Gastrointestinal Protein Hydrolysis Kinetics: Opportunities for Further Infant Formula Improvement
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Description
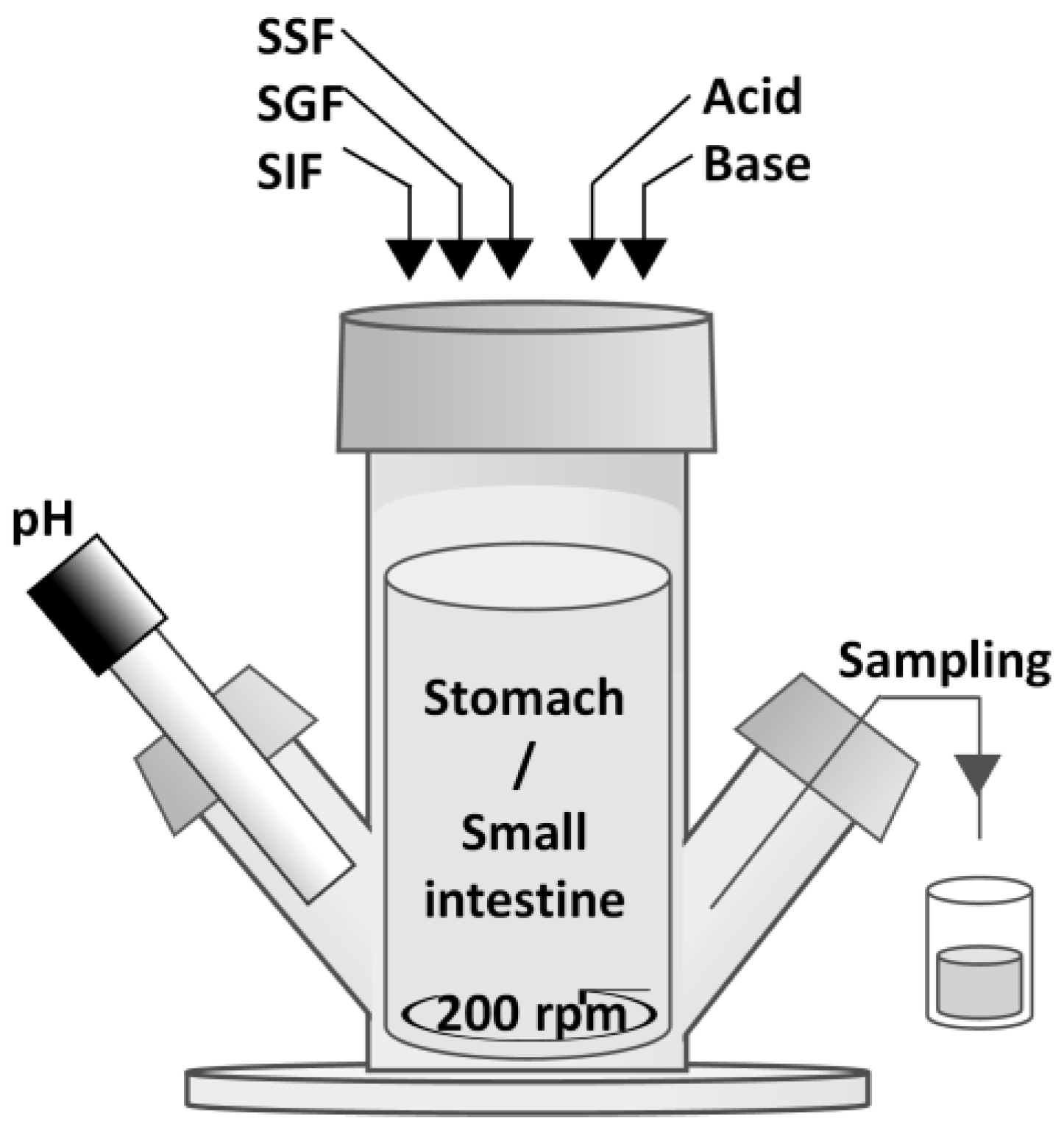
2.3. Semi Dynamic In Vitro Simulation of Infant Gastrointestinal Tract (SIM)
2.4. O-Phthalaldehyde Method (OPA)
2.5. Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS-PAGE)
2.6. Ultra High-Performance Liquid Chromatography-Fluorescence (UPLC-FLR)
2.7. High-Performance Size Exclusion Chromatography (HP-SEC)
2.8. Data Analysis
3. Results
3.1. Characterization of Human Milk and Infant Formula
3.2. Gastrointestinal Protein Hydrolysis in SIM
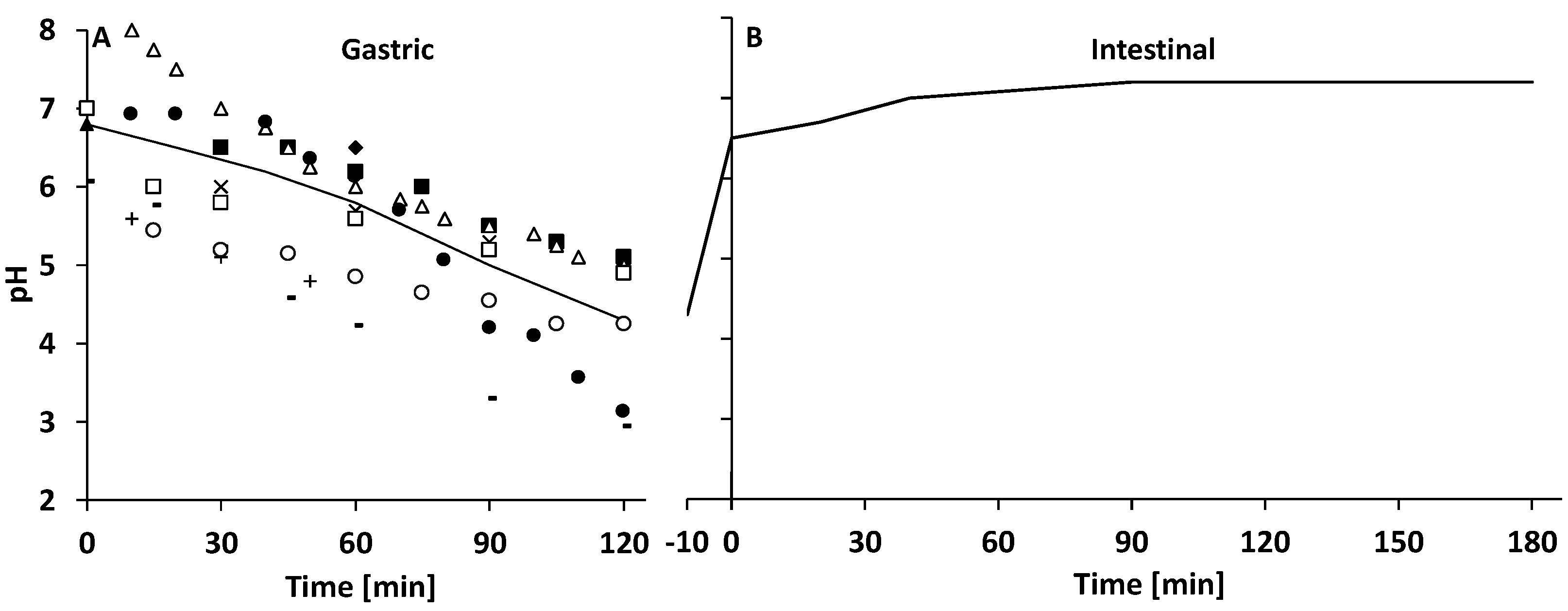
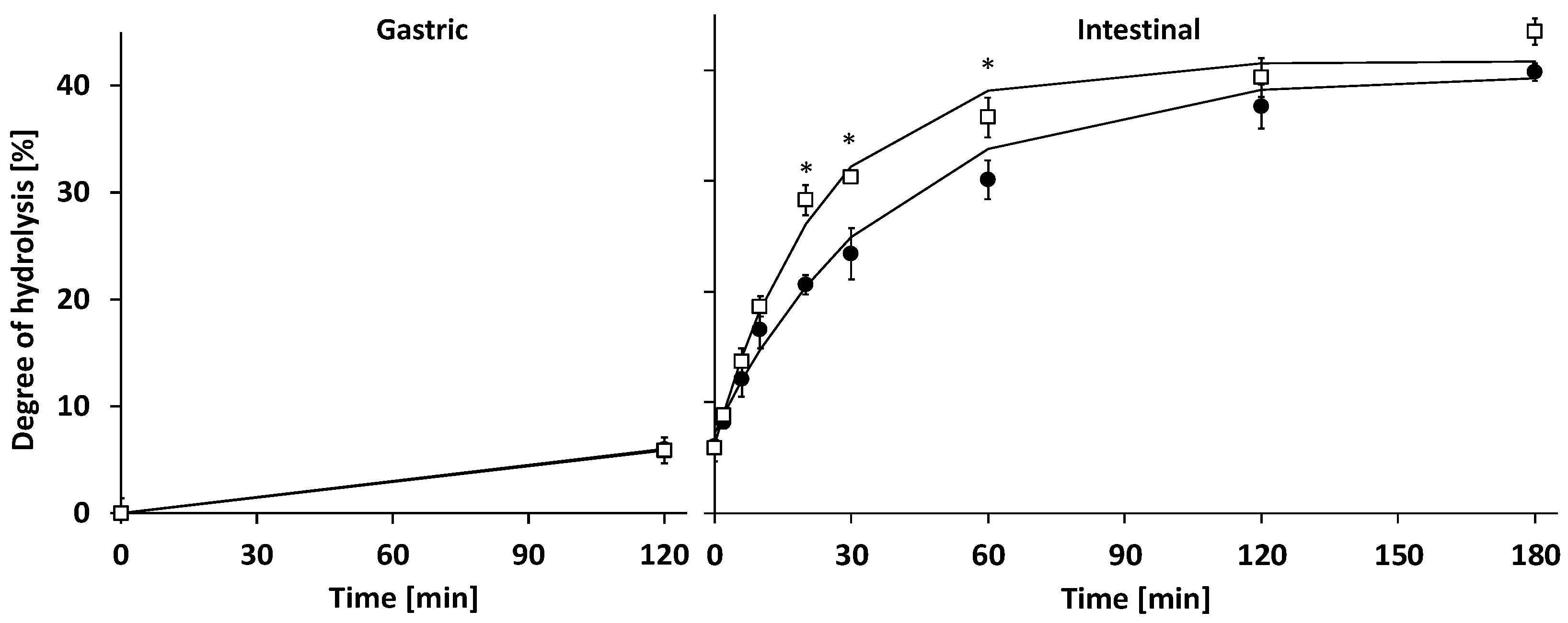
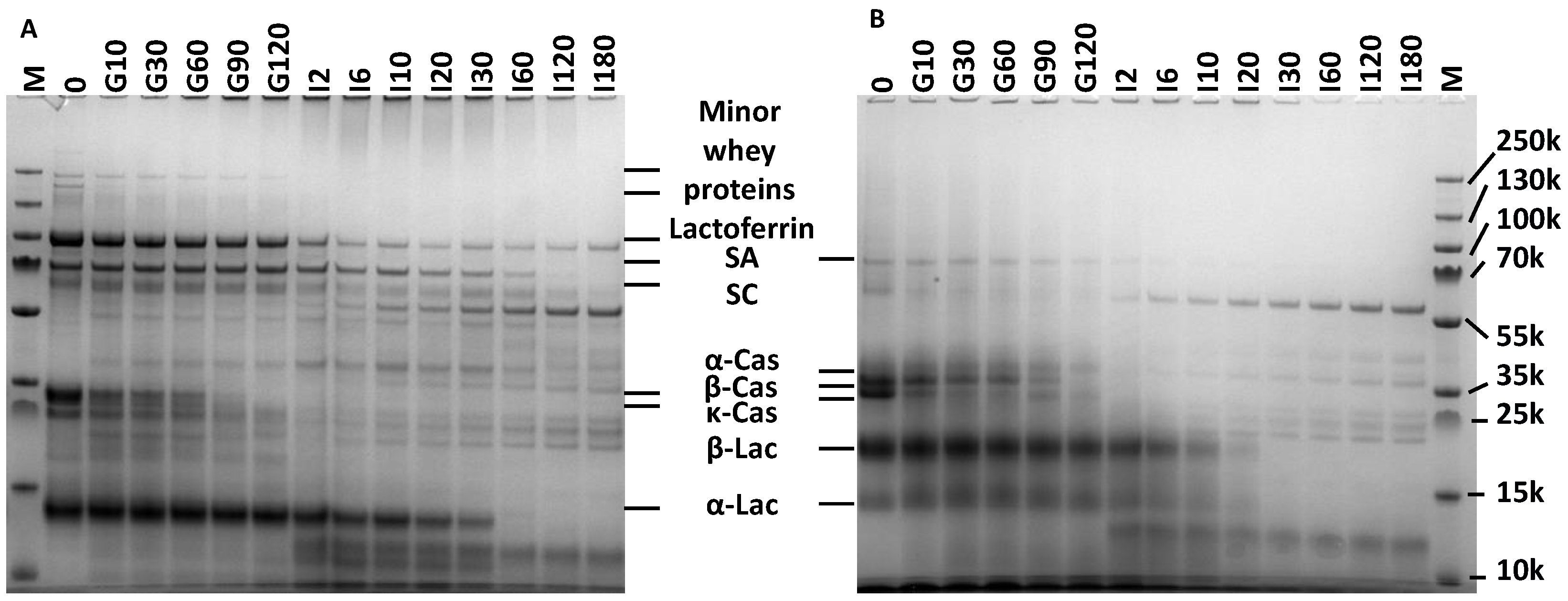
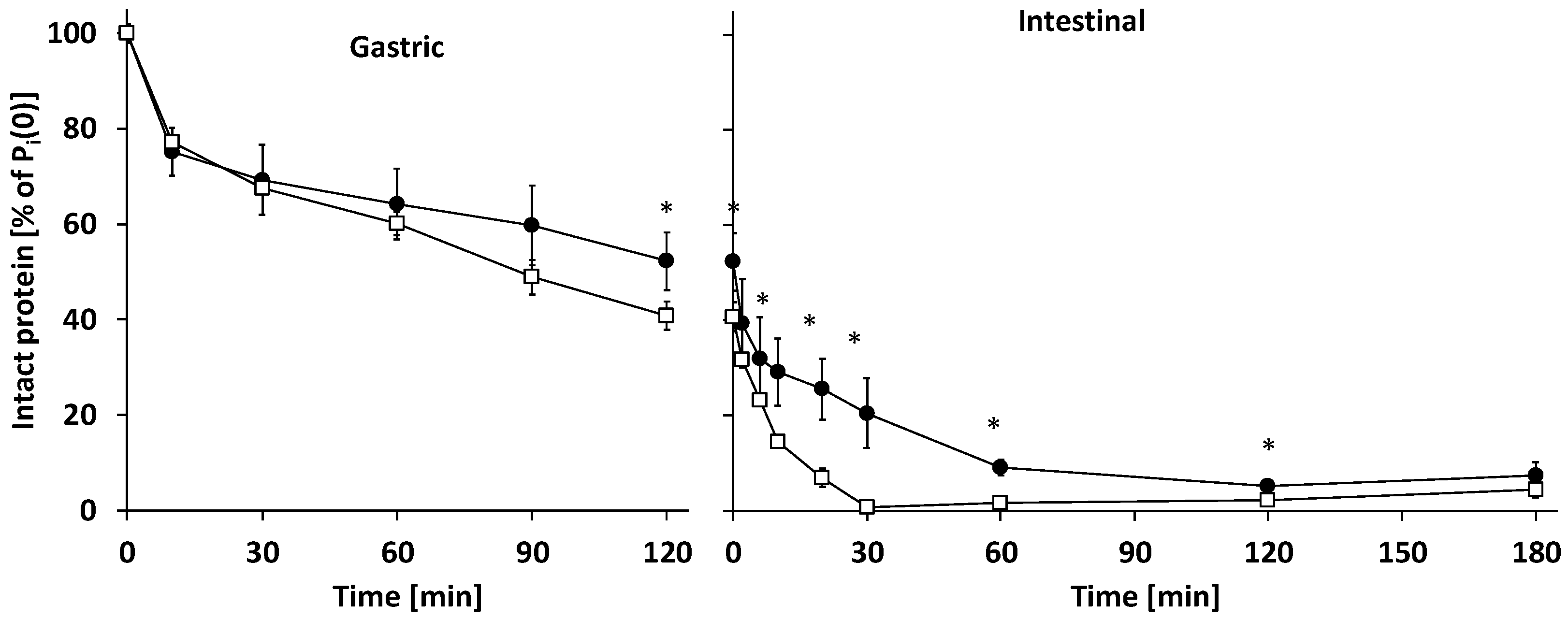
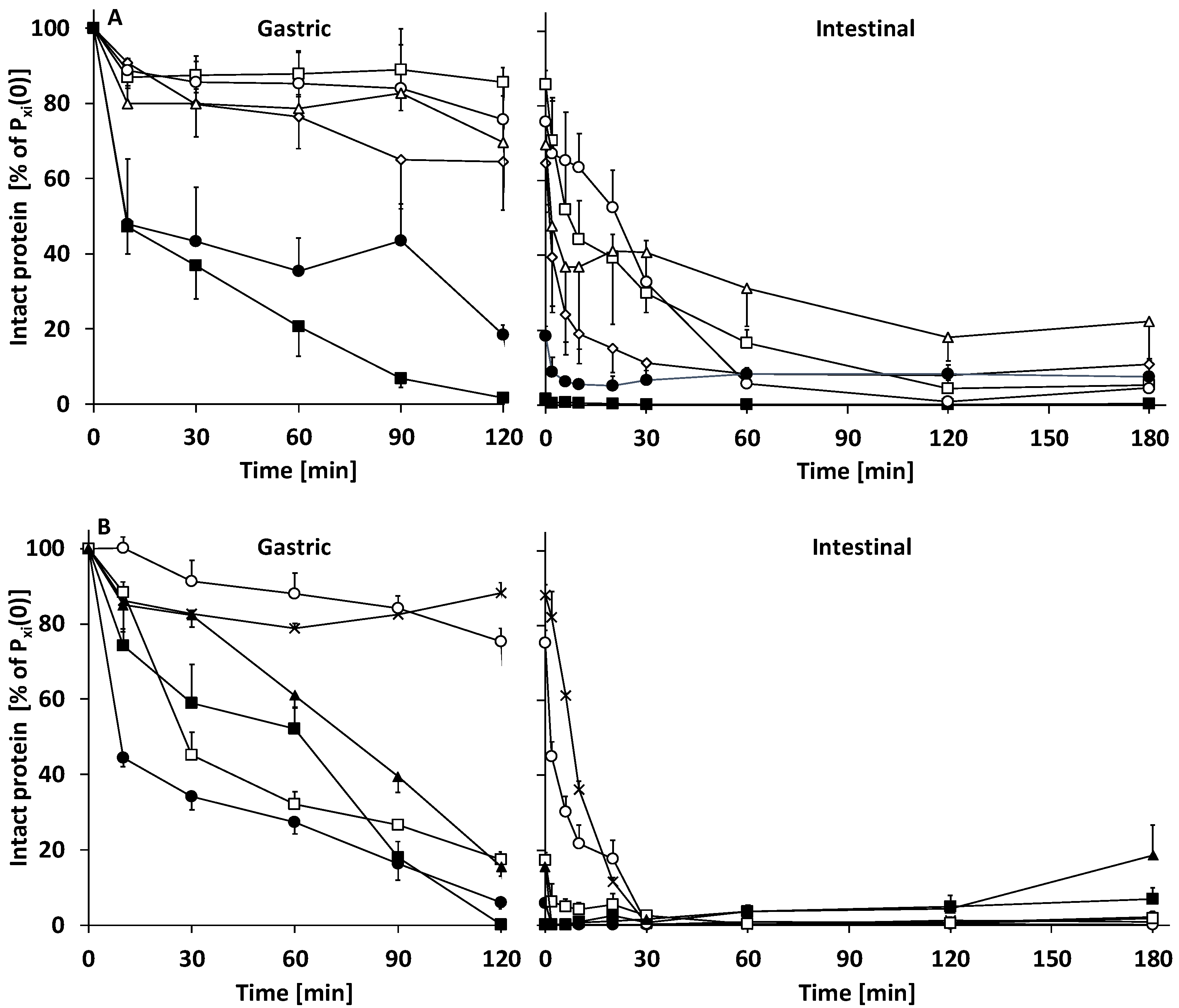
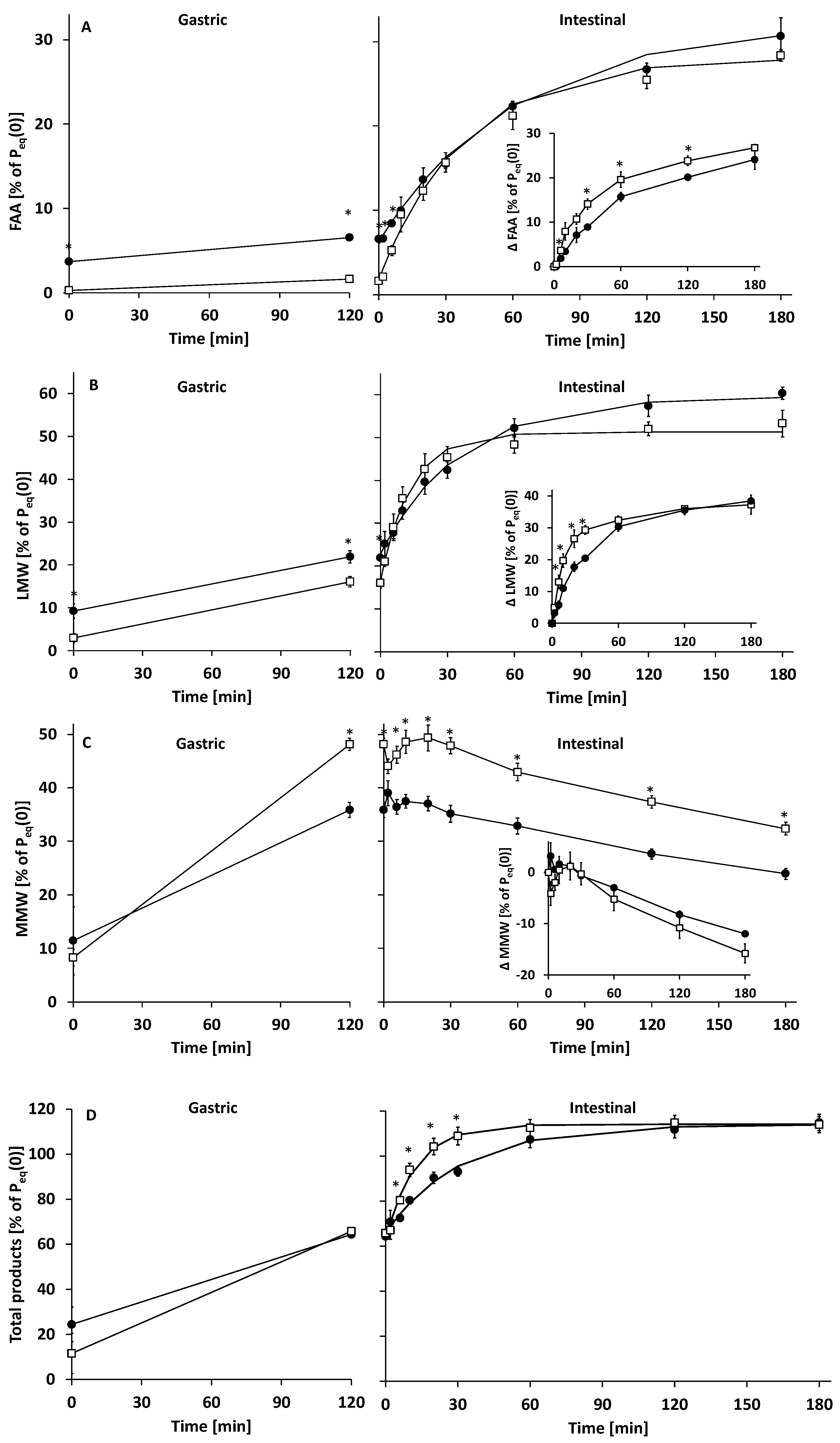
3.2.1. Gastric Digestion
3.2.2. Intestinal Digestion
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Global Strategy for Infant and Young Child Feeding; World Health Organization: Geneva, Switzerland, 2003.
- Inglingstad, R.A.; Devold, T.G.; Eriksen, E.K.; Holm, H.; Jacobsen, M.; Liland, K.H.; Rukke, E.O.; Vegarud, G.E. Comparison of the digestion of caseins and whey proteins in equine, bovine, caprine and human milks by human gastrointestinal enzymes. Dairy Sci. Technol. 2010, 90, 549–563. [Google Scholar] [CrossRef] [Green Version]
- Wada, Y.; Lönnerdal, B. Effects of Different Industrial Heating Processes of Milk on Site-Specific Protein Modifications and Their Relationship to In Vitro and In vivo Digestibility. J. Agric. Food Chem. 2014, 62, 4175–4185. [Google Scholar] [CrossRef] [PubMed]
- Mackie, A.; Macierzanka, A. Colloidal aspects of protein digestion. Curr. Opin. Colloid Interface Sci. 2010, 15, 102–108. [Google Scholar] [CrossRef]
