The Lifestyle Profile of Individuals with Cardiovascular and Endocrine Diseases in Cyprus: A Hierarchical, Classification Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Setting
2.3. Sampling
2.4. Participants’ Characteristics
2.4.1. Sociodemographic Characteristics
2.4.2. Anthropometric Characteristics
2.4.3. Smoking Habits
2.4.4. Physical Activity Assessment
2.4.5. Dietary Habits Assessment
2.4.6. Quality of Sleep Assessment
2.4.7. Participants’ Medical History
2.5. Ethics Approval
2.6. Statistical Analysis
3. Results
3.1. Participants’ Characteristics
3.2. Participants’ Characteristics by Cardiovascular and Endocrine Diseases
3.3. Lifestyle Factors
3.4. Profile of Cardiovascular and Endocrine Individuals
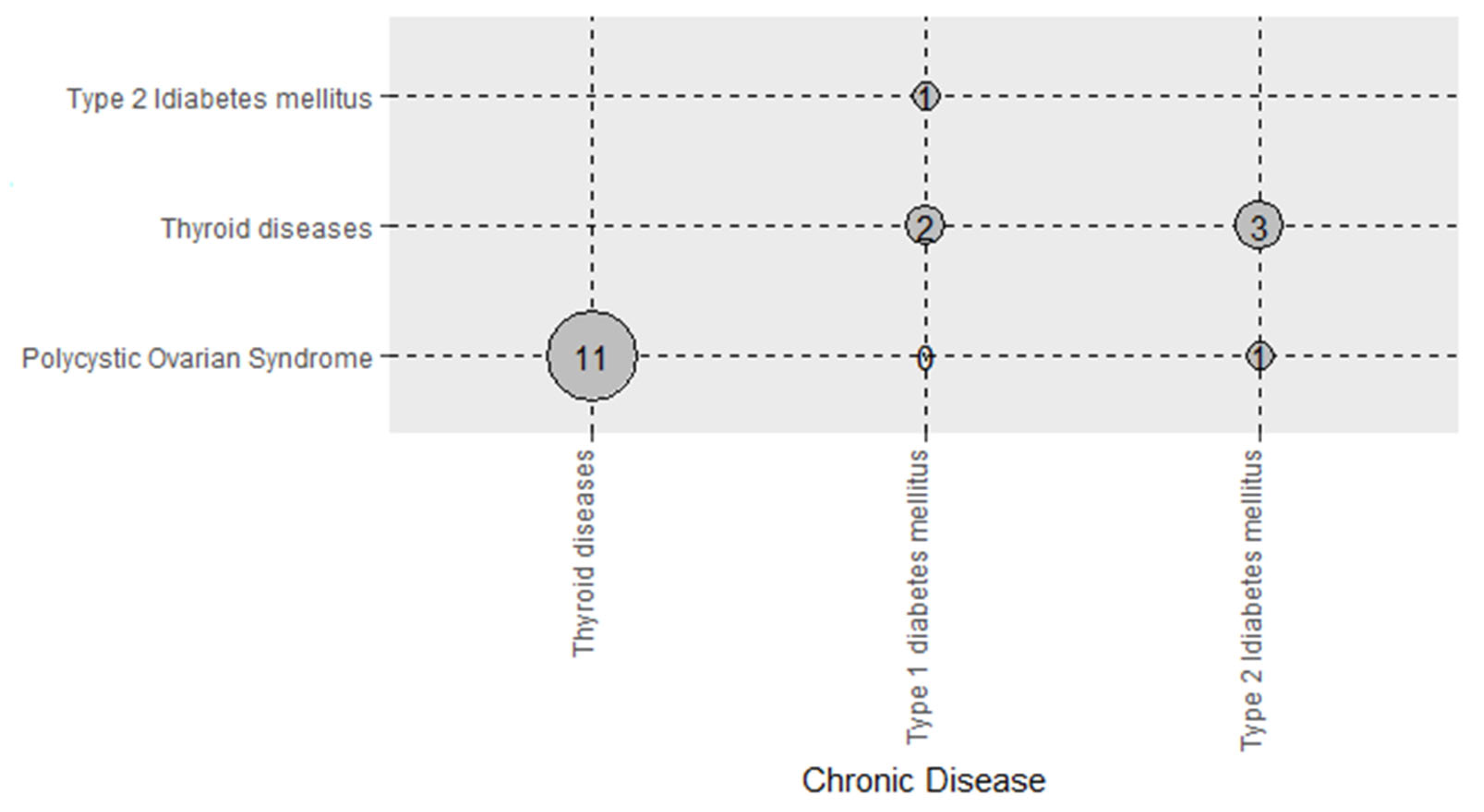
3.5. Combinations of Cardiovascular and Endocrine Diseases
4. Discussion
Limitation and Strengths
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cleven, L.; Krell-Roesch, J.; Nigg, C.R.; Woll, A. The association between physical activity with incident obesity, coronary heart disease, diabetes and hypertension in adults: A systematic review of longitudinal studies published after 2012. BMC Public Health 2020, 20, 726. [Google Scholar] [CrossRef] [PubMed]
- Déruaz-Luyet, A.; N’Goran, A.A.; Senn, N.; Bodenmann, P.; Pasquier, J.; Widmer, D.; Tandjung, R.; Rosemann, T.; Frey, P.; Streit, S.; et al. Multimorbidity and patterns of chronic conditions in a primary care population in Switzerland: A cross-sectional study. BMJ Open 2017, 7, e013664. [Google Scholar] [CrossRef] [PubMed]
- Excoffier, S.; Herzig, L.; N’Goran, A.A.; Déruaz-Luyet, A.; Haller, D.M. Prevalence of multimorbidity in general practice: A cross-sectional study within the Swiss Sentinel Surveillance System (Sentinella). BMJ Open 2018, 8, e019616. [Google Scholar] [CrossRef]
- Hussain, M.A.; Huxley, R.R.; Al Mamun, A. Multimorbidity prevalence and pattern in Indonesian adults: An exploratory study using national survey data. BMJ Open 2015, 5, e009810. [Google Scholar] [CrossRef] [PubMed]
- Ornstein, S.M.; Nietert, P.J.; Jenkins, R.G.; Litvin, C.B. The Prevalence of Chronic Diseases and Multimorbidity in Primary Care Practice: A PPRNet Report. J. Am. Board Fam. Med. 2013, 26, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Kyprianidou, M.; Panagiotakos, D.; Faka, A.; Kambanaros, M.; Makris, K.C.; Christophi, C.A. Prevalence of multimorbidity in the Cypriot population; A cross-sectional study (2018–2019). PLoS ONE 2020, 15, e0239835. [Google Scholar] [CrossRef]
- Gholami, F.; Khoramdad, M.; Esmailnasab, N.; Moradi, G.; Nouri, B.; Safiri, S.; Alimohamadi, Y. The effect of dairy consumption on the prevention of cardiovascular diseases: A meta-analysis of prospective studies. J. Cardiovasc. Thorac. Res. 2017, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Yaribeygi, H.; Sathyapalan, T.; Atkin, S.L.; Sahebkar, A. Molecular Mechanisms Linking Oxidative Stress and Diabetes Mellitus. Oxid. Med. Cell. Longev. 2020, 2020, 8609213. [Google Scholar] [CrossRef]
- WHO. WHO Cardiovascular Diseases. Available online: https://www.who.int/health-topics/cardiovascular-diseases#tab=tab_1 (accessed on 19 January 2022).
