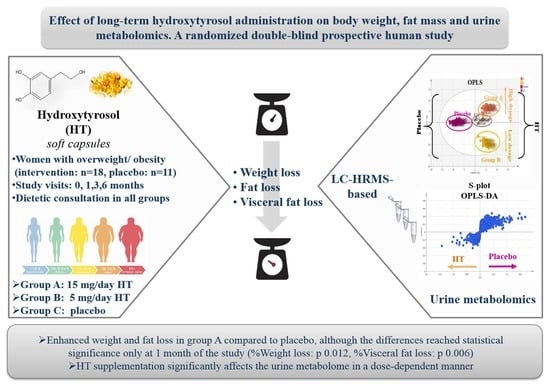
Effect of Long-Term Hydroxytyrosol Administration on Body Weight, Fat Mass and Urine Metabolomics: A Randomized Double-Blind Prospective Human Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Instrumentation
2.3. Capsules Quantitative and Qualitative Analysis
2.4. Hydroxytyrosol Capsule Administration to Women with Overweight/Obesity
2.4.1. Study Design
2.4.2. Sample Size Analysis
2.4.3. Sample Characteristics and Study Protocol
| Group | Group A | Group B | Group C |
|---|---|---|---|
| HT Intake | 15 mg HT/Day | 5 mg HT/Day | 0 mg/Day |
| Capsule consumption (per day) | 6 HT capsules (2 HT capsules before three main meals) | 2 HT and 4 placebo capsules (1 HT and 1 placebo capsule before breakfast, 2 placebo capsules before lunch, 1 HT and 1 placebo capsule before dinner) | 6 placebo capsules (2 placebo capsules before three main meals) |
| Number of volunteers | 9 | 9 | 11 |
2.4.4. Studied Anthropometric Parameters and Statistical Analysis
2.4.5. Urine Collection and Sample Preparation
2.5. Untargeted Metabolomics Analysis of Urine Samples
2.5.1. Samples
2.5.2. UPLC-HRMS Analysis
2.5.3. Statistical Process and Chemometrics
3. Results and Discussion
3.1. Quantitative and Qualitative Capsule Analysis
| m/z Exp | m/z Theor | RT (min) | MF | RDBeq. | δ (ppm) | Compound | |
|---|---|---|---|---|---|---|---|
| 1 | 153.0561 | 153.0546 | 10.05 | C8H10O3 | 4.5 | 1.650 | Hydroxytyrosol |
| 2 | 183.0665 | 183.0652 | 12.06 | C9H12O4 | 4.5 | 0.917 | Dialdehydic form of decarboxymethyl elenolic acid |
| 3 | 195.0656 | 195.0652 | 13.19 | C10H12O4 | 5.5 | 0.963 | Hydroxytyrosol acetate |
| 4 | 313.2379 | 313.2373 | 18.82 | C18H34O4 | 2.5 | −1.861 | Octadecanedioic acid |
| 5 | 279.2325 | 279.2319 | 20.09 | C18H32O2 | 3.5 | −1.588 | Linoleic acid |
| 6 | 255.2328 | 255.2319 | 21.45 | C16H34O2 | 1.5 | −0.719 | Palmitic acid |
| 7 | 281.2483 | 281.2475 | 21.60 | C18H34O2 | 2.5 | −1.222 | Oleic acid |

3.2. Effect of the Intervention in Anthropometric Parameters
| Mean Body Weight Loss (kg) | 1 Month (T1) | 3 Months (T3) | 6 Months (T6) |
|---|---|---|---|
| Group A | −4.31 (SD 1.83) | −7.97 (SD 4.24) | −10.14 (SD 5.41) |
| Group Β | −1.22 (SD 1.03) | −2.78 (SD 2.12) | −2.74 (SD 2.43) |
| Intervention group (groups A and B) | −2.76 (SD 2.15) | −5.52 (SD 4.25) | −6.44 (SD 5.57) |
| Group C (placebo) | −2.45 (SD 1.11) | −5.04 (SD 2.69) | −5.44 (SD 5.69) |
| p | 0.604 § 0.019 | 0.714 § 0.096 | 0.685 § 0.113 |
| Mean Visceral Fat Loss (%) | 1 Month (t1) | 3 Months (t3) | 6 Months (t6) |
| Group A | −1.67 (SD 1.20) | −2.67 (SD 1.44) | −3.00 (SD 2.15) |
| Group β | −0.22 (SD 0.44) | −0.62 (SD 0.83) | −0.56 (SD 1.14) |
| Intervention Group (GROUPS A and B) | −0.94 (SD 1.15) | −1.71 (SD 1.56) | −1.78 (SD 2.09) |
| Group C | −0.41 (SD 0.70) | −1.50 (SD 1.26) | −1.94 (SD 1.86) |