- Boirie, Y.; Dangin, M.; Gachon, P.; Vasson, M.-P.; Maubois, J.-L.; Beaufrère, B. Slow and fast dietary proteins differently modulate postprandial protein accretion. Proc. Natl. Acad. Sci. USA 1997, 94, 14930–14935. [Google Scholar] [CrossRef] [Green Version]
- Erickson, R.H.; Kim, Y.S. Digestion and Absorption of Dietary Protein. Annu. Rev. Med. 1990, 41, 133–139. [Google Scholar] [CrossRef]
- Moro, G.; Minoli, I.; Boehm, G.; Georgi, G.; Jelinek, J.; Sawatzki, G. Postprandial plasma amino acids in preterm infants: Influence of the protein source. Acta Paediatr. 1999, 88, 885–889. [Google Scholar] [CrossRef]
- Bourlieu, C.; Ménard, O.; Bouzerzour, K.; Mandalari, G.; Macierzanka, A.; Mackie, A.R.; Dupont, D. Specificity of Infant Digestive Conditions: Some Clues for Developing Relevant In Vitro Models. Crit. Rev. Food Sci. Nutr. 2014, 54, 1427–1457. [Google Scholar] [CrossRef]
- Lönnerdal, B. Nutritional and physiologic significance of human milk proteins. Am. J. Clin. Nutr. 2003, 77, 1537S–1543S. [Google Scholar] [CrossRef]
- Lindberg, T.; Engberg, S.; Sjöberg, L.B.; Lönnerdal, B. In Vitro Digestion of Proteins in Human Milk Fortifiers and in Preterm Formula. J. Pediatr. Gastroenterol. Nutr. 1998, 27, 30–36. [Google Scholar] [CrossRef]
- Chatterton, D.E.W.; Rasmussen, J.T.; Heegaard, C.W.; Sørensen, E.S.; Petersen, T.E. In Vitro Digestion of Novel Milk Protein Ingredients for Use in Infant Formulas: Research on Biological Functions. Trends Food Sci. Technol. 2004, 15, 373–383. [Google Scholar] [CrossRef]
- Davidson, L.A.; Lönnerdal, B. Persistence of Human Milk Proteins in the Breast-Fed Infant. Acta Paediatr. 1987, 76, 733–740. [Google Scholar] [CrossRef] [PubMed]