- World Health Organization. Global Strategy on Diet, Physical Activity and Health; WHO: Geneva, Switzerland, 2004. [Google Scholar]
- World Health Organization (WHO). Noncommunicable Diseases N.D. Available online: https://www.who.int/news-room/fact-sheets/detail/noncommunicable-diseases (accessed on 10 March 2022).
- Ba, H.C.B.; Harrison, C.; Miller, G.C.; Knox, S.A. Prevalence and patterns of multimorbidity in Australia. Med. J. Aust. 2008, 189, 72–77. [Google Scholar] [CrossRef]
- Hadjipanayis, A.; Hadjichristodoulou, C.; Kallias, M.; Sava, K.; Petsa, A.; Demetriadou, K.; Christodoulou, C.; Constantinou, A.; Sidera, M. Prevalence of antibodies to hepatitis A among children and adolescents in Larnaca area, Cyprus. Eur. J. Epidemiol. 1999, 15, 903–905. [Google Scholar] [CrossRef]
- Kadam, U.T.; Croft, P.R.; North Staffordshire GP Consortium Group. Clinical multimorbidity and physical function in older adults: A record and health status linkage study in general practice. Fam. Pract. 2007, 24, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Country Profiles-European Union. State of Health in the EU Cyprus. 2019. Available online: https://ec.europa.eu/health/state-health-eu/country-health-profiles_en (accessed on 10 March 2022).
- Pingali, P. Westernization of Asian diets and the transformation of food systems: Implications for research and policy. Food Policy 2006, 32, 281–298. [Google Scholar] [CrossRef]
- Ozemek, C.; Tiwari, S.; Sabbahi, A.; Carbone, S.; Lavie, C.J. Impact of therapeutic lifestyle changes in resistant hypertension. Prog. Cardiovasc. Dis. 2020, 63, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Forouzanfar, M.H.; Alexander, L.; Anderson, H.R.; Bachman, V.F.; Biryukov, S.; Brauer, M.; Burnett, R.; Casey, D.; Coates, M.; GBD 2013 Risk Factors Collaborators; et al. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks in 188 countries, 1990–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015, 386, 2287–2323. [Google Scholar] [CrossRef]
- Colpani, V.; Baena, C.P.; Jaspers, L.; Van Dijk, G.M.; Farajzadegan, Z.; Dhana, K.; Tielemans, M.J.; Voortman, T.; Freak-Poli, R.; Veloso, G.G.V.; et al. Lifestyle factors, cardiovascular disease and all-cause mortality in middle-aged and elderly women: A systematic review and meta-analysis. Eur. J. Epidemiol. 2018, 33, 831–845. [Google Scholar] [CrossRef]
- Nyberg, S.T.; Batty, G.; Pentti, J.; Virtanen, M.; Alfredsson, L.; Fransson, E.I.; Goldberg, M.; Heikkila, K.; Jokela, M.; Knutsson, A.; et al. Obesity and loss of disease-free years owing to major non-communicable diseases: A multicohort study. Lancet Public Health 2018, 3, e490–e497. [Google Scholar] [CrossRef]
- Li, Y.; Schoufour, J.; Wang, D.D.; Dhana, K.; Pan, A.; Liu, X.; Song, M.; Liu, G.; Shin, H.J.; Sun, Q.; et al. Healthy lifestyle and life expectancy free of cancer, cardiovascular disease, and type 2 diabetes: Prospective cohort study. BMJ 2020, 368, l6669. [Google Scholar] [CrossRef]
- Schlesinger, S.; Neuenschwander, M.; Ballon, A.; Nöthlings, U.; Barbaresko, J. Adherence to healthy lifestyles and incidence of diabetes and mortality among individuals with diabetes: A systematic review and meta-analysis of prospective studies. J. Epidemiol. Community Health 2020, 74, 481–487. [Google Scholar] [CrossRef]
- Ferrie, J.E.; Kivimäki, M.; Akbaraly, T.N.; Singh-Manoux, A.; Miller, M.A.; Gimeno, D.; Kumari, M.; Smith, G.D.; Shipley, M.J. Associations Between Change in Sleep Duration and Inflammation: Findings on C-reactive Protein and Interleukin 6 in the Whitehall II Study. Am. J. Epidemiol. 2013, 178, 956–961. [Google Scholar] [CrossRef]
- Aldabal, L.; Bahammam, A.S. Metabolic, endocrine, and immune consequences of sleep deprivation. Open Respir. Med. J. 2011, 5, 31–43. [Google Scholar] [CrossRef]
- Harding, K.; Feldman, M. Sleep Disorders and Sleep Deprivation: An Unmet Public Health Problem. J. Am. Acad. Child Adolesc. Psychiatry 2008, 47, 473–474. [Google Scholar] [CrossRef]
- Vélez, J.C.; Souza, A.; Traslaviña, S.; Barbosa, C.; Wosu, A.; Andrade, A.; Frye, M.; Fitzpatrick, A.L.; Gelaye, B.; Williams, M.A. The Epidemiology of Sleep Quality and Consumption of Stimulant Beverages among Patagonian Chilean College Students. Sleep Disord. 2013, 2013, 910104. [Google Scholar] [CrossRef] [PubMed]
- Kyprianidou, M.; Panagiotakos, D.; Kambanaros, M.; Makris, K.C.; Christophi, C.A. Quality of Sleep in the Cypriot Population and Its Association With Multimorbidity: A Cross-Sectional Study. Front. Public Health 2021, 9, 693332. [Google Scholar] [CrossRef] [PubMed]
- Statistical Service of Cyprus, N.D. Available online: https://www.cystat.gov.cy/el/default (accessed on 10 March 2022).
