium Benzoate
3.3. Sodium Benzoate in Schizophrenia
3.3.1. Pathogenesis of Schizophrenia
3.3.2. Effects of Sodium Benzoate
3.3.3. Animal Models Studies
3.3.4. Human Studies
3.4. Sodium Benzoate in Neurodegenerative Diseases
3.4.1. Pathogenesis of Neurodegenerative Diseases
3.4.2. Effects of Sodium Benzoate in Neurodegenerative Diseases
3.4.3. Effects of Sodium Benzoate on Alzheimer’s Disease
3.4.4. Effects of Sodium Benzoate in Parkinson’s Disease
3.4.5. Effects of Sodium Benzoate on Multiple Sclerosis
3.5. Sodium Benzoate in Pain Relief
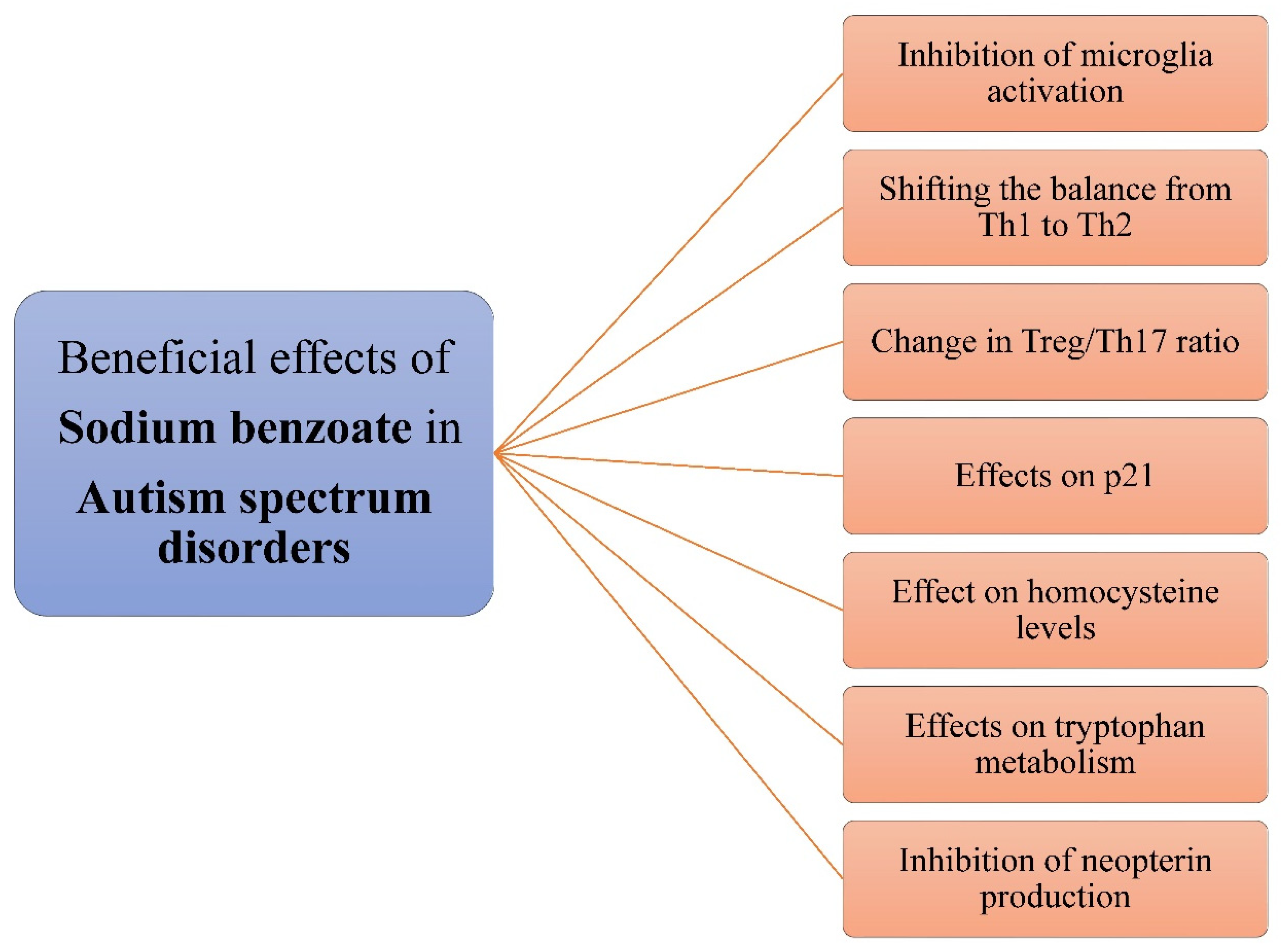
3.6. Sodium Benzoate in Autism Spectrum Disorder
3.6.1. Pathogenesis of Autism Spectrum Disorder
3.6.2. Effects of Sodium Benzoate
4. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hartmann, S.; Klaschka, U. Interested Consumers’ Awareness of Harmful Chemicals in Everyday Products. Environ. Sci. Eur. 2017, 29, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hartmann, S.; Klaschka, U. Do Consumers Care about Substances of Very High Concern in Articles? Environ. Sci. Eur. 2018, 30, 29. [Google Scholar] [CrossRef] [PubMed]
- Asioli, D.; Aschemann-Witzel, J.; Caputo, V.; Vecchio, R.; Annunziata, A.; Næs, T.; Varela, P. Making Sense of the “Clean Label” Trends: A Review of Consumer Food Choice Behavior and Discussion of Industry Implications. Food Res. Int. 2017, 99, 58–71. [Google Scholar] [CrossRef] [PubMed]
- Cegiełka, A. “Clean Label” as One of the Leading Trends in the Meat Industry in the World and in Poland—A Review. Rocz. Panstw. Zakl. Hig. 2020, 71, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Cheung, T.T.L.; Junghans, A.F.; Dijksterhuis, G.B.; Kroese, F.; Johansson, P.; Hall, L.; De Ridder, D.T.D. Consumers’ Choice-Blindness to Ingredient Information. Appetite 2016, 106, 2–12. [Google Scholar] [CrossRef] [Green Version]
- Davidson, P.M.; Taylor, T.M.; David, J.R.D. Antimicrobials in Food, 4th ed.; CRC Press: Boca Raton, FL, USA, 2021; ISBN 978-0-367-17878-9. [Google Scholar]
- CFR—Code of Federal Regulations Title 21. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/cfrsearch.cfm?fr=184.1733 (accessed on 14 November 2021).
- Lennerz, B.; Vafai, S.B.; Delaney, N.F.; Clish, C.B.; Deik, A.A.; Pierce, K.A.; Ludwig, D.S.; Mootha, V.K. Effects of Sodium Benzoate, a Widely Used Food Preservative, on Glucose Homeostasis and Metabolic Profiles in Humans. Mol. Genet. Metab. 2015, 114, 73–79. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Ma, Y.; Ma, W. Pharmacokinetics and Bioavailability of Cinnamic Acid after Oral Administration of Ramulus Cinnamomi in Rats. Eur. J. Drug Metab. Pharmacokinet. 2009, 34, 51–56. [Google Scholar] [CrossRef]
- Zhao, K.; Chen, Y.; Hong, S.; Yang, Y.; Xu, J.; Yang, H.; Zhu, L.; Liu, M.; Xie, Q.; Tang, X.; et al. Characteristics of β-Oxidative and Reductive Metabolism on the Acyl Side Chain of Cinnamic Acid and Its Analogues in Rats. Acta Pharmacol. Sin. 2019, 40, 1106–1118. [Google Scholar] [CrossRef]
- Shahmohammadi, M.; Javadi, M.; Nassiri-Asl, M. An Overview on the Effects of Sodium Benzoate as a Preservative in Food Products. Biotechnol. Health Sci. 2016, 3, 7–11. [Google Scholar] [CrossRef] [Green Version]
- BUPHENYL® (Sodium Phenylbutyrate) Tablets BUPHENYL® (Sodium Phenylbutyrate) Powder. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2009/020572s016,020573s015lbl.pdf (accessed on 10 February 2022).
- AMMONUL. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2005/020645lbl.pdf (accessed on 10 February 2022).