- Chowanadisai, W.; Lönnerdal, B. Alpha(1)-antitrypsin and antichymotrypsin in human milk: Origin, concentrations, and stability. Am. J. Clin. Nutr. 2002, 76, 828–833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Lieshout, G.A.; Lambers, T.T.; Bragt, M.C.; Hettinga, K.A. How processing may affect milk protein digestion and overall physiological outcomes: A systematic review. Crit. Rev. Food Sci. Nutr. 2020, 60, 2422–2445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernfeld, P. Amylases, α and β, in Methods in Enzymology; Academic Press: New York, NY, USA, 1955; pp. 149–158. [Google Scholar]
- Medicine, I.O. Food Chemicals Codex, 5th ed.; The National Academies Press: Washington, DC, USA, 2003; p. 998. [Google Scholar]
- Anson, M.L. The estimation of pepsin, trypsin, papain, and cathepsin with hemoglobin. J. Gen. Physiol. 1938, 22, 79–89. [Google Scholar] [CrossRef]
- Luiking, Y.C.; Abrahamse, E.; Ludwig, T.; Boirie, Y.; Verlaan, S. Protein type and caloric density of protein supplements modulate postprandial amino acid profile through changes in gastrointestinal behaviour: A randomized trial. Clin. Nutr. 2016, 35, 48–58. [Google Scholar] [CrossRef] [Green Version]
- Braak, C.C.V.D.; Klebach, M.; Abrahamse, E.; Minor, M.; Hofman, Z.; Knol, J.; Ludwig, T. A novel protein mixture containing vegetable proteins renders enteral nutrition products non-coagulating after In Vitro gastric digestion. Clin. Nutr. 2013, 32, 765–771. [Google Scholar] [CrossRef]
- Ménard, O.; Bourlieu, C.; De Oliveira, S.; Dellarosa, N.; Laghi, L.; Carrière, F.; Capozzi, F.; Dupont, D.; Deglaire, A. A first step towards a consensus static In Vitro model for simulating full-term infant digestion. Food Chem. 2018, 240, 338–345. [Google Scholar] [CrossRef]
- Mason, S. Some Aspects of Gastric Function in the Newborn. Arch. Dis. Child. 1962, 37, 387–391. [Google Scholar] [CrossRef] [Green Version]