- Lamnisos, D.; Giannakou, K.; Jakovljevic, M.M. Demographic forecasting of population aging in Greece and Cyprus: One big challenge for the Mediterranean health and social system long-term sustainability. Health Res. Policy Syst. 2021, 19, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Panagiotakos, D.B.; Georgousopoulou, E.N.; Pitsavos, C.; Chrysohoou, C.; Metaxa, V.; Georgiopoulos, G.A.; Kalogeropoulou, K.; Tousoulis, D.; Stefanadis, C. Ten-year (2002–2012) cardiovascular disease incidence and all-cause mortality, in urban Greek population: The ATTICA Study. Int. J. Cardiol. 2014, 180, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Bountziouka, V.; Bathrellou, E.; Giotopoulou, A.; Katsagoni, C.N.; Bonou, M.; Vallianou, N.; Barbetseas, J.; Avgerinos, P.; Panagiotakos, D. Development, repeatability and validity regarding energy and macronutrient intake of a semi-quantitative food frequency questionnaire: Methodological considerations. Nutr. Metab. Cardiovasc. Dis. 2012, 22, 659–667. [Google Scholar] [CrossRef]
- Panagiotakos, D.B.; Pitsavos, C.; Chrysohoou, C.; Skoumas, I.; Stefanadis, C. Five-year incidence of cardiovascular disease and its predictors in Greece: The ATTICA study. Vasc. Med. 2008, 13, 113–121. [Google Scholar] [CrossRef]
- Reynolds, C.F.; Hoch; Yeager, A.L.; Kupfer, D.J.; Buysse, D.J.; Monk, T.H. Quantification of Subjective Sleep Quality in Healthy Elderly Men and Women Using the Pittsburgh Sleep Quality Index (PSQI). Sleep 1991, 14, 331–338. [Google Scholar] [CrossRef]
- Antonogeorgos, G.; Panagiotakos, D.B.; Priftis, K.N.; Tzonou, A. Logistic Regression and Linear Discriminant Analyses in Evaluating Factors Associated with Asthma Prevalence among 10- to 12-Years-Old Children: Divergence and Similarity of the Two Statistical Methods. Int. J. Pediatr. 2009, 2009, 952042. [Google Scholar] [CrossRef]
- Hinton, W.; McGovern, A.; Coyle, R.; Han, T.S.; Sharma, P.; Correa, A.; Ferreira, F.I.M.; De Lusignan, S. Incidence and prevalence of cardiovascular disease in English primary care: A cross-sectional and follow-up study of the Royal College of General Practitioners (RCGP) Research and Surveillance Centre (RSC). BMJ Open 2018, 8, e020282. [Google Scholar] [CrossRef]
- Reddy, K.S.; Mathur, M.R. Global Burden of CVD. Handb. Glob. Health 2021, 423–437. [Google Scholar] [CrossRef]
- Andersson, C.; Vasan, R.S. Epidemiology of cardiovascular disease in young individuals. Nat. Rev. Cardiol. 2018, 15, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Druss, B.G.; Marcus, S.C.; Olfson, M.; Tanielian, T.; Elinson, L.; Pincus, H.A. Comparing The National Economic Burden Of Five Chronic Conditions. Health Aff. 2001, 20, 233–241. [Google Scholar] [CrossRef]
- Féart, C. Adherence to a Mediterranean Diet, Cognitive Decline, and Risk of Dementia. JAMA 2009, 302, 638–648. [Google Scholar] [CrossRef]
- Kastorini, C.-M.; Milionis, H.J.; Esposito, K.; Giugliano, D.; Goudevenos, J.A.; Panagiotakos, D.B. The Effect of Mediterranean Diet on Metabolic Syndrome and its Components: A Meta-Analysis of 50 Studies and 534,906 Individuals. J. Am. Coll. Cardiol. 2011, 57, 1299–1313. [Google Scholar] [CrossRef] [PubMed]
- Bots, S.H.; Peters, S.A.E.; Woodward, M. Sex differences in coronary heart disease and stroke mortality: A global assessment of the effect of ageing between 1980 and 2010. BMJ Glob. Health 2017, 2, e000298. [Google Scholar] [CrossRef]
- Gao, Z.; Chen, Z.; Sun, A.; Deng, X. Gender differences in cardiovascular disease. Med. Nov. Technol. Devices 2019, 4, 100025. [Google Scholar] [CrossRef]
- Weidner, G. Why Do Men Get More Heart Disease Than Women? An International Perspective. J. Am. Coll. Health 2000, 48, 291–294. [Google Scholar] [CrossRef]
- World Health Organizatin. 10 Facts on Gender and Tobacco; World Health Organizatin: Geneva, Switzerland, 2010; Volume 4. [Google Scholar]