- Weber, R.W.; Hoffman, M.; Raine, D.A.; Nelson, H.S. Incidence of Bronchoconstriction Due to Aspirin, Azo Dyes, Non-Azo Dyes, and Preservatives in a Population of Perennial Asthmatics. J. Allergy Clin. Immunol. 1979, 64, 32–37. [Google Scholar] [CrossRef]
- Settipane, G.A. Aspirin and Allergic Diseases: A Review. Am. J. Med. 1983, 74, 102–109. [Google Scholar] [CrossRef]
- Balatsinou, L.; Di Gioacchino, G.; Sabatino, G.; Cavallucci, E.; Caruso, R.; Gabriele, E.; Ramondo, S.; Di Giampaolo, L.; Verna, N.; Di Gioacchino, M. Asthma Worsened by Benzoate Contained in Some Antiasthmatic Drugs. Int. J. Immunopathol. Pharmacol. 2004, 17, 225–226. [Google Scholar] [CrossRef]
- Piper, J.D.; Piper, P.W. Benzoate and Sorbate Salts: A Systematic Review of the Potential Hazards of These Invaluable Preservatives and the Expanding Spectrum of Clinical Uses for Sodium Benzoate. Compr. Rev. Food Sci. Food Saf. 2017, 16, 868–880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zengin, N.; Yüzbaşıoğlu, D.; Unal, F.; Yılmaz, S.; Aksoy, H. The Evaluation of the Genotoxicity of Two Food Preservatives: Sodium Benzoate and Potassium Benzoate. Food Chem. Toxicol. 2011, 49, 763–769. [Google Scholar] [CrossRef] [PubMed]
- Pongsavee, M. Effect of Sodium Benzoate Preservative on Micronucleus Induction, Chromosome Break, and Ala40Thr Superoxide Dismutase Gene Mutation in Lymphocytes. BioMed Res. Int. 2015, 2015, 103512. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S. Chapter Two—Oxidative Stress, Inflammation, and Disease. In Oxidative Stress and Biomaterials; Dziubla, T., Butterfield, D.A., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 35–58. ISBN 978-0-12-803269-5. [Google Scholar]
- Khansari, N.; Shakiba, Y.; Mahmoudi, M. Chronic Inflammation and Oxidative Stress as a Major Cause of Age-Related Diseases and Cancer. Recent Pat. Inflamm. Allergy Drug Discov. 2009, 3, 73–80. [Google Scholar] [CrossRef]
- Pole, A.; Dimri, M.; Dimri, G. Oxidative Stress, Cellular Senescence and Ageing. AIMS Mol. Sci. 2016, 3, 300–324. [Google Scholar] [CrossRef]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative Stress, Aging, and Diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef] [Green Version]
- Yetuk, G.; Pandir, D.; Bas, H. Protective Role of Catechin and Quercetin in Sodium Benzoate-Induced Lipid Peroxidation and the Antioxidant System in Human Erythrocytes In Vitro. Sci. World J. 2014, 2014, e874824. [Google Scholar] [CrossRef] [Green Version]
- El-Shennawy, L.; Kamel, M.A.E.; Khalaf, A.H.Y.; Yousef, M.I. Dose-Dependent Reproductive Toxicity of Sodium Benzoate in Male Rats: Inflammation, Oxidative Stress and Apoptosis. Reprod. Toxicol. 2020, 98, 92–98. [Google Scholar] [CrossRef]
- Sabour, A.; Ibrahim, I.R. Effect of Sodium Benzoate on Corticosterone Hormone Level, Oxidative Stress Indicators and Electrolytes in Immature Male Rats. Sci. J. Med. Res. 2019, 3, 101–106. [Google Scholar] [CrossRef]
- Olofinnade, A.T.; Onaolapo, A.Y.; Onaolapo, O.J.; Olowe, O.A. The Potential Toxicity of Food-Added Sodium Benzoate in Mice Is Concentration-Dependent. Toxicol. Res. 2021, 10, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.S.; Dar, K.B.; Ganie, S.A.; Ali, M.N. Toxicological Impact of Sodium Benzoate on Inflammatory Cytokines, Oxidative Stress and Biochemical Markers in Male Wistar Rats. Drug Chem. Toxicol. 2020, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.; Kumar, A.; Das, M.; Tripathi, A. Sodium Benzoate, a Food Preservative, Affects the Functional and Activation Status of Splenocytes at Non Cytotoxic Dose. Food Chem. Toxicol. 2016, 88, 40–47. [Google Scholar] [CrossRef]
- Tsay, H.-J.; Wang, Y.-H.; Chen, W.-L.; Huang, M.-Y.; Chen, Y.-H. Treatment with Sodium Benzoate Leads to Malformation of Zebrafish Larvae. Neurotoxicol. Teratol. 2007, 29, 562–569. [Google Scholar] [CrossRef]
- Chen, Q.; Huang, N.-N.; Huang, J.-T.; Chen, S.; Fan, J.; Li, C.; Xie, F.-K. Sodium Benzoate Exposure Downregulates the Expression of Tyrosine Hydroxylase and Dopamine Transporter in Dopaminergic Neurons in Developing Zebrafish. Birth Defects Res. B Dev. Reprod. Toxicol. 2009, 86, 85–91. [Google Scholar] [CrossRef]
- Saatci, C.; Erdem, Y.; Bayramov, R.; Akalın, H.; Tascioglu, N.; Ozkul, Y. Effect of Sodium Benzoate on DNA Breakage, Micronucleus Formation and Mitotic Index in Peripheral Blood of Pregnant Rats and Their Newborns. Biotechnol. Biotechnol. Equip. 2016, 30, 1179–1183. [Google Scholar] [CrossRef] [Green Version]
- Taheri, S.H.; Sohrabi, D. Teratogenic Effects of Sodium Benzoate on the Rat Fetus. J. Adv. Med. Biomed. Res. 2002, 10, 1–4. [Google Scholar]
- Ajpt, E. Fetal Malformations Due to Long Term Consumption of Sodium Benzoate in Pregnant Balb/c Mice. Asian J. Pharmacol. Toxicol. 2014, 2, 1–7. [Google Scholar]
- Afshar, M.; Moallem, S.A.; Khayatzadeh, J.; Shahsavan, M. Teratogenic Effects of Long Term Consumption of Potassium Benzoate on Eye Development in Balb/c Fetal Mice. Iran J. Basic Med. Sci. 2013, 16, 593–598. [Google Scholar]
- Afshar, M.; Moallem, S.A.; Taheri, M.H.; Shahsavan, M.; Sukhtanloo, F.; Salehi, F. Effect of Long Term Consumption of Sodium Benzoat before and during Pregnancy on Growth Indexes of Fetal Balb/c Mice. Modern Care J. 2012, 9, 173–180. [Google Scholar]
- Emon, S.T.; Orakdogen, M.; Uslu, S.; Somay, H. Effects of the Popular Food Additive Sodium Benzoate on Neural Tube Development in the Chicken Embryo. Turk. Neurosurg. 2015, 25, 294–297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jewo, P.I.; Oyeniran, D.A.; Ojekale, A.B.; Oguntola, J.A. Histological and Biochemical Studies of Germ Cell Toxicity in Male Rats Exposed to Sodium Benzoate. J. Adv. Med. Pharm. Sci. 2020, 22, 51–69. [Google Scholar] [CrossRef]
- Kehinde, O.S.; Christianah, O.I.; Oyetunji, O.A. Ascorbic Acid and Sodium Benzoate Synergistically Aggravates Testicular Dysfunction in Adult Wistar Rats. Int. J. Physiol. Pathophysiol. Pharmacol. 2018, 10, 39–46. [Google Scholar] [PubMed]
- Mahmoud, G.S.; Sayed, S.A.; Abdelmawla, S.N.; Amer, M.A. Positive Effects of Systemic Sodium Benzoate and Olanzapine Treatment on Activities of Daily Life, Spatial Learning and Working Memory in Ketamine-Induced Rat Model of Schizophrenia. Int. J. Physiol. Pathophysiol. Pharmacol. 2019, 11, 21–30. [Google Scholar] [PubMed]
- Sohrabi, D.; Alipour, M.; Gholami, M.R. The Effect of Sodium Benzoate on Testicular Tissue, Gonadotropins and Thyroid Hormones Level in Adult (Balb/C) Mice. KAUMS J. (FEYZ) 2008, 12, 7–11. [Google Scholar]
- Najah, A. Effect of Sodium Benzoate in the Level of Thyroid Stimulating Hormone and the Level of Thyroxin Hormone in Mature Albino Male Rats. J. Kerbala Univ. 2015, 13, 295–299. [Google Scholar]
- Khodaei, F.; Kholghipour, H.; Hosseinzadeh, M.; Rashedinia, M. Effect of Sodium Benzoate on Liver and Kidney Lipid Peroxidation and Antioxidant Enzymes in Mice. J. Rep. Pharm. Sci. 2019, 8, 217. [Google Scholar] [CrossRef]