- Rondini, G.; Pittaluga, F.; Sina, P.; Prigione, M.G. [Lipid digestion and absorption in 1-month-old infants fed with an acidified and non-acidified milk]. Minerva Pediatr. 1972, 24, 755–761. [Google Scholar]
- Pascale, J.; Mims, L.; Greenberg, M.; Alexander, J. Gastric Response in Low Birth Weight Infants Fed Various Formulas. Neonatol. 1978, 34, 150–154. [Google Scholar] [CrossRef]
- Cavell, B. Postprandial gastric acid secretion in infants. Acta Paediatr. 1983, 72, 857–860. [Google Scholar] [CrossRef] [PubMed]
- Sondheimer, J.M.; Clark, D.A.; Gervaise, E.P. Continuous Gastric pH Measurement in Young and Older Healthy Preterm Infants Receiving Formula and Clear Liquid Feedings. J. Pediatr. Gastroenterol. Nutr. 1985, 4, 352–355. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.J.; Kaminsky, S.; D’Souza, S.W. Neonatal Fat Digestion and Lingual Lipase. Acta Paediatr. 1986, 75, 913–918. [Google Scholar] [CrossRef]
- Armand, M.; Hamosh, M.; Mehta, N.R.; Angelus, P.A.; Philpott, J.R.; Henderson, T.R.; Dwyer, N.K.; Lairon, D.; Hamosh, P. Effect of Human Milk or Formula on Gastric Function and Fat Digestion in the Premature Infant1. Pediatr. Res. 1996, 40, 429–437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitchell, D.J.; McClure, B.G.; Tubman, T.R.J. Simultaneous monitoring of gastric and oesophageal pH reveals limitations of conventional oesophageal pH monitoring in milk fed infants. Arch. Dis. Child. 2001, 84, 273–276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Omari, T.I.; Davidson, G.P. Multipoint measurement of intragastric pH in healthy preterm infants. Arch. Dis. Child.-Fetal Neonatal Ed. 2003, 88, F517. [Google Scholar] [CrossRef] [Green Version]
- Roman, C.; Carriere, F.; Villeneuve, P.; Pina, M.; Millet, V.; Simeoni, U.; Sarles, J. Quantitative and Qualitative Study of Gastric Lipolysis in Premature Infants: Do MCT-Enriched Infant Formulas Improve Fat Digestion? Pediatr. Res. 2007, 61, 83–88. [Google Scholar] [CrossRef] [Green Version]