| p | 0.176 § 0.009 # 0.497 | 0.718 § 0.069 # 0.107 | 0.847 § 0.295 # 0.09 |
| Mean Body Fat Loss (kg) | 1 Month (T1) | 3 Months (T3) | 6 Months (T6) |
|---|---|---|---|
| Group A | −3.24 (SD 0.92) | −6.29 (SD 2.80) | −8.16 (SD 4.20) |
| Group Β | −1.10 (SD 0.99) | −2.68 (SD 1.85) | −2.06 (SD 1.73) |
| Intervention group (groups A and B) | −2.18 (SD 1.43) | −4.59 (SD 3.01) | −5.11 (SD 4.46) |
| Group C | −2.48 (SD 1.46) | −4.17 (SD 2.49) | −4.35 (SD 5.12) |
| p | 0.586 § 0.192 | 0.707 § 0.094 | 0.711 § 0.129 |
| Month 1 (T1) | Month 3 (T3) | Month 6 (T6) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean % Variations ± SD | Intervention Group | Group C (Placebo) | p | Intervention Group | Group C (Placebo) | p | Intervention Group | Group C (Placebo) | p |
| %Weight | Group A: −4.82 ± 1.77 Group B: −1.67 ± 1.44 | −2.98 ± 1.33 | 0.694 # 0.065 § 0.012 | Group A: −8.80 ± 4.18 Group B: −3.84 ± 2.92 | −6.15 ± 3.31 | 0.829 # 0.170 § 0.106 | Group A: −11.03 ± 5.67 Group B: −3.80 ± 3.33 | −6.54 ± 6.76 | 0.760 # 0.325 § 0.114 |
| %Visceral fat mass | Group A: −11.65 ± 7.30 Group B: −1.89 ± 3.78 | −3.74 ± 6.06 | 0.245 # 0.494 § 0.006 | Group A: −18.21 ± 7.70 Group B: −5.58 ± 7.68 | −12.25 ± 10.45 | 0.996 # 0.120 § 0.148 | Group A: −19.16 ± 13.68 Group B: −4.75 ± 10.93 | −15.82 ± 15.59 | 0.547 # 0.109 § 0.620 |
| %Fat mass | Group A: −8.75 ± 2.31 Group B: −4.15 ± 3.50 | −6.88 ± 4.28 | 0.538 # 0.066 § 0.516 | Group A: −16.58 ± 6.79 Group B: −10.17 ± 6.67 | −12.18 ± 7.08 | 0.623 # 0.535 § 0.167 | Group A: −20.88 ± 11.06 Group B: −7.89 ± 6.03 | −11.87 ± 13.89 | 0.663 # 0.470 § 0.111 |
3.3. Metabolomic Analysis of Urine Samples via UPLC-Orbitrap-MS
3.3.1. Samples for Metabolomics Study and Validation Aspects
3.3.2. Chemometrics in Urine Samples

| m/z Exp | Suggested Molecule | MF | RDB eq | VIP | Group | |
|---|---|---|---|---|---|---|
| Ur1 | 178.0512 | Hippuric acid | C9H9O3N | 6.5 | 20.3404 | HT |
| Ur2 | 194.0462 | Hydroxyhippuric acid | C9H9O4N | 6.5 | 6.30864 | Placebo |
| Ur3 | 367.1586 | Epitestosterone sulfate | C19H28O5S | 6.5 | 5.59170 | Placebo |
| Ur4 | 369.1733 | 5a-Dihydrotestosterone sulfate | C19H30O5S | 5.5 | 5.29357 | Placebo |
| Ur5 | 181.0505 | Homovanillic acid | C9H10O4 | 5.5 | 5.20725 | Placebo |
| Ur6 | 145.0616 | Glutamine | C5H10O3N2 | 2.5 | 5.08479 | HT |
| Ur7 | 261.0079 | Homovanillic acid sulfate | C9H10O7S | 5.5 | 4.62802 | Placebo |
| Ur8 | 187.0073 | p-cresol sulfate | C7H8O4S | 4.5 | 4.15471 | HT |
| Ur9 | 195.0523 | 1,3-Dimethyluric acid | C7H8O3N4 | 6.5 | 4.05979 | Placebo |
| Ur10 | 245.0128 | Homovanillic aldehyde sulfate | C9H10O6S | 5.5 | 3.97235 | Placebo |
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- World Health Organization. Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 12 July 2021).