- Zeghib, K.; Boutlelis, D.A. Food Additive (Sodium Benzoate)-Induced Damage on Renal Function and Glomerular Cells in Rats; Modulating Effect of Aqueous Extract of Atriplex halimus L. Iran J. Pharm. Res. 2021, 20, 296–306. [Google Scholar] [CrossRef]
- Ibekwe, E.S.; Uwakwe, A.A.; Monanu, O.M. In Vivo Effects of Sodium Benzoate on Plasma Aspartate Amino Transferase and Alkaline Phosphatase of Wistar Albino Rats. Sci. Res. Essays 2007, 2, 10–12. [Google Scholar] [CrossRef]
- Tomoko Fujitani Short-Term Effect of Sodium Benzoate in F344 Rats and B6C3F1 Mice. Toxicol. Lett. 1993, 69, 171–179. [CrossRef]
- Radwan, E.H.; Elghazaly, M.M.; Aziz, K.A.; Barakat, A.I.; Hussein, H.K. The Possible Effects of Sodium Nitrite and Sodium Benzoate as Food Additives on The Liver in Male Rats. J. Adv. Biol. 2020, 13, 14–30. [Google Scholar] [CrossRef]
- Oyewole, O.I.; Dere, F.A.; Okoro, O.E. Sodium Benzoate Mediated Hepatorenal Toxicity in Wistar Rat: Modulatory Effects of Azadirachta Indica (Neem) Leaf. Eur. J. Med. Plants 2012, 2, 11–18. [Google Scholar] [CrossRef]
- Agarwal, A.; Sharma, A.; Nigam, G.L.; Gupta, A.; Pandey, V.D.; Malik, A.; Yadav, A. Histological profile of liver of albino rats on oral administration of sodium benzoate. J. Anat. Sci. 2016, 24, 29–32. [Google Scholar]
- McDougal, E.; Gracie, H.; Oldridge, J.; Stewart, T.M.; Booth, J.N.; Rhodes, S.M. Relationships between Cognition and Literacy in Children with Attention-Deficit/Hyperactivity Disorder: A Systematic Review and Meta-Analysis. Br. J. Dev. Psychol. 2022, 40, 130–150. [Google Scholar] [CrossRef]
- Bateman, B.; Warner, J.; Hutchinson, E.; Dean, T.; Rowlandson, P.; Gant, C.; Grundy, J.; Fitzgerald, C.; Stevenson, J. The Effects of a Double Blind, Placebo Controlled, Artificial Food Colourings and Benzoate Preservative Challenge on Hyperactivity in a General Population Sample of Preschool Children. Arch. Dis. Child. 2004, 89, 506–511. [Google Scholar] [CrossRef] [Green Version]
- McCann, D.; Barrett, A.; Cooper, A.; Crumpler, D.; Dalen, L.; Grimshaw, K.; Kitchin, E.; Lok, K.; Porteous, L.; Prince, E.; et al. Food Additives and Hyperactive Behaviour in 3-Year-Old and 8/9-Year-Old Children in the Community: A Randomised, Double-Blinded, Placebo-Controlled Trial. Lancet 2007, 370, 1560–1567. [Google Scholar] [CrossRef]
- Beezhold, B.L.; Johnston, C.S.; Nochta, K.A. Sodium Benzoate-Rich Beverage Consumption Is Associated with Increased Reporting of ADHD Symptoms in College Students: A Pilot Investigation. J. Atten. Disord. 2014, 18, 236–241. [Google Scholar] [CrossRef]
- Van West, D.; Claes, S.; Deboutte, D. Differences in Hypothalamic-Pituitary-Adrenal Axis Functioning among Children with ADHD Predominantly Inattentive and Combined Types. Eur. Child. Adolesc. Psychiatry 2009, 18, 543–553. [Google Scholar] [CrossRef] [Green Version]
- Corominas-Roso, M.; Palomar, G.; Ferrer, R.; Real, A.; Nogueira, M.; Corrales, M.; Casas, M.; Ramos-Quiroga, J.A. Cortisol Response to Stress in Adults with Attention Deficit Hyperactivity Disorder. Int. J. Neuropsychopharmacol. 2015, 18, pyv027. [Google Scholar] [CrossRef] [Green Version]
- Maier, E.; Kurz, K.; Jenny, M.; Schennach, H.; Ueberall, F.; Fuchs, D. Food Preservatives Sodium Benzoate and Propionic Acid and Colorant Curcumin Suppress Th1-Type Immune Response in Vitro. Food Chem. Toxicol. 2010, 48, 1950–1956. [Google Scholar] [CrossRef] [PubMed]
- Suwan, P.; Akaramethathip, D.; Noipayak, P. Association between Allergic Sensitization and Attention Deficit Hyperactivity Disorder (ADHD). Asian Pac. J. Allergy Immunol. 2011, 29, 57–65. [Google Scholar] [PubMed]
- Miyazaki, C.; Koyama, M.; Ota, E.; Swa, T.; Mlunde, L.B.; Amiya, R.M.; Tachibana, Y.; Yamamoto-Hanada, K.; Mori, R. Allergic Diseases in Children with Attention Deficit Hyperactivity Disorder: A Systematic Review and Meta-Analysis. BMC Psychiatry 2017, 17, 120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dunn, G.A.; Nigg, J.T.; Sullivan, E.L. Neuroinflammation as a Risk Factor for Attention Deficit Hyperactivity Disorder. Pharmacol. Biochem. Behav. 2019, 182, 22–34. [Google Scholar] [CrossRef]
- Joseph, N.; Zhang-James, Y.; Perl, A.; Faraone, S.V. Oxidative Stress and ADHD: A Meta-Analysis. J. Atten. Disord. 2015, 19, 915–924. [Google Scholar] [CrossRef]
- Pinho, R.; Wang, B.; Becker, A.; Rothenberger, A.; Outeiro, T.F.; Herrmann-Lingen, C.; Meyer, T. Attention-Deficit/Hyperactivity Disorder Is Associated with Reduced Levels of Serum Low-Density Lipoprotein Cholesterol in Adolescents. Data from the Population-Based German KiGGS Study. World J. Biol. Psychiatry 2019, 20, 496–504. [Google Scholar] [CrossRef]
- Efekemo, O.; Akaninwor, J.O.; Essien, E.B. Effect of Oral Intake of Sodium Benzoate on Serum Cholesterol and Proinflammatory Cytokine (Tumor Necrosis Factor Alpha [TNF-α] and Interleukin-6 [IL-6]) Levels in the Heart Tissue of Wistar Rats. Asian J. Res. Biochem. 2019, 5, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Stoodley, C.J. Distinct Regions of the Cerebellum Show Gray Matter Decreases in Autism, ADHD, and Developmental Dyslexia. Front. Syst. Neurosci. 2014, 8, 92. [Google Scholar] [CrossRef]
- Noorafshan, A.; Erfanizadeh, M.; Karbalay-Doust, S. Stereological Studies of the Effects of Sodium Benzoate or Ascorbic Acid on Rats’ Cerebellum. Saudi Med. J. 2014, 35, 1494–1500. [Google Scholar]
- Schaubschläger, W.W.; Becker, W.M.; Schade, U.; Zabel, P.; Schlaak, M. Release of Mediators from Human Gastric Mucosa and Blood in Adverse Reactions to Benzoate. Int. Arch. Allergy Appl. Immunol. 1991, 96, 97–101. [Google Scholar] [CrossRef]
- Mcneal, T.P.; Nyman, P.J.; Diachenko, G.W.; Hollifield, H.C. Survey of Benzene in Foods by Using Headspace Concentration Techniques and Capillary Gas Chromatography. J. AOAC Int. 1993, 76, 1213–1219. [Google Scholar] [CrossRef] [PubMed]
- Heshmati, A.; Ghadimi, S.; Mousavi Khaneghah, A.; Barba, F.J.; Lorenzo, J.M.; Nazemi, F.; Fakhri, Y. Risk Assessment of Benzene in Food Samples of Iran’s Market. Food Chem. Toxicol. 2018, 114, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Salviano dos Santos, V.P.; Medeiros Salgado, A.; Guedes Torres, A.; Signori Pereira, K. Benzene as a Chemical Hazard in Processed Foods. Int. J. Food Sci. 2015, 2015, e545640. [Google Scholar] [CrossRef] [PubMed]
- Azuma, S.L.; Quartey, N.K.-A.; Ofosu, I.W. Sodium Benzoate in Non-Alcoholic Carbonated (Soft) Drinks: Exposure and Health Risks. Sci. Afr. 2020, 10, e00611. [Google Scholar] [CrossRef]
- Gardner, L.K.; Lawrence, G.D. Benzene Production from Decarboxylation of Benzoic Acid in the Presence of Ascorbic Acid and a Transition-Metal Catalyst. J. Agric. Food Chem. 1993, 41, 693–695. [Google Scholar] [CrossRef]
- Nyman, P.J.; Wamer, W.G.; Begley, T.H.; Diachenko, G.W.; Perfetti, G.A. Evaluation of Accelerated UV and Thermal Testing for Benzene Formation in Beverages Containing Benzoate and Ascorbic Acid. J. Food Sci. 2010, 75, C263–C267. [Google Scholar] [CrossRef]