- Blanquet, S.; Zeijdner, E.; Beyssac, E.; Meunier, J.-P.; Denis, S.; Havenaar, R.; Alric, M. A Dynamic Artificial Gastrointestinal System for Studying the Behavior of Orally Administered Drug Dosage Forms Under Various Physiological Conditions. Pharm. Res. 2004, 21, 585–591. [Google Scholar] [CrossRef]
- Wierenga, P.A.; Meinders, M.B.J.; Egmond, M.R.; Voragen, F.A.G.J.; de Jongh, H.H.J. Protein Exposed Hydrophobicity Reduces the Kinetic Barrier for Adsorption of Ovalbumin to the Air−Water Interface. Langmuir 2003, 19, 8964–8970. [Google Scholar] [CrossRef]
- Kuipers, B.J.H.; Gruppen, H. Prediction of Molar Extinction Coefficients of Proteins and Peptides Using UV Absorption of the Constituent Amino Acids at 214 nm To Enable Quantitative Reverse Phase High-Performance Liquid Chromatography−Mass Spectrometry Analysis. J. Agric. Food Chem. 2007, 55, 5445–5451. [Google Scholar] [CrossRef]
- Bodin, A.; Framboisier, X.; Alonso, D.; Marc, I.; Kapel, R. Size-exclusion HPLC as a sensitive and calibrationless method for complex peptide mixtures quantification. J. Chromatogr. B 2015, 1006, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Maathuis, A.; Havenaar, R.; He, T.; Bellmann, S. Protein Digestion and Quality of Goat and Cow Milk Infant Formula and Human Milk Under Simulated Infant Conditions. J. Pediatr. Gastroenterol. Nutr. 2017, 65, 661–666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dallas, D.C.; Underwood, M.A.; Zivkovic, A.M.; German, J.B. Digestion of Protein in Premature and Term Infants. J. Nutr. Disord. Ther. 2012, 2, 112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elwakiel, M.; Boeren, S.; Wang, W.; Schols, H.A.; Hettinga, K.A. Degradation of Proteins From Colostrum and Mature Milk From Chinese Mothers Using an In Vitro Infant Digestion Model. Front. Nutr. 2020, 7, 162. [Google Scholar] [CrossRef]