- Mitchell, N.S.; Catenacci, V.A.; Wyatt, H.R.; Hill, J.O. Obesity: Overview of an epidemic. Psychiatr. Clin. N. Am. 2011, 34, 717–732. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wang, N.; Ma, Y.; Wen, D. Hydroxytyrosol improves obesity and insulin resistance by modulating gut microbiota in high-fat diet-induced obese mice. Front. Microbiol. 2019, 10, 390. [Google Scholar] [CrossRef] [PubMed]
- Bray, G.A.; Heisel, W.E.; Afshin, A.; Jensen, M.D.; Dietz, W.H.; Long, M.; Kushner, R.F.; Daniels, S.R.; Wadden, T.A.; Tsai, A.G.; et al. The Science of Obesity Management: An Endocrine Society Scientific Statement. Endocr. Rev. 2018, 39, 79–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castaner, O.; Goday, A.; Park, Y.-M.; Lee, S.-H.; Magkos, F.; Shiow, S.-A.T.E.; Schröder, H. The Gut Microbiome Profile in Obesity: A Systematic Review. Int. J. Endocrinol. 2018, 2018, 4095789. [Google Scholar] [CrossRef]
- Cani, P.D.; Osto, M.; Geurts, L.; Everard, A. Involvement of gut microbiota in the development of low-grade inflammation and type 2 diabetes associated with obesity. Gut Microbes 2012, 3, 279–288. [Google Scholar] [CrossRef] [Green Version]
- Boghossian, N.S.; Yeung, E.H.; Mumford, S.L.; Zhang, C.; Gaskins, A.J.; Wactawski-Wende, J.; Schisterman, E.F.; for the BioCycle Study Group. Adherence to the Mediterranean diet and body fat distribution in reproductive aged women. Eur. J. Clin. Nutr. 2013, 67, 289–294. [Google Scholar] [CrossRef] [Green Version]
- Bertoli, S.; Leone, A.; Vignati, L.; Bedogni, G.; Martinez-Gonzalez, M.A.; Bes-Rastrollo, M.; Spadafranca, A.; Vanzulli, A.; Battezzati, A. Adherence to the Mediterranean diet is inversely associated with visceral abdominal tissue in Caucasian subjects. Clin. Nutr. 2015, 34, 1266–1272. [Google Scholar] [CrossRef]
- Bendall, C.L.; Mayr, H.L.; Opie, R.S.; Itsiopoulos, C.; Mayr, H.L.; Opie, R.S.; Itsiopoulos, C. Central obesity and the Mediterranean diet: A systematic review of intervention trials. Crit. Rev. Food Sci. Nutr. 2018, 58, 3070–3084. [Google Scholar] [CrossRef]
- Tripoli, E.; Giammanco, M.; Tabacchi, G.; Di Majo, D.; Giammanco, S.; La Guardia, M. The phenolic compounds of olive oil: Structure, biological activity and beneficial effects on human health. Nutr. Res. Rev. 2005, 18, 98–112. [Google Scholar] [CrossRef]
- Cicerale, S.; Lucas, L.J.; Keast, R.S.J. Antimicrobial, antioxidant and anti-inflammatory phenolic activities in extra virgin olive oil. Curr. Opin. Biotechnol. 2012, 23, 129–135. [Google Scholar] [CrossRef]
- Lemonakis, N.; Poudyal, H.; Halabalaki, M.; Brown, L.; Tsarbopoulos, A.; Skaltsounis, A.-L.; Gikas, E. The LC-MS-based metabolomics of hydroxytyrosol administration in rats reveals amelioration of the metabolic syndrome. J. Chromatogr. B, Anal. Technol. Biomed. Life Sci. 2017, 1041–1042, 45–59. [Google Scholar] [CrossRef] [PubMed]
- Carito, V.; Ciafre, S.; Tarani, L.; Ceccanti, M.; Natella, F.; Iannitelli, A.; Tirassa, P.; Chaldakov, G.N.; Ceccanti, M.; Boccardo, C.; et al. TNF-alpha and IL-10 modulation induced by polyphenols extracted by olive pomace in a mouse model of paw inflammation. Ann. Ist. Super. Sanita 2015, 51, 382–386. [Google Scholar] [CrossRef] [PubMed]