- Silander, K.; Alanne, M.; Kristiansson, K.; Saarela, O.; Ripatti, S.; Auro, K.; Karvanen, J.; Kulathinal, S.; Niemelä, M.; Ellonen, P.; et al. Gender Differences in Genetic Risk Profiles for Cardiovascular Disease. PLoS ONE 2008, 3, e3615. [Google Scholar] [CrossRef]
- Mendelsohn, M.E.; Karas, R.H.; Yamamoto, Y.; Brady, M.P.; Lu, Z.P.; Maziasz, P.J.; Liu, C.T.; Pint, B.A.; More, K.L.; Meyer, H.M.; et al. Molecular and Cellular Basis of Cardiovascular Gender Differences. Science 2005, 308, 1583–1587. [Google Scholar] [CrossRef]
- Cowie, C.C.; Rust, K.F.; Byrd-Holt, D.D.; Eberhardt, M.S.; Flegal, K.M.; Engelgau, M.M.; Saydah, S.H.; Williams, D.E.; Geiss, L.S.; Gregg, E.W. Prevalence of Diabetes and Impaired Fasting Glucose in Adults in the U.S. Population: National Health and Nutrition Examination Survey 1999-2002. Diabetes Care 2006, 29, 1263–1268. [Google Scholar] [CrossRef]
- Golden, S.H.; Robinson, K.A.; Saldanha, I.; Anton, B.; Ladenson, P.W. Prevalence and Incidence of Endocrine and Metabolic Disorders in the United States: A Comprehensive Review. J. Clin. Endocrinol. Metab. 2009, 94, 1853–1878. [Google Scholar] [CrossRef]
- Ford, E.S.; Li, C.; Zhao, G.; Pearson, W.S.; Mokdad, A.H. Prevalence of the Metabolic Syndrome Among US Adolescents Using the International Diabetes Federation. J. Clin. Endocrinol. Metab. 2008, 31, 587–589. [Google Scholar] [CrossRef]
- Lauretta, R.; Sansone, M.; Sansone, A.; Romanelli, F.; Appetecchia, M. Gender in Endocrine Diseases: Role of Sex Gonadal Hormones. Int. J. Endocrinol. 2018, 2018, 4847376. [Google Scholar] [CrossRef]
- Kalra, S.; Owens, D.; Sahay, R. Geriatric endocrinology. Indian J. Endocrinol. Metab. 2011, 15, 71–72. [Google Scholar] [CrossRef]
- Dégano, I.R.; Marrugat, J.; Grau, M.; Salvador-González, B.; Ramos, R.; Zamora, A.; Martí, R.; Elosua, R. The association between education and cardiovascular disease incidence is mediated by hypertension, diabetes, and body mass index. Sci. Rep. 2017, 7, 71–72. [Google Scholar] [CrossRef]
- Lynch, J.; Smith, G.D.; Harper, S.; Bainbridge, K. Explaining the social gradient in coronary heart disease: Comparing relative and absolute risk approaches. J. Epidemiol. Community Health 2006, 60, 436–441. [Google Scholar] [CrossRef]
- Yarnell, J.; Yu, S.; McCrum, E.; Arveiler, D.; Hass, B.; Dallongeville, J.; Montaye, M.; Amouyel, P.; Ferrieres, J.; Ruidavets, J.-B.; et al. Education, socioeconomic and lifestyle factors, and risk of coronary heart disease: The PRIME Study. Int. J. Epidemiol. 2004, 34, 268–275. [Google Scholar] [CrossRef]
- Cavelaars, A.E.J.M.; Kunst, A.E.; Geurts, J.J.M.; Crialesi, R.; Grötvedt, L.; Helmert, U.; Lahelma, E.; Lundberg, O.; Matheson, J.; Mielck, A.; et al. Educational differences in smoking: International comparison. BMJ 2000, 320, 1102–1107. [Google Scholar] [CrossRef]
- Liu, K.; Cedres, L.B.; Stamler, J.; Dyer, A.; Stamler, R.; Nanas, S.; Berkson, D.M.; Paul, O.; Lepper, M.; Lindberg, H.A.; et al. Relationship of education to major risk factors and death from coronary heart disease, cardiovascular diseases and all causes, Findings of three Chicago epidemiologic studies. Circulation 1982, 66, 1308–1314. [Google Scholar] [CrossRef]
- Berger, M.C.; Leigh, J.P. Schooling, Self-Selection, and Health. J. Hum. Resour. 1989, 24, 433–455. [Google Scholar] [CrossRef]
- Dyer, A.R.; Stamler, J.; Shekelle, R.B.; Schoenberger, J. The relationship of education to blood pressure: Findings on 40,000 employed Chicagoans. Circulation 1976, 54, 987–992. [Google Scholar] [CrossRef] [PubMed]
- Matthews, K.A.; Kelsey, S.F.; Meilahn, E.N.; Kuller, L.H.; Wing, R.R. Educational attainment and behavioral and biologic risk factors for coronary heart disease in middle-aged women. Am. J. Epidemiol. 1989, 129, 1132–1144. [Google Scholar] [CrossRef] [PubMed]