- Van Veen, H.A.; Geerts, M.E.; van Berkel, P.H.; Nuijens, J.H. The role of N-linked glycosylation in the protection of human and bovine lactoferrin against tryptic proteolysis. Eur. J. Biochem. 2004, 271, 678–684. [Google Scholar] [CrossRef]
- Dupont, D.; Boutrou, R.; Ménard, O.; Jardin, J.; Tanguy, G.; Schuck, P.; Haab, B.B.; Leonil, J. Heat Treatment of Milk During Powder Manufacture Increases Casein Resistance to Simulated Infant Digestion. Food Dig. 2010, 1, 28–39. [Google Scholar] [CrossRef]
- Reddy, I.M.; Kella, N.K.D.; Kinsella, J.E. Structural and conformational basis of the resistance of.beta.-lactoglobulin to peptic and chymotryptic digestion. J. Agric. Food Chem. 1988, 36, 737–741. [Google Scholar] [CrossRef]
- Guo, M.; Fox, P.; Flynn, A.; Kindstedt, P. Susceptibility of β-Lactoglobulin and Sodium Caseinate to Proteolysis by Pepsin and Trypsin. J. Dairy Sci. 1995, 78, 2336–2344. [Google Scholar] [CrossRef]
- Leeb, E.; Götz, A.; Letzel, T.; Cheison, S.C.; Kulozik, U. Influence of denaturation and aggregation of β-lactoglobulin on its tryptic hydrolysis and the release of functional peptides. Food Chem. 2015, 187, 545–554. [Google Scholar] [CrossRef]
- Adler-Nissen, J. Enzymatic hydrolysis of proteins for increased solubility. J. Agric. Food Chem. 1976, 24, 1090–1093. [Google Scholar] [CrossRef]
- McCarthy, N.A.; Kelly, A.; O’Mahony, J.A.; Hickey, D.K.; Chaurin, V.; Fenelon, M.A. Effect of protein content on emulsion stability of a model infant formula. Int. Dairy J. 2012, 25, 80–86. [Google Scholar] [CrossRef]
- Bourlieu, C.; Ménard, O.; De La Chevasnerie, A.; Sams, L.; Rousseau, F.; Madec, M.-N.; Robert, B.; Deglaire, A.; Pezennec, S.; Bouhallab, S.; et al. The structure of infant formulas impacts their lipolysis, proteolysis and disintegration during In Vitro gastric digestion. Food Chem. 2015, 182, 224–235. [Google Scholar] [CrossRef] [PubMed]