- Cao, K.; Xu, J.; Zou, X.; Li, Y.; Chen, C.; Zheng, A.; Li, H.; Li, H.; Szeto, I.M.-Y.; Shi, Y.; et al. Hydroxytyrosol prevents diet-induced metabolic syndrome and attenuates mitochondrial abnormalities in obese mice. Free Radic. Biol. Med. 2014, 67, 396–407. [Google Scholar] [CrossRef] [PubMed]
- EFSA EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on the substantiation of health claims related to polyphenols in olive and protection of LDL particles from oxidative damage (ID 1333, 1638, 1639, 1696, 2865), maintenance. EFSA J. 2011, 9, 2033–2058. [Google Scholar] [CrossRef]
- El, S.N.; Karakaya, S. Olive tree (Olea europaea) leaves: Potential beneficial effects on human health. Nutr. Rev. 2009, 67, 632–638. [Google Scholar] [CrossRef]
- Kanakis, P.; Termentzi, A.; Michel, T.; Gikas, E.; Halabalaki, M.; Skaltsounis, A.-L. From olive drupes to olive oil. An HPLC-orbitrap-based qualitative and quantitative exploration of olive key metabolites. Planta Med. 2013, 79, 1576–1587. [Google Scholar] [CrossRef] [Green Version]
- Vilaplana-Pérez, C.; Auñón, D.; García-Flores, L.A.; Gil-Izquierdo, A. Hydroxytyrosol and Potential Uses in Cardiovascular Diseases, Cancer, and AIDS. Front. Nutr. 2014, 1, 18. [Google Scholar]
- Mučaji, P.; Nagy, M.; Záhradníková, A.; Holková, I.; Bezáková, L.; Švajdlenka, E.; Liptaj, T.; Prónayová, N. Polar constituents of Ligustrum vulgare L. and their effect on lipoxygenase activity. Chem. Pap. 2011, 65, 367–372. [Google Scholar] [CrossRef]
- Rodríguez-morató, J.; Boronat, A.; Kotronoulas, A.; Pastor, A.; Olesti, E.; Pérez-mañá, C.; Khymenets, O.; Farré, M.; De Torre, R.; Boronat, A.; et al. Metabolic disposition and biological significance of simple phenols of dietary origin: Hydroxytyrosol and tyrosol. Drug Metab. Rev. Metab. Rev. 2016, 48, 218–236. [Google Scholar] [CrossRef] [Green Version]
- Robles-Almazan, M.; Pulido-Moran, M.; Moreno-Fernandez, J.; Ramirez-Tortosa, C.; Rodriguez-Garcia, C.; Quiles, J.L.; Ramirez-Tortosa, M.C. Hydroxytyrosol: Bioavailability, toxicity, and clinical applications. Food Res. Int. 2018, 105, 654–667. [Google Scholar] [CrossRef]
- Tuck, K.L.; Freeman, M.P.; Hayball, P.J.; Stretch, G.L.; Stupans, I. The in vivo fate of hydroxytyrosol and tyrosol, antioxidant phenolic constituents of olive oil, after intravenous and oral dosing of labeled compounds to rats. J. Nutr. 2001, 131, 1993–1996. [Google Scholar] [CrossRef] [Green Version]
- Dominguez-Perles, R.; Aunon, D.; Ferreres, F.; Gil-Izquierdo, A. Gender differences in plasma and urine metabolites from Sprague-Dawley rats after oral administration of normal and high doses of hydroxytyrosol, hydroxytyrosol acetate, and DOPAC. Eur. J. Nutr. 2017, 56, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Granados-Principal, S.; El-Azem, N.; Pamplona, R.; Ramirez-Tortosa, C.; Pulido-Moran, M.; Vera-Ramirez, L.; Quiles, J.L.; Sanchez-Rovira, P.; Naudí, A.; Portero-Otin, M.; et al. Hydroxytyrosol ameliorates oxidative stress and mitochondrial dysfunction in doxorubicin-induced cardiotoxicity in rats with breast cancer. Biochem. Pharmacol. 2014, 90, 25–33. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, S.; Manna, C.; Migliardi, V.; Mazzoni, O.; Morrica, P.; Capasso, G.; Pontoni, G.; Galletti, P.; Zappia, V. Pharmacokinetics and metabolism of hydroxytyrosol, a natural antioxidant from olive oil. Drug Metab. Dispos. 2001, 29, 1492–1498. [Google Scholar] [PubMed]