- Millar, W.J.; Wigle, D.T. Socioeconomic disparities in risk factors for cardiovascular disease. Can. Med. Assoc. J. 1986, 134, 127–132. [Google Scholar]
- Wagenknecht, L.E.; Perkins, L.L.; Cutter, G.R.; Sidney, S.; Burke, G.L.; Manolio, T.A.; Jacobs, D.R.; Liu, K.; Friedman, G.D.; Hughes, G.H.; et al. Cigarette smoking behavior is strongly related to educational status: The cardia study. Prev. Med. 1990, 19, 158–169. [Google Scholar] [CrossRef]
- Jacobsen, B.K.; Thelle, D.S. Risk factors for coronary heart disease and level of education. The Tromsø Heart Study. Am. J. Epidemiol. 1988, 127, 923–932. [Google Scholar] [CrossRef]
- Opiyo, P.O.; Agong, S.G. Nexus between Urban Food System and Other Urban Systems: Exploring Nexus between Urban Food System and Other Urban Systems: Exploring Opportunities for Improving Food Security in Kisumu, Kenya. Soc. Econ. Geogr. 2020, 5, 20–28. [Google Scholar] [CrossRef]
- Xu, Y.; Geng, Y.; Gao, Z.; Xiao, S.; Zhang, C.; Zhuang, M. Accounting greenhouse gas emissions of food consumption between urban and rural residents in China: A whole production perspective. Front. Energy 2021, 15, 1–18. [Google Scholar] [CrossRef]
- Kwok, C.S.; Kontopantelis, E.; Kuligowski, G.; Gray, M.; Muhyaldeen, A.; Gale, C.P.; Peat, G.M.; Cleator, J.; Chew-Graham, C.; Loke, Y.K.; et al. Self-Reported Sleep Duration and Quality and Cardiovascular Disease and Mortality: A Dose-Response Meta-Analysis. J. Am. Heart Assoc. 2018, 7, e008552. [Google Scholar] [CrossRef]
- Lao, X.Q.; Liu, X.; Deng, H.-B.; Chan, T.-C.; Ho, K.F.; Wang, F.; Vermeulen, R.; Tam, T.; Wong, M.; Tse, L.A.; et al. Sleep Quality, Sleep Duration, and the Risk of Coronary Heart Disease: A Prospective Cohort Study With 60,586 Adults. J. Clin. Sleep Med. 2018, 14, 109–117. [Google Scholar] [CrossRef]
- Helbig, A.K.; Stöckl, D.; Heier, M.; Thorand, B.; Schulz, H.; Peters, A.; Ladwig, K.-H.; Meisinger, C. Relationship between sleep disturbances and multimorbidity among community-dwelling men and women aged 65–93 years: Results from the KORA Age Study. Sleep Med. 2017, 33, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Castell, M.; Makovski, T.T.; Bocquet, V.; Stranges, S. Sleep duration and multimorbidity in Luxembourg: Results from the European Health Examination Survey in Luxembourg, 2013–2015. BMJ Open 2019, 9, e026942. [Google Scholar] [CrossRef] [PubMed]
- Koren, D.; O’Sullivan, K.L.; Mokhlesi, B. Metabolic and glycemic sequelae of sleep disturbances in children and adults. Curr. Diabetes Rep. 2014, 15, 562. [Google Scholar] [CrossRef] [PubMed]
- Barakat, S.; Abujbara, M.; Banimustafa, R.; Batieha, A.; Ajlouni, K. Sleep Quality in Patients With Type 2 Diabetes Mellitus. J. Clin. Med. Res. 2019, 11, 261–266. [Google Scholar] [CrossRef]
- Holtermann, A.; Schnohr, P.; Nordestgaard, B.G.; Marott, J.L. The physical activity paradox in cardiovascular disease and all-cause mortality: The contemporary Copenhagen General Population Study with 104 046 adults. Eur. Heart J. 2021, 42, 1499–1511. [Google Scholar] [CrossRef]
- Sattelmair, J.; Pertman, J.; Ding, E.L.; Kohl, H.W.; Haskell, W.; Lee, I.-M. Dose Response Between Physical Activity and Risk of Coronary Heart Disease. Circulation 2011, 124, 789–795. [Google Scholar] [CrossRef]
- Aune, D.; Norat, T.; Leitzmann, M.; Tonstad, S.; Vatten, L.J. Physical activity and the risk of type 2 diabetes: A systematic review and dose–response meta-analysis. Eur. J. Epidemiol. 2015, 30, 529–542. [Google Scholar] [CrossRef]
- Lavie, C.J.; De Schutter, A.; Parto, P.; Jahangir, E.; Kokkinos, P.; Ortega, F.B.; Arena, R.; Milani, R.V. Obesity and Prevalence of Cardiovascular Diseases and Prognosis—The Obesity Paradox Updated. Prog. Cardiovasc. Dis. 2016, 58, 537–547. [Google Scholar] [CrossRef]
- Lavie, C.J.; Sharma, A.; Alpert, M.A.; De Schutter, A.; Lopez-Jimenez, F.; Milani, R.V.; Ventura, H.O. Update on Obesity and Obesity Paradox in Heart Failure. Prog. Cardiovasc. Dis. 2015, 58, 393–400. [Google Scholar] [CrossRef]