- Tunick, M.H.; Ren, D.; Van Hekken, D.L.; Bonnaillie, L.; Paul, M.; Kwoczak, R.; Tomasula, P.M. Effect of heat and homogenization on In Vitro digestion of milk. J. Dairy Sci. 2016, 99, 4124–4139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ozorio, L.; Mellinger-Silva, C.; Cabral, L.M.C.; Jardin, J.; Boudry, G.; Dupont, D. The Influence of Peptidases in Intestinal Brush Border Membranes on the Absorption of Oligopeptides from Whey Protein Hydrolysate: An Ex Vivo Study Using an Ussing Chamber. Foods 2020, 9, 1415. [Google Scholar] [CrossRef] [PubMed]
- Picariello, G.; Miralles, B.; Mamone, G.; Sánchez-Rivera, L.; Recio, I.; Addeo, F.; Ferranti, P. Role of intestinal brush border peptidases in the simulated digestion of milk proteins. Mol. Nutr. Food Res. 2015, 59, 948–956. [Google Scholar] [CrossRef] [Green Version]
| g/L | HM | IF 7 |
|---|---|---|
| N | 1.72 ± 0.01 4 | 1.66 ± 0.03 |
| NPN | 0.28 ± 0.10 4 | 0.16 ± 0.02 |
| True protein 2 | 9.00 ± 0.68 4 | 9.38 ± 0.35 |
| Free AA | 0.35 ± 0.02 4 | 0.02 ± 0.01 |
| Protein equivalent 3 | 9.35 ± 0.69 4 | 9.40 ± 0.47 |
| Fat | 34.00 ± 1.05 5 | 29.60 6 |
| Carbohydrates | 59.50 ± 1.79 5 | 63.60 6 |
| % of Total Protein Band Intensity | HM | IF |
|---|---|---|
| Minor whey proteins | 4.50 ± 0.31 | - |
| Lactoferrin | 22.54 ± 1.29 | - |
| Serum albumin | 10.70 ± 0.31 | 4.91 ± 0.38 |
| Secretory component of Ig | 11.06 ± 2.00 | - |
| α-casein | - | 11.61 ± 0.23 |
| β-casein | 17.81 ± 1.34 | 20.35 ± 0.68 |
| κ-casein | 8.60 ± 1.04 | 19.36 ± 0.49 |
| β-lactoglobulin | - | 31.18 ± 0.31 |
| α-lactalbumin | 24.78 ± 1.15 | 12.59 ± 0.51 |
| HM | IF | |
|---|---|---|
| DH | ||
| kDH (×103) (min−1) | 26.28 ± 6.27 a | 42.92 ± 2.70 b |