- Kamel, M.M.; Razek, A.H. Neurobehavioral Alterations in Male Rats Exposed to Sodium Benzoate. Life Sci. J. 2013, 10, 722–726. [Google Scholar]
- Noorafshan, A.; Erfanizadeh, M.; Karbalay-doust, S. Sodium Benzoate, a Food Preservative, Induces Anxiety and Motor Impairment in Rats. Neurosciences J. 2014, 19, 24–28. [Google Scholar]
- Olofinnade, A.T.; Onaolapo, A.Y.; Onaolapo, O.J. Anxiogenic, Memory-Impairing, pro-Oxidant and pro-Inflammatory Effects of Sodium Benzoate in the Mouse Brain. Dusunen Adam J. Psychiatry Neurol. Sci. 2021, 34, 14. [Google Scholar] [CrossRef]
- Gaur, H.; Purushothaman, S.; Pullaguri, N.; Bhargava, Y.; Bhargava, A. Sodium Benzoate Induced Developmental Defects, Oxidative Stress and Anxiety-like Behaviour in Zebrafish Larva. Biochem. Biophys. Res. Commun. 2018, 502, 364–369. [Google Scholar] [CrossRef]
- Khoshnoud, M.J.; Siavashpour, A.; Bakhshizadeh, M.; Rashedinia, M. Effects of Sodium Benzoate, a Commonly Used Food Preservative, on Learning, Memory, and Oxidative Stress in Brain of Mice. J. Biochem. Mol. Toxicol. 2018, 32, e22022. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, P.; Nandakumar, N.; Rengarajan, T.; Palaniswami, R.; Gnanadhas, E.N.; Lakshminarasaiah, U.; Gopas, J.; Nishigaki, I. Antioxidants and Human Diseases. Clin. Chim. Acta 2014, 436, 332–347. [Google Scholar] [CrossRef] [PubMed]
- Brahmachari, S.; Jana, A.; Pahan, K. Sodium Benzoate, a Metabolite of Cinnamon and a Food Additive, Reduces Microglial and Astroglial Inflammatory Responses. J. Immunol. 2009, 183, 5917–5927. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walia, D.; Kaur, G.; Jaggi, A.S.; Bali, A. Exploring the Therapeutic Potential of Sodium Benzoate in Acetic Acid-Induced Ulcerative Colitis in Rats. J. Basic Clin. Physiol. Pharmacol. 2019, 30. [Google Scholar] [CrossRef]
- Xu, W.; Li, T.; Gao, L.; Lenahan, C.; Zheng, J.; Yan, J.; Shao, A.; Zhang, J. Sodium Benzoate Attenuates Secondary Brain Injury by Inhibiting Neuronal Apoptosis and Reducing Mitochondria-Mediated Oxidative Stress in a Rat Model of Intracerebral Hemorrhage: Possible Involvement of DJ-1/Akt/IKK/NFκB Pathway. Front. Mol. Neurosci. 2019, 12, 105. [Google Scholar] [CrossRef]
- Molero-Luis, M.; Casas-Alba, D.; Orellana, G.; Ormazabal, A.; Sierra, C.; Oliva, C.; Valls, A.; Velasco, J.; Launes, C.; Cuadras, D.; et al. Cerebrospinal Fluid Neopterin as a Biomarker of Neuroinflammatory Diseases. Sci. Rep. 2020, 10, 18291. [Google Scholar] [CrossRef]
- Williams, R.E.; Lock, E.A. Sodium Benzoate Attenuates D-Serine Induced Nephrotoxicity in the Rat. Toxicology 2005, 207, 35–48. [Google Scholar] [CrossRef]
- Arabsolghar, R.; Saberzadeh, J.; Khodaei, F.; Borojeni, R.A.; Khorsand, M.; Rashedinia, M. The Protective Effect of Sodium Benzoate on Aluminum Toxicity in PC12 Cell Line. Res. Pharm. Sci. 2017, 12, 391–400. [Google Scholar] [CrossRef] [Green Version]
- Yilmaz, B.; Karabay, A.Z. Food Additive Sodium Benzoate (NaB) Activates NFκB and Induces Apoptosis in HCT116 Cells. Molecules 2018, 23, 723. [Google Scholar] [CrossRef] [Green Version]
- GBD Results Tool. GHDx. Available online: http://ghdx.healthdata.org/gbd-results-tool?params=gbd-api-2019-permalink/d780dffbe8a381b25e1416884959e88b (accessed on 14 November 2021).
- Bains, N.; Abdijadid, S. Major Depressive Disorder. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Nobis, A.; Zalewski, D.; Waszkiewicz, N. Peripheral Markers of Depression. J. Clin. Med. 2020, 9, 3793. [Google Scholar] [CrossRef]
- Adell, A. Brain NMDA Receptors in Schizophrenia and Depression. Biomolecules 2020, 10, 947. [Google Scholar] [CrossRef] [PubMed]
- Otte, D.-M.; de Arellano, M.L.B.; Bilkei-Gorzo, A.; Albayram, Ö.; Imbeault, S.; Jeung, H.; Alferink, J.; Zimmer, A. Effects of Chronic D-Serine Elevation on Animal Models of Depression and Anxiety-Related Behavior. PLoS ONE 2013, 8, e67131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belujon, P.; Grace, A.A. Dopamine System Dysregulation in Major Depressive Disorders. Int. J. Neuropsychopharmacol. 2017, 20, 1036–1046. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Betts, J.F.; Schweimer, J.V.; Burnham, K.E.; Burnet, P.W.J.; Sharp, T.; Harrison, P.J. D-Amino Acid Oxidase Is Expressed in the Ventral Tegmental Area and Modulates Cortical Dopamine. Front. Synaptic Neurosci. 2014, 6, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, F.; Zhang, Z.; Liang, Y.; Yang, R.; Tan, Y. Exploring the Role and Mechanism of Sodium Benzoate in CUMS-Induced Depression Model of Rats. Neuroendocrinol. Lett. 2020, 41, 205–212. [Google Scholar]
- Lai, C.-H. Sodium Benzoate, a D-Amino Acid Oxidase Inhibitor, Increased Volumes of Thalamus, Amygdala, and Brainstem in a Drug-Naïve Patient With Major Depression. J. Neuropsychiatry Clin. Neurosci. 2013, 25, E50–E51. [Google Scholar] [CrossRef]
- Lai, C.-H.; Lane, H.-Y.; Tsai, G.E. Clinical and Cerebral Volumetric Effects of Sodium Benzoate, a D-Amino Acid Oxidase Inhibitor, in a Drug-Naïve Patient with Major Depression. Biol. Psychiatry 2012, 71, e9–e10. [Google Scholar] [CrossRef]
- Hou, Y.-C.; Lai, C.-H. A Kind of D-Amino Acid Oxidase Inhibitor, Sodium Benzoate, Might Relieve Panic Symptoms in a First-Episode, Drug-Naïve Panic-Disorder Patient. J. Neuropsychiatry Clin. Neurosci. 2013, 25, E07–E08. [Google Scholar] [CrossRef]
- Modi, K.K.; Roy, A.; Brahmachari, S.; Rangasamy, S.B.; Pahan, K. Cinnamon and Its Metabolite Sodium Benzoate Attenuate the Activation of P21rac and Protect Memory and Learning in an Animal Model of Alzheimer’s Disease. PLoS ONE 2015, 10, e0130398. [Google Scholar] [CrossRef]
- Esnafoglu, E.; Ozturan, D.D. The Relationship of Severity of Depression with Homocysteine, Folate, Vitamin B12, and Vitamin D Levels in Children and Adolescents. Child. Adolesc. Ment. Health 2020, 25, 249–255. [Google Scholar] [CrossRef]
- Chung, K.-H.; Chiou, H.-Y.; Chen, Y.-H. Associations between Serum Homocysteine Levels and Anxiety and Depression among Children and Adolescents in Taiwan. Sci. Rep. 2017, 7, 8330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monje, F.J.; Cabatic, M.; Divisch, I.; Kim, E.-J.; Herkner, K.R.; Binder, B.R.; Pollak, D.D. Constant Darkness Induces IL-6-Dependent Depression-Like Behavior through the NF-ΚB Signaling Pathway. J. Neurosci. 2011, 31, 9075–9083. [Google Scholar] [CrossRef] [PubMed]
- Koo, J.; Marangell, L.b.; Nakamura, M.; Armstrong, A.; Jeon, C.; Bhutani, T.; Wu, J.j. Depression and Suicidality in Psoriasis: Review of the Literature Including the Cytokine Theory of Depression. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 1999–2009. [Google Scholar] [CrossRef] [PubMed]
- Makhija, K.; Karunakaran, S. The Role of Inflammatory Cytokines on the Aetiopathogenesis of Depression. Aust. New Zealand J. Psychiatry 2013, 47, 828–839. [Google Scholar] [CrossRef]