- Van Hulsteijn, L.T.; Pasquali, R.; Casanueva, F.; Haluzik, M.; LeDoux, S.; Monteiro, M.; Salvador, J.; Santini, F.; Toplak, H.; Dekkers, O.M. Prevalence of endocrine disorders in obese patients: Systematic review and meta-analysis. Eur. J. Endocrinol. 2020, 182, 11–21. [Google Scholar] [CrossRef]
- Kavouras, S.A.; Panagiotakos, D.B.; Pitsavos, C.; Chrysohoou, C.; Anastasiou, C.A.; Lentzas, Y.; Stefanadis, C. Physical Activity, Obesity Status, and Glycemic Control. Med. Sci. Sports Exerc. 2007, 39, 606–611. [Google Scholar] [CrossRef] [PubMed]
- Baier, J.M.; Funck, K.L.; Vernstrøm, L.; Laugesen, E.; Poulsen, P.L. Low physical activity is associated with impaired endothelial function in patients with type 2 diabetes and controls after 5 years of follow-up. BMC Endocr. Disord. 2021, 21, 189. [Google Scholar] [CrossRef] [PubMed]
- Bijnen, F.C.H.; Caspersen, C.J.; Feskens, E.J.M.; Saris, W.H.M.; Mosterd, W.L.; Kromhout, D. Physical Activity and 10-Year Mortality From Cardiovascular Diseases and All Causes. Arch. Intern. Med. 1998, 158, 1499–1505. [Google Scholar] [CrossRef] [PubMed]
- Rossi, A.; Dikareva, A.; Bacon, S.L.; Daskalopoulou, S.S. The impact of physical activity on mortality in patients with high blood pressure. J. Hypertens. 2012, 30, 1277–1288. [Google Scholar] [CrossRef]
- Kouvari, M.; Panagiotakos, D.B.; Chrysohoou, C.; Georgousopoulou, E.; Notara, V.; Tousoulis, D.; Pitsavos, C.; ATTICA & GREECS Studies Investigators. Gender-specific, Lifestyle-related Factors and 10-year Cardiovascular Disease Risk; the ATTICA and GREECS Cohort Studies. Curr. Vasc. Pharmacol. 2019, 17, 401–410. [Google Scholar] [CrossRef] [PubMed]

| Cardiovascular Diseases | Endocrine Diseases | ||||||
|---|---|---|---|---|---|---|---|
| Characteristics | Overall (n = 1140) | No (n = 856) | Yes (n = 283) | p-Value | No (n = 944) | Yes (n = 196) | p-Value |
| Age (years), Mean (SD) | 40.8 ± 16.9 | 35.9 ± 14.1 | 55.4 ± 16.3 | <0.01f | 39.5 ± 16.3 | 47.2 ± 18.1 | <0.01f |
| Age group, n a (%) | |||||||
| 18–24 | 167 (14.7) | 161 (96.4) | 6 (3.6) | <0.01g | 156 (93.4) | 11 (6.6) | <0.01g |
| 25–44 | 524 (46.0) | 464 (88.5) | 60 (11.5) | 441 (84.2) | 83 (15.8) | ||
| 45–64 | 314 (27.5) | 188 (59.9) | 126 (40.1) | 254 (80.9) | 60 (19.1) | ||
| 65+ | 135 (11.8) | 44 (32.6) | 91 (67.4) | 93 (68.9) | 42 (31.1) | ||
| Sex, n b (%) | |||||||
| Women | 642 (56.4) | 509 (79.3) | 133 (20.7) | <0.01g | 488 (76.0) | 154 (24.0) | <0.01g |
| Men | 497 (43.6) | 347 (69.8) | 150 (30.2) | 455 (91.5) | 42 (8.5) | ||
| Geographical area, n c (%) | |||||||
| Nicosia | 493 (43.3) | 366 (74.2) | 127 (25.8) | 0.03g | 410 (83.2) | 83 (16.8) | 0.06 g |
| Limassol | 311 (27.3) | 222 (71.4) | 89 (28.6) | 243 (78.1) | 68 (21.9) | ||
| Larnaca | 171 (15.0) | 137 (80.1) | 34 (19.9) | 149 (87.1) | 22 (12.9) | ||
| Paphos | 113 (9.9) | 95 (84.1) | 18 (15.9) | 99 (87.6) | 14 (12.4) | ||
| Ammochostos | 50 (4.5) | 35 (70.0) | 15 (30.0) | 41 (82.0) | 9 (18.0) | ||
| Residency, n d (%) | |||||||
| Urban | 864 (76.3) | 639 (75.1) | 225 (79.8) | 0.11 g | 715 (76.3) | 149 (76.0) | 0.93 g |
| Rural | 269 (23.7) | 212 (24.9) | 57 (20.2) | 222 (23.7) | 47 (24.0) | ||
| Educational status, n d (%) | |||||||
| Primary education | 66 (5.8) | 21 (31.8) | 45 (68.2) | <0.01g | 43 (65.2) | 23 (34.8) | <0.01g |
| Secondary education | 338 (29.8) | 240 (71.0) | 98 (29.0) | 282 (83.4) | 56 (16.6) | ||
| Higher education | 729 (64.4) | 591 (81.1) | 138 (18.9) | 613 (84.1) | 116 (15.9) | ||
| Job status, n e (%) | |||||||
| Private employee | 432 (39.9) | 362 (83.8) | 70 (16.2) | <0.01g | 369 (85.4) | 63 (14.6) | <0.01g |
| State employee | 218 (20.1) | 157 (72.0) | 61 (28.0) | 179 (82.1) | 39 (17.9) | ||
| Freelance | 100 (9.2) | 79 (79.0) | 21 (21.0) | 86 (86.0) | 14 (14.0) | ||