| bDH (%) | 7.26 ± 0.28 | 6.20 ± 0.96 |
| mDH (%) | 32.31 ± 2.31 | 34.61 ± 1.35 |
| R2DH | 0.977 ± 0.001 | 0.974 ± 0.001 |
| Free AA | ||
| kFAA (×103) (min−1) | 16.44 ± 2.90 | 26.24 ± 5.23 |
| bFAA (% of Peq(0)) | 6.24 ± 0.23 a | 1.76 ± 0.42 b |
| mFAA (% of Peq(0)) | 25.82 ± 3.21 | 26.27 ± 1.21 |
| R2FAA | 0.988 ± 0.007 | 0.982 ± 0.012 |
| LMW | ||
| kLMW (×103) (min−1) | 27.84 ± 1.42 a | 72.32 ± 1.22 b |
| bLMW (% of Peq(0)) | 22.77 ± 1.81 a | 16.52 ± 0.78 b |
| mLMW (% of Peq(0)) | 36.84 ± 1.78 | 34.76 ± 1.77 |
| R2LMW | 0.988 ± 0.003 | 0.984 ± 0.005 |
| Total products | ||
| kTP (×103) (min−1) | 31.94 ± 2.04 a | 79.65 ± 9.51 b |
| bTP (% of Peq(0)) | 65.88 ± 2.32 | 62.98 ± 0.93 |
| mTP (% of Peq(0)) | 48.17 ± 2.39 | 51.21 ± 0.93 |
| R2TP | 0.981 ± 0.008 | 0.979 ± 0.005 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abrahamse, E.; Thomassen, G.G.M.; Renes, I.B.; Wierenga, P.A.; Hettinga, K.A. Gastrointestinal Protein Hydrolysis Kinetics: Opportunities for Further Infant Formula Improvement. Nutrients 2022, 14, 1512. https://doi.org/10.3390/nu14071512
Abrahamse E, Thomassen GGM, Renes IB, Wierenga PA, Hettinga KA. Gastrointestinal Protein Hydrolysis Kinetics: Opportunities for Further Infant Formula Improvement. Nutrients. 2022; 14(7):1512. https://doi.org/10.3390/nu14071512
Chicago/Turabian StyleAbrahamse, Evan, Gabriël G. M. Thomassen, Ingrid B. Renes, Peter A. Wierenga, and Kasper A. Hettinga. 2022. "Gastrointestinal Protein Hydrolysis Kinetics: Opportunities for Further Infant Formula Improvement" Nutrients 14, no. 7: 1512. https://doi.org/10.3390/nu14071512
APA StyleAbrahamse, E., Thomassen, G. G. M., Renes, I. B., Wierenga, P. A., & Hettinga, K. A. (2022). Gastrointestinal Protein Hydrolysis Kinetics: Opportunities for Further Infant Formula Improvement. Nutrients, 14(7), 1512. https://doi.org/10.3390/nu14071512