- Stefanon, B.; Colitti, M. Original Research: Hydroxytyrosol, an ingredient of olive oil, reduces triglyceride accumulation and promotes lipolysis in human primary visceral adipocytes during differentiation. Exp. Biol. Med. 2016, 241, 1796–1802. [Google Scholar] [CrossRef]
- Voigt, A.; Ribot, J.; Sabater, A.G.; Palou, A.; Bonet, M.L.; Klaus, S. Identification of Mest/Peg1 gene expression as a predictive biomarker of adipose tissue expansion sensitive to dietary anti-obesity interventions. Genes Nutr. 2015, 10, 27. [Google Scholar] [CrossRef] [Green Version]
- González-Santiago, M.; Martín-Bautista, E.; Carrero, J.J.; Fonollá, J.; Baró, L.; Bartolomé, M.V.; Gil-Loyzaga, P.; López-Huertas, E. One-month administration of hydroxytyrosol, a phenolic antioxidant present in olive oil, to hyperlipemic rabbits improves blood lipid profile, antioxidant status and reduces atherosclerosis development. Atherosclerosis 2006, 188, 35–42. [Google Scholar] [CrossRef]
- Jemai, H.; Fki, I.; Bouaziz, M.; Bouallagui, Z.; El Feki, A.; Isoda, H.; Sayadi, S. Lipid-lowering and antioxidant effects of hydroxytyrosol and its triacetylated derivative recovered from olive tree leaves in cholesterol-fed rats. J. Agric. Food Chem. 2008, 56, 2630–2636. [Google Scholar] [CrossRef]
- Jemai, H.; Feki, A.E.L.; Sayadi, S. Antidiabetic and antioxidant effects of hydroxytyrosol and oleuropein from olive leaves in alloxan-diabetic rats. J. Agric. Food Chem. 2009, 57, 8798–8804. [Google Scholar] [CrossRef]
- Lopez-Huertas, E.; Fonolla, J. Hydroxytyrosol supplementation increases vitamin C levels in vivo. A human volunteer trial. Redox Biol. 2017, 11, 384–389. [Google Scholar] [CrossRef]
- de Bock, M.; Derraik, J.G.B.; Brennan, C.M.; Biggs, J.B.; Morgan, P.E.; Hodgkinson, S.C.; Hofman, P.L.; Cutfield, W.S. Olive (Olea europaea L.) leaf polyphenols improve insulin sensitivity in middle-aged overweight men: A randomized, placebo-controlled, crossover trial. PLoS ONE 2013, 8, e57622. [Google Scholar] [CrossRef] [PubMed]
- Santangelo, C.; Filesi, C.; Varì, R.; Scazzocchio, B.; Filardi, T.; Fogliano, V.; D’Archivio, M.; Giovannini, C.; Lenzi, A.; Morano, S.; et al. Consumption of extra-virgin olive oil rich in phenolic compounds improves metabolic control in patients with type 2 diabetes mellitus: A possible involvement of reduced levels of circulating visfatin. J. Endocrinol. Investig. 2016, 39, 1295–1301. [Google Scholar] [CrossRef] [PubMed]
- Colica, C.; Di Renzo, L.; Trombetta, D.; Smeriglio, A.; Bernardini, S.; Cioccoloni, G.; Costa De Miranda, R.; Gualtieri, P.; Sinibaldi Salimei, P.; De Lorenzo, A. Antioxidant Effects of a Hydroxytyrosol-Based Pharmaceutical Formulation on Body Composition, Metabolic State, and Gene Expression: A Randomized Double-Blinded, Placebo-Controlled Crossover Trial. Oxid. Med. Cell. Longev. 2017, 2017, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Boccellino, M.; D’Angelo, S. Anti-Obesity Effects of Polyphenol Intake: Current Status and Future Possibilities. Int. J. Mol. Sci. 2020, 21, 5642. [Google Scholar] [CrossRef]
- Rodríguez-López, P.; Lozano-Sanchez, J.; Borrás-Linares, I.; Emanuelli, T.; Menéndez, J.A.; Segura-Carretero, A. Structure–biological activity relationships of extra-virgin olive oil phenolic compounds: Health properties and bioavailability. Antioxidants 2020, 9, 685. [Google Scholar] [CrossRef]