| Unemployed | 205 (18.9) | 175 (85.4) | 30 (14.6) | 178 (86.8) | 27 (13.2) | ||
| Retired | 129 (11.9) | 40 (31.0) | 89 (69.0) | 86 (66.7) | 43 (33.3) | ||
| Cardiovascular Diseases | Endocrine Diseases | ||||||
|---|---|---|---|---|---|---|---|
| Characteristics | Overall (n = 1139) | No (n = 856) | Yes (n = 283) | p-Value | No (n = 944) | Yes (n = 196) | p-Value |
| Smoking habits | |||||||
| Age of starting smoking a, Mean (SD) | 18.7 ± 4.6 | 18.6 ± 4.1 | 19.1 ± 5.6 | 0.31 i | 18.8 ± 4.8 | 18.3 ± 3.4 | 0.41 i |
| Smoking status, n a (%) | |||||||
| Non-smoker | 731 (64.5) | 561 (76.7) | 170 (23.3) | 0.09 j | 598 (81.8) | 133 (18.2) | 0.24 j |
| Current smoker | 402 (35.5) | 290 (72.1) | 112 (27.9) | 340 (84.6) | 62 (15.4) | ||
| Physical activity level | |||||||
| Physical activity, n b (%) | |||||||
| Not adequately physical active | 591 (52.0) | 413 (69.9) | 178 (30.1) | <0.01j | 471 (79.7) | 120 (20.3) | <0.01j |
| Physical active | 545 (48.0) | 438 (80.4) | 108 (19.6) | 470 (86.2) | 76 (13.8) | ||
| Type of exercise, n c (%) | |||||||
| Gym | 146 (26.8) | 127 (87.0) | 19 (13.0) | <0.01j | 127 (87.0) | 19 (13.0) | 0.58 j |
| Combination | 189 (34.7) | 156 (82.5) | 33 (17.5) | 164 (86.8) | 25 (13.2) | ||
| Walking/Gait | 62 (11.4) | 36 (58.1) | 26 (41.9) | 48 (77.4) | 14 (22.6) | ||
| Jogging | 27 (5.0) | 22 (81.5) | 5 (18.5) | 26 (96.3) | 1 (3.7) | ||
| Swimming | 22 (4.0) | 16 (72.7) | 6 (27.3) | 18 (81.8) | 4 (18.2) | ||
| Football | 21 (3.9) | 14 (66.7) | 7 (33.3) | 20 (95.2) | 1 (4.8) | ||
| Pilates/Yoga | 18 (3.3) | 13 (72.2) | 5 (27.8) | 14 (77.8) | 4 (22.2) | ||
| Dance/Zumba | 10 (1.8) | 10 (100.0) | 0 (0.0) | 9 (90.0) | 1 (10.0) | ||
| Martial arts | 10 (1.8) | 8 (80.0) | 2 (20.0) | 9 (90.0) | 1 (10.0) | ||
| Cycling | 10 (1.8) | 9 (90.0) | 1 (10.0) | 9 (90.0) | 1 (10.0) | ||
| Basketball | 8 (1.5) | 6 (75.0) | 2 (25.0) | 6 (75.0) | 2 (25.0) | ||
| Volleyball | 5 (0.9) | 5 (100.0) | 0 (0.0) | 5 (100.0) | 0 (0.0) | ||
| Cross fit/TRX | 5 (0.9) | 5 (100.0) | 0 (0.0) | 4 (80.0) | 1 (20.0) | ||
| Handball | 5 (0.9) | 5 (100.0) | 0 (0.0) | 5 (100.0) | 0 (0.0) | ||
| Other d | 7 (1.3) | 5 (71.4) | 2 (28.6) | 6 (85.7) | 1 (14.3) | ||
| Hours of exercise per week, n e (%) | |||||||
| Less than 1 h | 58 (11.3) | 39 (67.2) | 19 (32.8) | <0.01j | 45 (77.6) | 13 (22.4) | 0.17j |
| 1–3 h | 208 (39.3) | 169 (81.2) | 39 (18.8) | 178 (85.6) | 30 (14.4) | ||
| 3–6 h | 151 (28.5) | 121 (80.1) | 30 (19.9) | 131 (86.7) | 20 (13.3) | ||
| 6–9 h | 77 (14.6) | 70 (90.9) | 7 (9.1) | 70 (90.9) | 7 (9.1) | ||
| More than 9 h | 33 (6.3) | 26 (78.8) | 7 (21.2) | 31 (93.9) | 2 (6.1) | ||
| Dietary habits | |||||||
| MedDietScore (range: 0–55), Median (IQR) | |||||||
| MedDietScore | 15 (13, 18) | 15 (13, 18) | 16 (13, 18) | 0.91 k | 16 (13, 18) | 15 (13, 18) | 0.30 k |
| Mediterranean Diet adherence, n f (%) | |||||||
| Low (≤13) | 370 (32.9) | 286 (77.3) | 84 (22.7) | 0.204 j | 298 (80.5) | 72 (19.5) | 0.381 j |
| Moderate (14–18) | 511 (45.4) | 372 (72.8) | 139 (27.2) | 429 (84.0) | 82 (16.0) | ||
| High (≥19) | 245 (21.7) | 190 (77.5) | 55 (22.5) | 205 (83.7) | 40 (16.3) | ||
| Food consumption (0: no consumption—5: daily), Median (IQR) | |||||||
| Full-fat dairy products | 2.5 (1.5, 3) | 2.5 (1.5, 3) | 2.5 (1.5, 3) | 0.95 k | 2.5 (1.5, 3) | 2.5 (1.5, 3) | 0.88 k |
| Non-refined cereals | 2 (1.3, 2.3) | 2 (1.3, 2.3) | 1.7 (1, 2.3) | 0.16 k | 2 (1.3, 2.3) | 2 (1.3, 2.3) | 0.74 k |
| Meat and meat products | 2.5 (2.2, 3.2) | 2.7 (2.2, 3.2) | 2.2 (2, 3) | <0.01k | 2.7 (2.2, 3.2) | 2.5 (2, 3) | 0.03k |
| Poultry | 3 (2, 4) | 3 (2, 4) | 3 (2, 3) | 0.01k | 3 (2, 4) | 3 (2, 3) | 0.58 k |
| Fish | 2 (1.5, 2) | 2 (1.5, 2) | 2 (1.5, 2.5) | 0.04k | 2 (1.5, 2) | 2 (1.5, 2) | 0.80 k |
| Vegetables | 3 (2.2, 3.5) | 3 (2.2, 3.5) | 3 (2.7, 3.7) | 0.17 k | 3 (2.2, 3.5) | 3 (2.2, 3.5) | 1.0 k |
