Inhibition of In Vitro Infection of Hepatitis B Virus by Human Breastmilk
Abstract
:1. Introduction
2. Materials and Methods
2.1. Milk and Serum Samples and Preparation of Whey Fractions
2.2. Immunoassays for HBsAg and Hepatitis B e Antigen (HBeAg)
2.3. Competitive Inhibition Immunoassay for HBsAg-Binding Activity
2.4. Quantitative Assay of HBV DNA
2.5. Far-Western Blotting
2.6. Mass Spectrometry Analysis
2.7. Generation of HepG2-NTCP Cells
2.8. In Vitro HBV Culture and Inhibition of HBV Infection
2.9. Statistical Analysis
3. Results
3.1. Inhibition of Binding of HBsAg to Anti-HBs by Human Breastmilk
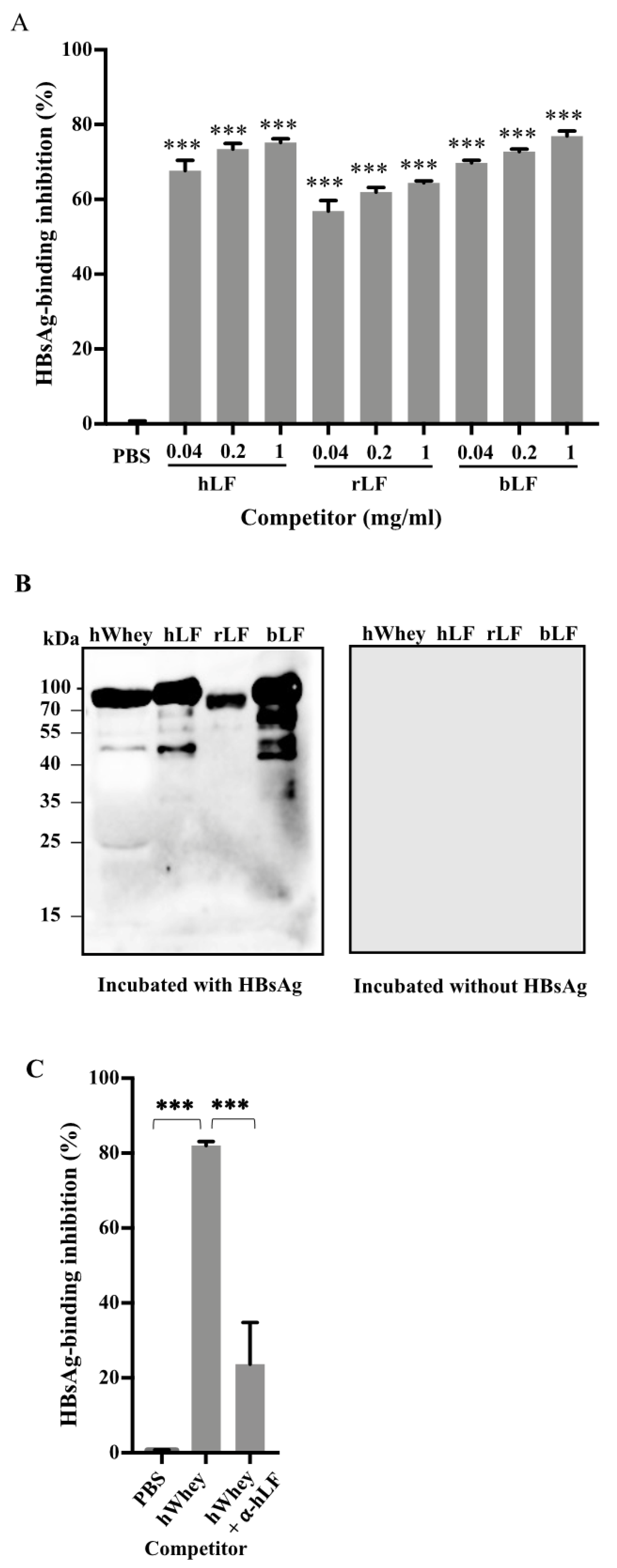
3.2. Identification of Lactoferrin as a Component in Human Whey to Bind to HBsAg