- Jenkins, T.A.; Nguyen, J.C.D.; Polglaze, K.E.; Bertrand, P.P. Influence of Tryptophan and Serotonin on Mood and Cognition with a Possible Role of the Gut-Brain Axis. Nutrients 2016, 8, 56. [Google Scholar] [CrossRef]
- Lindseth, G.; Helland, B.; Caspers, J. The Effects of Dietary Tryptophan on Affective Disorders. Arch. Psychiatr. Nurs. 2015, 29, 102–107. [Google Scholar] [CrossRef] [Green Version]
- Dantzer, R. Role of the Kynurenine Metabolism Pathway in Inflammation-Induced Depression: Preclinical Approaches. In Inflammation-Associated Depression: Evidence, Mechanisms and Implications; Aktualne Tematy w Neuronaukach Behawioralnych; Dantzer, R., Capuron, L., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 117–138. ISBN 978-3-319-51152-8. [Google Scholar]
- Muszyńska, B.; Łojewski, M.; Rojowski, J.; Opoka, W.; Sułkowska-Ziaja, K. Surowce naturalne mające znaczenie w profilaktyce i wspomagające leczenie depresji [Natural products of relevance in the prevention and supportive treatment of depression]. Psychiatria Polska 2015, 49, 435–453. [Google Scholar] [CrossRef]
- Widner, B.; Laich, A.; Sperner-Unterweger, B.; Ledochowski, M.; Fuchs, D. Neopterin Production, Tryptophan Degradation, and Mental Depression—What Is the Link? Brain Behav. Immun. 2002, 16, 590–595. [Google Scholar] [CrossRef]
- Celik, C.; Erdem, M.; Caycı, T.; Ozdemir, B.; Ozgur Akgul, E.; Kurt, Y.G.; Yaman, H.; Isıntas, M.; Ozgen, F.; Ozsahin, A. The Association between Serum Levels of Neopterin and Number of Depressive Episodes of Major Depression. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2010, 34, 372–375. [Google Scholar] [CrossRef]
- Ciardi, C.; Jenny, M.; Tschoner, A.; Ueberall, F.; Patsch, J.; Pedrini, M.; Ebenbichler, C.; Fuchs, D. Food Additives Such as Sodium Sulphite, Sodium Benzoate and Curcumin Inhibit Leptin Release in Lipopolysaccharide-Treated Murine Adipocytes in Vitro. Br. J. Nutr. 2012, 107, 826–833. [Google Scholar] [CrossRef] [Green Version]
- Ambrus, L.; Westling, S. Leptin, Anxiety Symptoms, and Hypothalamic-Pituitary-Adrenal Axis Activity among Drug-Free, Female Suicide Attempters. Neuropsychobiology 2019, 78, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Mikulska, J.; Juszczyk, G.; Gawrońska-Grzywacz, M.; Herbet, M. HPA Axis in the Pathomechanism of Depression and Schizophrenia: New Therapeutic Strategies Based on Its Participation. Brain Sci. 2021, 11, 1298. [Google Scholar] [CrossRef] [PubMed]
- Pasquin, S.; Sharma, M.; Gauchat, J.-F. Ciliary Neurotrophic Factor (CNTF): New Facets of an Old Molecule for Treating Neurodegenerative and Metabolic Syndrome Pathologies. Cytokine Growth Factor Rev. 2015, 26, 507–515. [Google Scholar] [CrossRef]
- Modi, K.K.; Jana, M.; Mondal, S.; Pahan, K. Sodium Benzoate, a Metabolite of Cinnamon and a Food Additive, Upregulates Ciliary Neurotrophic Factor in Astrocytes and Oligodendrocytes. Neurochem. Res. 2015, 40, 2333–2347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peruga, I.; Hartwig, S.; Merkler, D.; Thöne, J.; Hovemann, B.; Juckel, G.; Gold, R.; Linker, R.A. Endogenous Ciliary Neurotrophic Factor Modulates Anxiety and Depressive-like Behavior. Behav. Brain Res. 2012, 229, 325–332. [Google Scholar] [CrossRef]
- Uzbekov, M.; Shikhov, S. Ciliary Neurotrophic Factor Disturbances in Patients with Melancholic Depression. Biomed. J. Sci. Technol. Res. 2019, 13, 10016–10017. [Google Scholar] [CrossRef]
- Jia, C.; Brown, R.W.; Malone, H.M.; Burgess, K.C.; Gill, D.W.; Keasey, M.P.; Hagg, T. Ciliary Neurotrophic Factor Is a Key Sex-Specific Regulator of Depressive-like Behavior in Mice. Psychoneuroendocrinology 2019, 100, 96–105. [Google Scholar] [CrossRef]
- Lin, C.-H.; Chen, P.-K.; Wang, S.-H.; Lane, H.-Y. Effect of Sodium Benzoate on Cognitive Function Among Patients With Behavioral and Psychological Symptoms of Dementia. JAMA Netw. Open 2021, 4, e216156. [Google Scholar] [CrossRef]
- Jarema, M. Psychiatria w Praktyce; Oficyna Wydawnicza Medical Education Sp. z o.o.: Warszawa, Poland, 2011; ISBN 978-83-62510-06-1. [Google Scholar]
- Jarema, M. Psychiatria, 2nd ed.; PZWL: Warszawa, Poland, 2016; ISBN 978-83-200-4180-4. [Google Scholar]
- Harrison’s Principles of Internal Medicine, 20e. AccessMedicine. McGraw Hill Medical. Available online: https://accessmedicine.mhmedical.com/book.aspx?bookid=2129&isMissingChapter=true (accessed on 23 November 2021).
- Wu, Q.; Wang, X.; Wang, Y.; Long, Y.-J.; Zhao, J.-P.; Wu, R.-R. Developments in Biological Mechanisms and Treatments for Negative Symptoms and Cognitive Dysfunction of Schizophrenia. Neurosci. Bull. 2021, 37, 1609–1624. [Google Scholar] [CrossRef]
- Raj, K.S.; Williams, N.; DeBattista, C. Schizophrenia Spectrum Disorders. In Current Medical Diagnosis & Treatment 2022; Papadakis, M.A., McPhee, S.J., Rabow, M.W., McQuaid, K.R., Eds.; McGraw-Hill Education: New York, NY, USA, 2022. [Google Scholar]
- Sklar, P. Schizophrenia. In Clinical Genomics: Practical Applications in Adult Patient Care; Murray, M.F., Babyatsky, M.W., Giovanni, M.A., Alkuraya, F.S., Stewart, D.R., Eds.; McGraw-Hill Education: New York, NY, USA, 2014. [Google Scholar]
- Radhakrishnan, R.; Ganesh, S.; Meltzer, H.Y.; Bobo, W.V.; Heckers, S.H.; Fatemi, H.S.; D’Souza, D.C. Schizophrenia. In Current Diagnosis & Treatment: Psychiatry; Ebert, M.H., Leckman, J.F., Petrakis, I.L., Eds.; McGraw-Hill Education: New York, NY, USA, 2019. [Google Scholar]
- Smith, S.M.; Uslaner, J.M.; Hutson, P.H. The Therapeutic Potential of D-Amino Acid Oxidase (DAAO) Inhibitors. Open Med. Chem. J. 2010, 4, 3–9. [Google Scholar] [CrossRef] [Green Version]
- Burnet, P.; Eastwood, S.; Bristow, G.; Godlewska, B.; Sikka, P.; Walker, M.; Harrison, P. D-Amino Acid Oxidase (DAO) Activity and Expression Are Increased in Schizophrenia. Mol. Psychiatry 2008, 13, 658–660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caldinelli, L.; Molla, G.; Bracci, L.; Lelli, B.; Pileri, S.; Cappelletti, P.; Sacchi, S.; Pollegioni, L. Effect of Ligand Binding on Human D-Amino Acid Oxidase: Implications for the Development of New Drugs for Schizophrenia Treatment. Protein Sci. 2010, 19, 1500–1512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Howley, E.; Bestwick, M.; Fradley, R.; Harrison, H.; Leveridge, M.; Okada, K.; Fieldhouse, C.; Farnaby, W.; Canning, H.; Sykes, A.P.; et al. Assessment of the Target Engagement and D-Serine Biomarker Profiles of the D-Amino Acid Oxidase Inhibitors Sodium Benzoate and PGM030756. Neurochem. Res. 2017, 42, 3279–3288. [Google Scholar] [CrossRef] [PubMed]
- MacKay, M.-A.B.; Kravtsenyuk, M.; Thomas, R.; Mitchell, N.D.; Dursun, S.M.; Baker, G.B. D-Serine: Potential Therapeutic Agent and/or Biomarker in Schizophrenia and Depression? Front. Psychiatry 2019, 10, 25. [Google Scholar] [CrossRef] [Green Version]
- Saleem, S.; Shaukat, F.; Gul, A.; Arooj, M.; Malik, A. Potential Role of Amino Acids in Pathogenesis of Schizophrenia. Int. J. Health Sci. 2017, 11, 63–68. [Google Scholar]