| Potatoes | 3 (2.3) | 3 (2.3) | 3 (2.3) | 0.97 k | 3 (2.3) | 3 (2.3) | 0.99 k |
| Fruits | 2.8 (2, 3.6) | 2.8 (2, 3.6) | 3 (2.2, 3.8) | 0.06 k | 2.8 (2, 3.6) | 3 (2.25, 3.6) | 0.59 k |
| Legumes | 3 (2.3) | 3 (2.3) | 3 (3, 4) | 0.23 k | 3 (2, 3) | 3 (3, 3) | 0.99 k |
| Olive oil | 4 (3, 5) | 4 (2, 5) | 4 (3, 5) | <0.01k | 4 (3, 5) | 4 (2, 5) | 0.28 k |
| Alcohol intake | 1 (1, 2) | 1 (1, 2) | 1 (1, 2) | <0.01k | 1 (1, 2) | 1 (1, 2) | 0.02k |
| Quality of sleep | |||||||
| Quality of sleep score (range: 0–21), Median (IQR) | |||||||
| Quality of sleep score | 5 (3, 7) | 5 (3, 7) | 5 (3, 8) | <0.01k | 5 (3, 7) | 5 (3, 8) | 0.01k |
| Quality of sleep, n g (%) | |||||||
| Good (≤5) | 695 (61.0) | 547 (78.7) | 148 (21.3) | <0.01j | 590 (84.9) | 105 (15.1) | 0.02j |
| Poor (>5) | 445 (39.0) | 310 (69.7) | 135 (30.3) | 354 (79.5) | 91 (20.5) | ||
| Hours of sleeping, Mean (SD) | 6.6 ± 1.4 | 6.6 ± 1.4 | 6.3 ± 1.4 | <0.01i | 6.6 ± 1.4 | 6.4 ± 1.5 | 0.19 i |
| Minutes to fall asleep, Mean (SD) | 20.3 ± 23.8 | 19.9 ± 23.8 | 21.8 ± 23.7 | 0.24 i | 19.6 ± 22.9 | 23.9 ± 27.3 | 0.02i |
| Obesity | |||||||
| BMI (kg/m2), Mean (SD) | 25.0 ± 4.6 | 24.4 ± 14.7 | 26.7 ± 4.3 | <0.01i | 24.7 ± 4.4 | 26.2 ± 5.5 | <0.01i |
| BMI, n h (%) | |||||||
| Normal | 565 (50.4) | 467 (82.6) | 98 (17.4) | <0.01j | 486 (86.0) | 79 (14.0) | 0.01j |
| Underweight | 42 (3.7) | 41 (97.6) | 1 (2.4) | 36 (85.7) | 6 (14.3) | ||
| Overweight | 362 (32.3) | 241 (66.6) | 121 (33.4) | 290 (80.1) | 72 (19.9) | ||
| Obese | 152 (13.6) | 94 (61.8) | 58 (38.2) | 116 (76.3) | 36 (23.7) | ||
| Overall | Gender | Age Group | |||||
|---|---|---|---|---|---|---|---|
| Women | Men | 18–24 | 25–44 | 45–64 | 65+ | ||
| Cardiovascular diseases | |||||||
| Mediterranean diet score (per 1 unit) | 0.19 | 0.31 | 0.02 | 0.07 | 0.12 | 0.44 | 0.24 |
| Quality of sleep score (per 1 unit) | 0.37 | 0.47 | 0.17 | 0.54 | 0.45 | 0.22 | 0.14 |
| Current smoker (Yes, No) | 0.12 | 0.10 | 0.18 | 0.11 | 0.21 | 0.09 | 0.11 |
| Physically active (Yes, No) | −0.36 | −0.39 | −0.44 | −0.33 | −0.33 | −0.39 | −0.50 |
| BMI (per 1 kg/m2) | 0.78 | 0.67 | 0.92 | 0.73 | 0.75 | 0.79 | 0.72 |
| Endocrine diseases | |||||||
| Mediterranean Diet score (per 1 unit) | −0.13 | 0.39 | 0.08 | −0.07 | −0.30 | 0.19 | 0.43 |
| Quality of sleep score (per 1 unit) | 0.58 | −0.41 | 0.71 | 0.69 | 0.57 | 0.51 | −0.33 |
| Current smoker (Yes, No) | −0.28 | 0.34 | −0.21 | −0.43 | −0.25 | −0.08 | 0.56 |
| Physically active (Yes, No) | −0.34 | 0.41 | −0.23 | −0.30 | −0.21 | −0.27 | 0.67 |
| BMI (per 1 kg/m2) | 0.63 | −0.56 | 0.64 | 0.38 | 0.71 | 0.73 | −0.20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kyprianidou, M.; Panagiotakos, D.; Makris, K.C.; Kambanaros, M.; Christophi, C.A.; Giannakou, K. The Lifestyle Profile of Individuals with Cardiovascular and Endocrine Diseases in Cyprus: A Hierarchical, Classification Analysis. Nutrients 2022, 14, 1559. https://doi.org/10.3390/nu14081559
Kyprianidou M, Panagiotakos D, Makris KC, Kambanaros M, Christophi CA, Giannakou K. The Lifestyle Profile of Individuals with Cardiovascular and Endocrine Diseases in Cyprus: A Hierarchical, Classification Analysis. Nutrients. 2022; 14(8):1559. https://doi.org/10.3390/nu14081559
Chicago/Turabian StyleKyprianidou, Maria, Demosthenes Panagiotakos, Konstantinos C. Makris, Maria Kambanaros, Costas A. Christophi, and Konstantinos Giannakou. 2022. "The Lifestyle Profile of Individuals with Cardiovascular and Endocrine Diseases in Cyprus: A Hierarchical, Classification Analysis" Nutrients 14, no. 8: 1559. https://doi.org/10.3390/nu14081559
APA StyleKyprianidou, M., Panagiotakos, D., Makris, K. C., Kambanaros, M., Christophi, C. A., & Giannakou, K. (2022). The Lifestyle Profile of Individuals with Cardiovascular and Endocrine Diseases in Cyprus: A Hierarchical, Classification Analysis. Nutrients, 14(8), 1559. https://doi.org/10.3390/nu14081559