- Miro-Casas, E.; Covas, M.-I.; Farre, M.; Fito, M.; Ortuño, J.; Weinbrenner, T.; Roset, P.; de la Torre, R. Hydroxytyrosol disposition in humans. Clin. Chem. 2003, 49, 945–952. [Google Scholar] [CrossRef] [Green Version]
- Cuyàs, E.; Verdura, S.; Lozano-Sánchez, J.; Viciano, I.; Llorach-Parés, L.; Nonell-Canals, A.; Bosch-Barrera, J.; Brunet, J.; Segura-Carretero, A.; Sanchez-Martinez, M.; et al. The extra virgin olive oil phenolic oleacein is a dual substrate-inhibitor of catechol-O-methyltransferase. Food Chem. Toxicol. 2019, 128, 35–45. [Google Scholar] [CrossRef]
- Cuyàs, E.; Castillo, D.; Llorach-Parés, L.; Lozano-Sánchez, J.; Verdura, S.; Nonell-Canals, A.; Brunet, J.; Bosch-Barrera, J.; Joven, J.; Valdés, R.; et al. Computational de-orphanization of the olive oil biophenol oleacein: Discovery of new metabolic and epigenetic targets. Food Chem. Toxicol. 2019, 131, 110529. [Google Scholar] [CrossRef]
- Xynos, N.; Abatis, D.; Argyropoulou, A.; Polychronopoulos, P.; Aligiannis, N.; Skaltsounis, A.-L. Development of a Sustainable Procedure for the Recovery of Hydroxytyrosol from Table Olive Processing Wastewater Using Adsorption Resin Technology and Centrifugal Partition Chromatography. Planta Med. 2015, 81, 1621–1627. [Google Scholar] [CrossRef]
- Visioli, F.; Galli, C.; Bornet, F.; Mattei, A.; Patelli, R.; Galli, G.; Caruso, D. Olive oil phenolics are dose-dependently absorbed in humans. FEBS Lett. 2000, 468, 159–160. [Google Scholar] [CrossRef]
- Calabriso, N.; Massaro, M.; Scoditti, E.; D’Amore, S.; Gnoni, A.; Pellegrino, M.; Storelli, C.; De Caterina, R.; Palasciano, G.; Carluccio, M.A. Extra virgin olive oil rich in polyphenols modulates VEGF-induced angiogenic responses by preventing NADPH oxidase activity and expression. J. Nutr. Biochem. 2016, 28, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Pereira-Caro, G.; Sarriá, B.; Madrona, A.; Espartero, J.L.; Escuderos, M.E.; Bravo, L.; Mateos, R. Digestive stability of hydroxytyrosol, hydroxytyrosyl acetate and alkyl hydroxytyrosyl ethers. Int. J. Food Sci. Nutr. 2012, 63, 703–707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Templeton, G.F. A Two-Step Approach for Transforming Continuous Variables to Normal: Implications and Recommendations for IS Research. Commun. Assoc. Inf. Syst. 2011, 28, 4. [Google Scholar] [CrossRef] [Green Version]
- Naz, S.; Vallejo, M.; García, A.; Barbas, C. Method validation strategies involved in non-targeted metabolomics. J. Chromatogr. A 2014, 1353, 99–105. [Google Scholar] [CrossRef] [PubMed]
- US Food and Drug Administration. Bioanalytical Method Validation Guidance for Industry; US FDA: White Oak, MD, USA, 2013. [Google Scholar]
- Lopez de las Hazas, M.-C.; Rubio, L.; Kotronoulas, A.; de la Torre, R.; Sola, R.; Motilva, M.-J. Dose effect on the uptake and accumulation of hydroxytyrosol and its metabolites in target tissues in rats. Mol. Nutr. Food Res. 2015, 59, 1395–1399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- US Food and Drug Administration. GRAS Notice (GRN) No. 600 for Hydroxytyrosol. Available online: https://www.fda.gov/media/96937/download (accessed on 11 October 2020).