- Enomoto, T.; Noda, Y.; Nabeshima, T. Phencyclidine and Genetic Animal Models of Schizophrenia Developed in Relation to the Glutamate Hypothesis. Methods Find. Exp. Clin. Pharmacol. 2007, 29, 291–301. [Google Scholar] [CrossRef]
- Matsuura, A.; Fujita, Y.; Iyo, M.; Hashimoto, K. Effects of Sodium Benzoate on Pre-Pulse Inhibition Deficits and Hyperlocomotion in Mice after Administration of Phencyclidine. Acta Neuropsychiatr. 2015, 27, 159–167. [Google Scholar] [CrossRef] [Green Version]
- Sershen, H.; Hashim, A.; Dunlop, D.S.; Suckow, R.F.; Cooper, T.B.; Javitt, D.C. Modulating NMDA Receptor Function with D-Amino Acid Oxidase Inhibitors: Understanding Functional Activity in PCP-Treated Mouse Model. Neurochem. Res. 2016, 41, 398–408. [Google Scholar] [CrossRef] [Green Version]
- De Luca, V.; Viggiano, E.; Messina, G.; Viggiano, A.; Borlido, C.; Viggiano, A.; Monda, M. Peripheral Amino Acid Levels in Schizophrenia and Antipsychotic Treatment. Psychiatry Investig. 2008, 5, 203–208. [Google Scholar] [CrossRef] [Green Version]
- Monte, A.S.; de Souza, G.C.; McIntyre, R.S.; Soczynska, J.K.; dos Santos, J.V.; Cordeiro, R.C.; Ribeiro, B.M.M.; de Lucena, D.F.; Vasconcelos, S.M.M.; de Sousa, F.C.F.; et al. Prevention and Reversal of Ketamine-Induced Schizophrenia Related Behavior by Minocycline in Mice: Possible Involvement of Antioxidant and Nitrergic Pathways. J. Psychopharmacol. 2013, 27, 1032–1043. [Google Scholar] [CrossRef]
- Becker, A.; Peters, B.; Schroeder, H.; Mann, T.; Huether, G.; Grecksch, G. Ketamine-Induced Changes in Rat Behaviour: A Possible Animal Model of Schizophrenia. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2003, 27, 687–700. [Google Scholar] [CrossRef]
- Lin, C.-Y.; Liang, S.-Y.; Chang, Y.-C.; Ting, S.-Y.; Kao, C.-L.; Wu, Y.-H.; Tsai, G.E.; Lane, H.-Y. Adjunctive Sarcosine plus Benzoate Improved Cognitive Function in Chronic Schizophrenia Patients with Constant Clinical Symptoms: A Randomised, Double-Blind, Placebo-Controlled Trial. World J. Biol. Psychiatry 2017, 18, 357–368. [Google Scholar] [CrossRef] [PubMed]
- Lane, H.-Y.; Lin, C.-H.; Green, M.F.; Hellemann, G.; Huang, C.-C.; Chen, P.-W.; Tun, R.; Chang, Y.-C.; Tsai, G.E. Add-on Treatment of Benzoate for Schizophrenia: A Randomized, Double-Blind, Placebo-Controlled Trial of D-Amino Acid Oxidase Inhibitor. JAMA Psychiatry 2013, 70, 1267–1275. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-H.; Lin, C.-H.; Chang, Y.-C.; Huang, Y.-J.; Chen, P.-W.; Yang, H.-T.; Lane, H.-Y. Sodium Benzoate, a D-Amino Acid Oxidase Inhibitor, Added to Clozapine for the Treatment of Schizophrenia: A Randomized, Double-Blind, Placebo-Controlled Trial. Biol. Psychiatry 2018, 84, 422–432. [Google Scholar] [CrossRef] [PubMed]
- Moustafa, A.A.; Hewedi, D.H.; Eissa, A.M.; Frydecka, D.; Misiak, B. Homocysteine Levels in Schizophrenia and Affective Disorders—Focus on Cognition. Front. Behav. Neurosci. 2014, 8, 343. [Google Scholar] [CrossRef] [Green Version]
- Volk, D.W.; Moroco, A.E.; Roman, K.M.; Edelson, J.R.; Lewis, D.A. The Role of the Nuclear Factor-ΚB Transcriptional Complex in Cortical Immune Activation in Schizophrenia. Biol. Psychiatry 2019, 85, 25–34. [Google Scholar] [CrossRef]
- Safa, A.; Badrlou, E.; Arsang-Jang, S.; Sayad, A.; Taheri, M.; Ghafouri-Fard, S. Expression of NF-ΚB Associated LncRNAs in Schizophrenia. Sci. Rep. 2020, 10, 18105. [Google Scholar] [CrossRef]
- Ermakov, E.A.; Dmitrieva, E.M.; Parshukova, D.A.; Kazantseva, D.V.; Vasilieva, A.R.; Smirnova, L.P. Oxidative Stress-Related Mechanisms in Schizophrenia Pathogenesis and New Treatment Perspectives. Oxid. Med. Cell. Longev. 2021, 2021, 8881770. [Google Scholar] [CrossRef]
- Chiappelli, J.; Postolache, T.T.; Kochunov, P.; Rowland, L.M.; Wijtenburg, S.A.; Shukla, D.K.; Tagamets, M.; Du, X.; Savransky, A.; Lowry, C.A.; et al. Tryptophan Metabolism and White Matter Integrity in Schizophrenia. Neuropsychopharmacology 2016, 41, 2587–2595. [Google Scholar] [CrossRef] [Green Version]
- Golightly, K.L.; Lloyd, J.A.; Hobson, J.E.; Gallagher, P.; Mercer, G.; Young, A.H. Acute Tryptophan Depletion in Schizophrenia. Psychol. Med. 2001, 31, 75–84. [Google Scholar] [CrossRef]
- Chittiprol, S.; Venkatasubramanian, G.; Neelakantachar, N.; Babu, S.V.S.; Reddy, N.A.; Shetty, K.T.; Gangadhar, B.N. Oxidative Stress and Neopterin Abnormalities in Schizophrenia: A Longitudinal Study. J. Psychiatr. Res. 2010, 44, 310–313. [Google Scholar] [CrossRef] [PubMed]
- Scott, J.G.; Baker, A.; Lim, C.C.W.; Foley, S.; Dark, F.; Gordon, A.; Ward, D.; Richardson, D.; Bruxner, G.; Beckmann, K.M.; et al. Effect of Sodium Benzoate vs Placebo Among Individuals With Early Psychosis. JAMA Netw. Open 2020, 3, e2024335. [Google Scholar] [CrossRef] [PubMed]
- Walczak-Nowicka, Ł.J.; Herbet, M. Acetylcholinesterase Inhibitors in the Treatment of Neurodegenerative Diseases and the Role of Acetylcholinesterase in Their Pathogenesis. Int. J. Mol. Sci. 2021, 22, 9290. [Google Scholar] [CrossRef] [PubMed]
- Serwer, P. Hypothesis for the Cause and Therapy of Neurodegenerative Diseases. Med. Hypotheses 2018, 110, 60–63. [Google Scholar] [CrossRef]
- Radi, E.; Formichi, P.; Battisti, C.; Federico, A. Apoptosis and Oxidative Stress in Neurodegenerative Diseases. J. Alzheimer’s Dis. 2014, 42, S125–S152. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Sidhu, J.; Goyal, A.; Tsao, J.W. Alzheimer Disease. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Kouli, A.; Torsney, K.M.; Kuan, W.-L. Parkinson’s Disease: Etiology, Neuropathology, and Pathogenesis. In Parkinson’s Disease: Pathogenesis and Clinical Aspects; Stoker, T.B., Greenland, J.C., Eds.; Codon Publications: Brisbane, Australia, 2018; ISBN 978-0-9944381-6-4. [Google Scholar]
- Zafar, S.; Yaddanapudi, S.S. Parkinson Disease. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Tafti, D.; Ehsan, M.; Xixis, K.L. Multiple Sclerosis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Ghasemi, N.; Razavi, S.; Nikzad, E. Multiple Sclerosis: Pathogenesis, Symptoms, Diagnoses and Cell-Based Therapy. Cell J. 2017, 19, 1–10. [Google Scholar]
- Jana, A.; Modi, K.K.; Roy, A.; Anderson, J.A.; van Breemen, R.B.; Pahan, K. Up-Regulation of Neurotrophic Factors by Cinnamon and Its Metabolite Sodium Benzoate: Therapeutic Implications for Neurodegenerative Disorders. J. Neuroimmune Pharmacol. 2013, 8, 739–755. [Google Scholar] [CrossRef] [Green Version]
- Sharma, M.; Tiwari, M.; Tiwari, R.K. Hyperhomocysteinemia: Impact on Neurodegenerative Diseases. Basic Clin. Pharmacol. Toxicol. 2015, 117, 287–296. [Google Scholar] [CrossRef]
- Herrmann, W.; Obeid, R. Homocysteine: A Biomarker in Neurodegenerative Diseases. Clin. Chem. Lab. Med. 2011, 49, 435–441. [Google Scholar] [CrossRef]
- Modi, K.K.; Rangasamy, S.B.; Dasarathi, S.; Roy, A.; Pahan, K. Cinnamon Converts Poor Learning Mice to Good Learners: Implications for Memory Improvement. J. Neuroimmune Pharmacol. 2016, 11, 693–707. [Google Scholar] [CrossRef] [Green Version]
- Kaur, N.; Lu, B.; Ward, S.m.; Halvorsen, S.w. Inducers of Oxidative Stress Block Ciliary Neurotrophic Factor Activation of Jak/STAT Signaling in Neurons. J. Neurochem. 2005, 92, 1521–1530. [Google Scholar] [CrossRef] [PubMed]
- Bloch, J.; Bachoud-Lévi, A.c.; Déglon, N.; Lefaucheur, J.p.; Winkel, L.; Palfi, S.; Nguyen, J.p.; Bourdet, C.; Remy, P.; Brugières, P.; et al. Neuroprotective Gene Therapy for Huntington’s Disease, Using Polymer-Encapsulated Cells Engineered to Secrete Human Ciliary Neurotrophic Factor: Results of a Phase I Study. Hum. Gene Ther. 2004, 15, 968–975. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Singh, T.G. Role of Nuclear Factor Kappa B (NF-ΚB) Signalling in Neurodegenerative Diseases: An Mechanistic Approach. Curr. Neuropharmacol. 2020, 18, 918–935. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Mizuta, H.; Rubinsztein, D.C. P21-Activated Kinase 1 Promotes Soluble Mutant Huntingtin Self-Interaction and Enhances Toxicity. Hum. Mol. Genet. 2008, 17, 895–905. [Google Scholar] [CrossRef]
- Vazquez-Villaseñor, I.; Garwood, C.J.; Heath, P.R.; Simpson, J.E.; Ince, P.G.; Wharton, S.B. Expression of P16 and P21 in the Frontal Association Cortex of ALS/MND Brains Suggests Neuronal Cell Cycle Dysregulation and Astrocyte Senescence in Early Stages of the Disease. Neuropathol. Appl. Neurobiol. 2020, 46, 171–185. [Google Scholar] [CrossRef]
- Blasko, I.; Knaus, G.; Weiss, E.; Kemmler, G.; Winkler, C.; Falkensammer, G.; Griesmacher, A.; Würzner, R.; Marksteiner, J.; Fuchs, D. Cognitive Deterioration in Alzheimer’s Disease Is Accompanied by Increase of Plasma Neopterin. J. Psychiatr. Res. 2007, 41, 694–701. [Google Scholar] [CrossRef]
- Widner, B.; Leblhuber, F.; Fuchs, D. Increased Neopterin Production and Tryptophan Degradation in Advanced Parkinson’s Disease. J. Neural Transm. 2002, 109, 181–189. [Google Scholar] [CrossRef]
- Stoy, N.; Mackay, G.M.; Forrest, C.M.; Christofides, J.; Egerton, M.; Stone, T.W.; Darlington, L.G. Tryptophan Metabolism and Oxidative Stress in Patients with Huntington’s Disease. J. Neurochem. 2005, 93, 611–623. [Google Scholar] [CrossRef]
- Lane, H.-Y.; Tu, C.-H.; Lin, W.-C.; Lin, C.-H. Brain Activity of Benzoate, a D-Amino Acid Oxidase Inhibitor, in Patients With Mild Cognitive Impairment in a Randomized, Double-Blind, Placebo Controlled Clinical Trial. Int. J. Neuropsychopharmacol. 2021, 24, 392–399. [Google Scholar] [CrossRef]
- Lin, C.-H.; Chen, P.-K.; Chang, Y.-C.; Chuo, L.-J.; Chen, Y.-S.; Tsai, G.E.; Lane, H.-Y. Benzoate, a D-Amino Acid Oxidase Inhibitor, for the Treatment of Early-Phase Alzheimer Disease: A Randomized, Double-Blind, Placebo-Controlled Trial. Biol. Psychiatry 2014, 75, 678–685. [Google Scholar] [CrossRef]
- Weaver, D.; Gupta, M.; Meek, A.; Wang, Y.; Wu, F. Alzheimer’s Disease as a Disorder of Tryptophan Metabolism (2745). Neurology 2020, 94, 2745. [Google Scholar]
- Lin, C.-H.; Yang, H.-T.; Chen, P.-K.; Wang, S.-H.; Lane, H.-Y. Precision Medicine of Sodium Benzoate for the Treatment of Behavioral and Psychological Symptoms of Dementia (BPSD). Neuropsychiatr. Dis. Treat. 2020, 16, 509–518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, C.-H.; Chen, P.-K.; Wang, S.-H.; Lane, H.-Y. Sodium Benzoate for the Treatment of Behavioral and Psychological Symptoms of Dementia (BPSD): A Randomized, Double-Blind, Placebo-Controlled, 6-Week Trial. J. Psychopharmacol. 2019, 33, 1030–1033. [Google Scholar] [CrossRef] [PubMed]
- Repici, M.; Giorgini, F. DJ-1 in Parkinson’s Disease: Clinical Insights and Therapeutic Perspectives. J. Clin. Med. 2019, 8, 1377. [Google Scholar] [CrossRef] [Green Version]
- Khasnavis, S.; Pahan, K. Sodium Benzoate, a Metabolite of Cinnamon and a Food Additive, Upregulates Neuroprotective Parkinson Disease Protein DJ-1 in Astrocytes and Neurons. J. Neuroimmune Pharmacol. 2012, 7, 424–435. [Google Scholar] [CrossRef] [Green Version]
- Patel, D.; Jana, A.; Roy, A.; Pahan, K. Cinnamon and Its Metabolite Protect the Nigrostriatum in a Mouse Model of Parkinson’s Disease via Astrocytic GDNF. J. Neuroimmune Pharmacol. 2019, 14, 503–518. [Google Scholar] [CrossRef]
- Chandra, G.; Roy, A.; Rangasamy, S.B.; Pahan, K. Induction of Adaptive Immunity Leads to Nigrostriatal Disease Progression in MPTP Mouse Model of Parkinson’s Disease. J. Immunol. 2017, 198, 4312–4326. [Google Scholar] [CrossRef]
- Rangasamy, S.B.; Dasarathi, S.; Nutakki, A.; Mukherjee, S.; Nellivalasa, R.; Pahan, K. Stimulation of Dopamine Production by Sodium Benzoate, a Metabolite of Cinnamon and a Food Additive. J. Alzheimer’s Dis. Rep. 2021, 5, 295–310. [Google Scholar] [CrossRef]
- Rzepiński, Ł.; Maciejek, Z. Heterogenność etiopatogenezy stwardnienia rozsianego w kontekście danych klinicznych, immunohistochemicznych, autopsyjnych oraz aktualnych możliwości terapeutycznych. Pol. Przegląd Neurol. 2018, 14, 1–9. [Google Scholar]
- Rezaei, N.; Amirghofran, Z.; Nikseresht, A.; Ashjazade, N.; Zoghi, S.; Tahvili, S.; Kamali-Sarvestani, E. In Vitro Effects of Sodium Benzoate on Th1/Th2 Deviation in Patients with Multiple Sclerosis. Immunol. Investig. 2016, 45, 679–691. [Google Scholar] [CrossRef]
- Brahmachari, S.; Pahan, K. Sodium Benzoate, a Food Additive and a Metabolite of Cinnamon, Modifies T Cells at Multiple Steps and Inhibits Adoptive Transfer of Experimental Allergic Encephalomyelitis. J. Immunol. 2007, 179, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S.; Pahan, K. Cinnamon Ameliorates Experimental Allergic Encephalomyelitis in Mice via Regulatory T Cells: Implications for Multiple Sclerosis Therapy. PLoS ONE 2015, 10, e0116566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Constantinescu, C.S.; Farooqi, N.; O’Brien, K.; Gran, B. Experimental Autoimmune Encephalomyelitis (EAE) as a Model for Multiple Sclerosis (MS). Br. J. Pharmacol. 2011, 164, 1079–1106. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.; Wang, H.; Yang, X.; Wu, Y.; Liao, C.; Xie, B.; Li, Y.; Zhang, W. Controlled Release of Ciliary Neurotrophic Factor from Bioactive Nerve Grafts Promotes Nerve Regeneration in Rats with Facial Nerve Injuries. J. Biomed. Mater. Res. Part A 2022, 110, 788–796. [Google Scholar] [CrossRef] [PubMed]
- Kimura, K. Regulatory T Cells in Multiple Sclerosis. Clin. Exp. Neuroimmunol. 2020, 11, 148–155. [Google Scholar] [CrossRef]
- Kundu, M.; Mondal, S.; Roy, A.; Martinson, J.L.; Pahan, K. Sodium Benzoate, a Food Additive and a Metabolite of Cinnamon, Enriches Regulatory T Cells via STAT6-Mediated Upregulation of TGF-β. J. Immunol. 2016, 197, 3099–3110. [Google Scholar] [CrossRef] [Green Version]
- Raffaeli, W.; Arnaudo, E. Pain as a Disease: An Overview. J. Pain Res. 2017, 10, 2003–2008. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.-L.; Li, X.-Y.; Qian, S.-B.; Wang, Y.-C.; Zhang, P.-Z.; Zhou, X.-J.; Wang, Y.-X. Down-Regulation of Spinal d-Amino Acid Oxidase Expression Blocks Formalin-Induced Tonic Pain. Biochem. Biophys. Res. Commun. 2012, 421, 501–507. [Google Scholar] [CrossRef]