| Parameter | Intervention Group (n = 18) | Group A (n = 9) | Group B (n = 9) | Group C (n = 11) | p | Overall Mean Values (n = 29) |
|---|---|---|---|---|---|---|
| Mean ViScan value (%) | 12.25 ± 3.23 | 13.83 ± 2.99 | 10.66 ± 2.76 | 12.73 ± 1.94 | 0.662 | 12.43 ± 2.78 (7.00 to 18.50) |
| Mean body weight (kg) | 80.40 ± 10.92 | 87.60 ± 10.77 | 73.2 ± 4.53 | 82.10 ± 7.44 | 0.438 | 81.04 ± 9.63 (65.4 to 100.60) |
| Mean fat mass (kg) | 31.98 ± 7.17 | 37.32 ± 5.67 | 26.82 ± 3.83 | 34.29 ± 5.76 | 0.373 | 32.85 ± 6.66 (19.1 to 45.3) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fytili, C.; Nikou, T.; Tentolouris, N.; Tseti, I.K.; Dimosthenopoulos, C.; Sfikakis, P.P.; Simos, D.; Kokkinos, A.; Skaltsounis, A.L.; Katsilambros, N.; et al. Effect of Long-Term Hydroxytyrosol Administration on Body Weight, Fat Mass and Urine Metabolomics: A Randomized Double-Blind Prospective Human Study. Nutrients 2022, 14, 1525. https://doi.org/10.3390/nu14071525
Fytili C, Nikou T, Tentolouris N, Tseti IK, Dimosthenopoulos C, Sfikakis PP, Simos D, Kokkinos A, Skaltsounis AL, Katsilambros N, et al. Effect of Long-Term Hydroxytyrosol Administration on Body Weight, Fat Mass and Urine Metabolomics: A Randomized Double-Blind Prospective Human Study. Nutrients. 2022; 14(7):1525. https://doi.org/10.3390/nu14071525
Chicago/Turabian StyleFytili, Christina, Theodora Nikou, Nikolaos Tentolouris, Ioulia K. Tseti, Charilaos Dimosthenopoulos, Petros P. Sfikakis, Dimitrios Simos, Alexandros Kokkinos, Alexios L. Skaltsounis, Nikolaos Katsilambros, and et al. 2022. "Effect of Long-Term Hydroxytyrosol Administration on Body Weight, Fat Mass and Urine Metabolomics: A Randomized Double-Blind Prospective Human Study" Nutrients 14, no. 7: 1525. https://doi.org/10.3390/nu14071525
APA StyleFytili, C., Nikou, T., Tentolouris, N., Tseti, I. K., Dimosthenopoulos, C., Sfikakis, P. P., Simos, D., Kokkinos, A., Skaltsounis, A. L., Katsilambros, N., & Halabalaki, M. (2022). Effect of Long-Term Hydroxytyrosol Administration on Body Weight, Fat Mass and Urine Metabolomics: A Randomized Double-Blind Prospective Human Study. Nutrients, 14(7), 1525. https://doi.org/10.3390/nu14071525