- Zhao, W.; Konno, R.; Zhou, X.-J.; Yin, M.; Wang, Y.-X. Inhibition of D-Amino-Acid Oxidase Activity Induces Pain Relief in Mice. Cell. Mol. Neurobiol. 2008, 28, 581–591. [Google Scholar] [CrossRef]
- Zhao, W.-J.; Gao, Z.-Y.; Wei, H.; Nie, H.-Z.; Zhao, Q.; Zhou, X.-J.; Wang, Y.-X. Spinal D-Amino Acid Oxidase Contributes to Neuropathic Pain in Rats. J. Pharmacol. Exp. Ther. 2010, 332, 248–254. [Google Scholar] [CrossRef] [Green Version]
- Gong, N.; Gao, Z.-Y.; Wang, Y.-C.; Li, X.-Y.; Huang, J.-L.; Hashimoto, K.; Wang, Y.-X. A Series of D-Amino Acid Oxidase Inhibitors Specifically Prevents and Reverses Formalin-Induced Tonic Pain in Rats. J. Pharmacol. Exp. Ther. 2011, 336, 282–293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, H.; Gong, N.; Huang, J.-L.; Fan, H.; Ma, A.-N.; Li, X.-Y.; Wang, Y.-X.; Pertovaara, A. Spinal D-Amino Acid Oxidase Contributes to Mechanical Pain Hypersensitivity Induced by Sleep Deprivation in the Rat. Pharmacol. Biochem. Behav. 2013, 111, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Blouin, M.; Han, Y.; Burch, J.; Farand, J.; Mellon, C.; Gaudreault, M.; Wrona, M.; Lévesque, J.-F.; Denis, D.; Mathieu, M.-C.; et al. The Discovery of 4-{1-[({2,5-Dimethyl-4-[4-(Trifluoromethyl)Benzyl]-3-Thienyl}carbonyl)Amino]Cyclopropyl}benzoic Acid (MK-2894), A Potent and Selective Prostaglandin E2 Subtype 4 Receptor Antagonist. J. Med. Chem. 2010, 53, 2227–2238. [Google Scholar] [CrossRef] [PubMed]
- Kramar, H.; Stepaniuk, H.; Voloshchuk, N.; Taran, I.; Kovalenko, S. Experimental study of pain-relieving mechanisms of 4-[4-oxo-(4h)-quinazolin-3-yl]-benzoic acid (PK-66 COMPOUND). Georgian Med. News 2018, 283, 148–154. [Google Scholar]
- Sheng, J.; Liu, S.; Wang, Y.; Cui, R.; Zhang, X. The Link between Depression and Chronic Pain: Neural Mechanisms in the Brain. Neural Plast. 2017, 2017, 9724371. [Google Scholar] [CrossRef]
- Holmes, A.; Christelis, N.; Arnold, C. Depression and Chronic Pain. Med. J. Aust. 2013, 199, S17–S20. [Google Scholar] [CrossRef]
- Brown, D.; Rosenthal, N.; Könning, A.; Wager, J. Intergenerational transmission of chronic pain-related disability: The explanatory effects of depressive symptoms. Pain 2021, 162, 653. [Google Scholar] [CrossRef]
- Lord, C.; Elsabbagh, M.; Baird, G.; Veenstra-Vanderweele, J. Autism Spectrum Disorder. Lancet 2018, 392, 508–520. [Google Scholar] [CrossRef]
- Lord, C.; Brugha, T.S.; Charman, T.; Cusack, J.; Dumas, G.; Frazier, T.; Jones, E.J.H.; Jones, R.M.; Pickles, A.; State, M.W.; et al. Autism Spectrum Disorder. Nat. Rev. Dis. Primers 2020, 6, 5. [Google Scholar] [CrossRef]
- Hughes, H.K.; Mills Ko, E.; Rose, D.; Ashwood, P. Immune Dysfunction and Autoimmunity as Pathological Mechanisms in Autism Spectrum Disorders. Front. Cell. Neurosci. 2018, 12, 405. [Google Scholar] [CrossRef] [Green Version]
- Tzang, R.-F.; Chang, C.-H.; Chang, Y.-C.; Lane, H.-Y. Autism Associated With Anti-NMDAR Encephalitis: Glutamate-Related Therapy. Front. Psychiatry 2019, 10, 440. [Google Scholar] [CrossRef] [PubMed]
- Moaaz, M.; Youssry, S.; Elfatatry, A.; El Rahman, M.A. Th17/Treg Cells Imbalance and Their Related Cytokines (IL-17, IL-10 and TGF-β) in Children with Autism Spectrum Disorder. J. Neuroimmunol. 2019, 337, 577071. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chauhn, A.; Shiekh, A.M.; Patil, S.; Chauhn, V.; Li, X.-M.; Ji, L.; Brown, T.; Malik, M. Elevated Immune Response in the Brain of Autistic Patients. J. Neuroimmunol. 2009, 207, 111–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, H.-F.; Wang, W.-Q.; Li, X.-M.; Rauw, G.; Baker, G.B. Body Fluid Levels of Neuroactive Amino Acids in Autism Spectrum Disorders: A Review of the Literature. Amino Acids 2017, 49, 57–65. [Google Scholar] [CrossRef] [Green Version]
- Yang, P. A Pilot Trial of Sodium Benzoate, a D-Amino Acid Oxidase Inhibitor, Added on Augmentative and Alternative Communication Intervention for Non-Communicative Children with Autism Spectrum Disorders. Transl. Med. 2017, 7, 1000192. [Google Scholar] [CrossRef]
- Görker, I.; Tüzün, Ü. Autistic-like Findings Associated with a Urea Cycle Disorder in a 4-Year-Old Girl. J. Psychiatry Neurosci. 2005, 30, 133–135. [Google Scholar]
- Kernohan, K.D.; McBride, A.; Hartley, T.; Rojas, S.K.; Care4Rare Canada Consortium; Dyment, D.A.; Boycott, K.M.; Dyack, S. P21 Protein-Activated Kinase 1 Is Associated with Severe Regressive Autism, and Epilepsy. Clin. Genet. 2019, 96, 449–455. [Google Scholar] [CrossRef]
- Fuentes-Albero, M.; Cauli, O. Homocysteine Levels in Autism Spectrum Disorder: A Clinical Update. Endocr Metab Immune Disord. Drug Targets 2018, 18, 289–296. [Google Scholar] [CrossRef]
- Guo, B.-Q.; Li, H.-B.; Ding, S.-B. Blood Homocysteine Levels in Children with Autism Spectrum Disorder: An Updated Systematic Review and Meta-Analysis. Psychiatry Res. 2020, 291, 113283. [Google Scholar] [CrossRef]
- Sweeten, T.L.; Posey, D.J.; McDougle, C.J. High Blood Monocyte Counts and Neopterin Levels in Children With Autistic Disorder. Am. J. Psychiatry 2003, 160, 1691–1693. [Google Scholar] [CrossRef]
- Zhao, H.; Yin, S.; Fan, J. High Plasma Neopterin Levels in Chinese Children with Autism Spectrum Disorders. Int. J. Dev. Neurosci. 2015, 41, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Boccuto, L.; Chen, C.-F.; Pittman, A.R.; Skinner, C.D.; McCartney, H.J.; Jones, K.; Bochner, B.R.; Stevenson, R.E.; Schwartz, C.E. Decreased Tryptophan Metabolism in Patients with Autism Spectrum Disorders. Mol. Autism 2013, 4, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kałużna-Czaplińska, J.; Jóźwik-Pruska, J.; Chirumbolo, S.; Bjørklund, G. Tryptophan Status in Autism Spectrum Disorder and the Influence of Supplementation on Its Level. Metab. Brain Dis. 2017, 32, 1585–1593. [Google Scholar] [CrossRef] [PubMed] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Walczak-Nowicka, Ł.J.; Herbet, M. Sodium Benzoate—Harmfulness and Potential Use in Therapies for Disorders Related to the Nervous System: A Review. Nutrients 2022, 14, 1497. https://doi.org/10.3390/nu14071497
Walczak-Nowicka ŁJ, Herbet M. Sodium Benzoate—Harmfulness and Potential Use in Therapies for Disorders Related to the Nervous System: A Review. Nutrients. 2022; 14(7):1497. https://doi.org/10.3390/nu14071497
Chicago/Turabian StyleWalczak-Nowicka, Łucja Justyna, and Mariola Herbet. 2022. "Sodium Benzoate—Harmfulness and Potential Use in Therapies for Disorders Related to the Nervous System: A Review" Nutrients 14, no. 7: 1497. https://doi.org/10.3390/nu14071497
APA StyleWalczak-Nowicka, Ł. J., & Herbet, M. (2022). Sodium Benzoate—Harmfulness and Potential Use in Therapies for Disorders Related to the Nervous System: A Review. Nutrients, 14(7), 1497. https://doi.org/10.3390/nu14071497