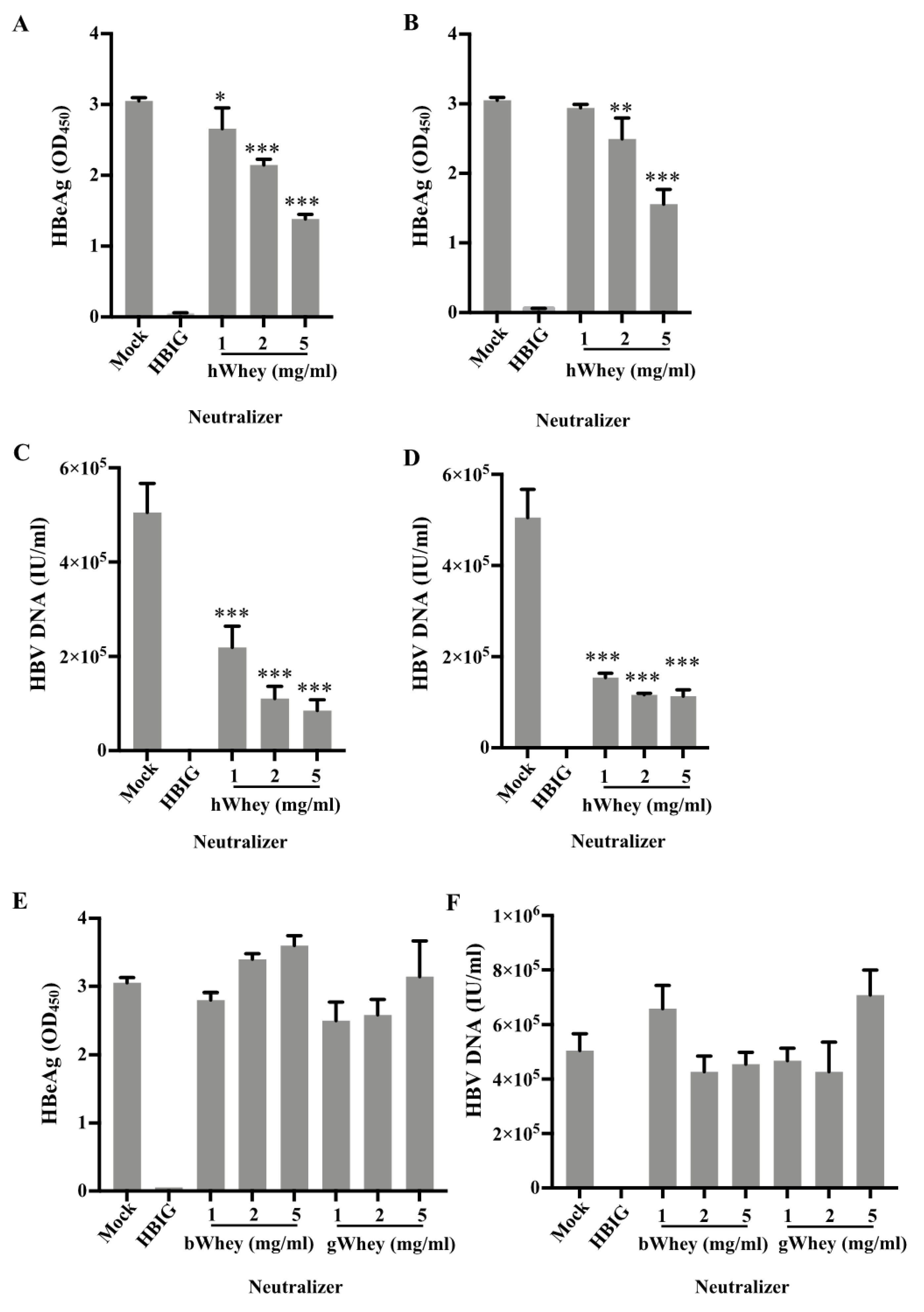
3.3. Inhibition of HBV Infectivity in HepG2-NTCP Cells by Human Whey
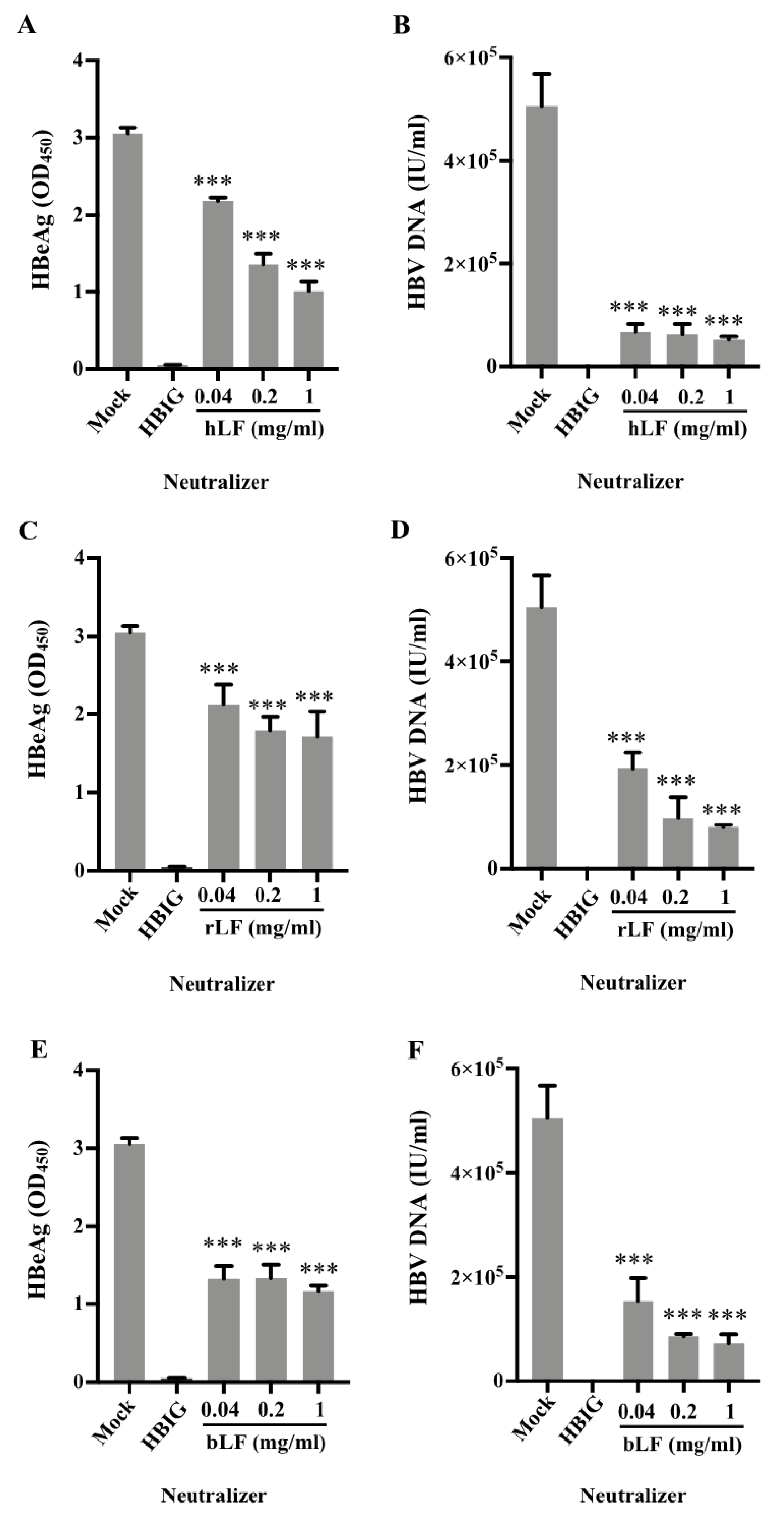
3.4. Inhibition of HBV Infectivity in HepG2-NTCP Cells by Human and Bovine Lactoferrin
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schweitzer, A.; Horn, J.; Mikolajczyk, R.T.; Krause, G.; Ott, J.J. Estimations of worldwide prevalence of chronic hepatitis B virus infection: A systematic review of data published between 1965 and 2013. Lancet 2015, 386, 1546–1555. [Google Scholar] [CrossRef]
- Tang, J.; Luo, Y.Q.; Zhou, Y.H. Elimination of hepatitis B virus infection in children: Experience and challenge in China. Chin. Med. J. 2021, 134, 2818–2824. [Google Scholar] [CrossRef] [PubMed]
- Linnemann, C.C., Jr.; Goldberg, S. Letter: HBAg in breast milk. Lancet 1974, 2, 155. [Google Scholar] [CrossRef]
- Lin, H.H.; Hsu, H.Y.; Chang, M.H.; Chen, P.J.; Chen, D.S. Hepatitis B virus in the colostra of HBeAg-positive carrier mothers. J. Pediatr. Gastroenterol. Nutr. 1993, 17, 207–210. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, P.R.; Yamamoto, A.Y.; de Souza, C.B.; de Araujo, N.M.; de Andrade Gomes, S.; Heck, A.R.; de Castro Figueiredo, J.F.; Mussi-Pinhata, M.M. Hepatitis B viral markers in banked human milk before and after Holder pasteurization. J. Clin. Virol. 2009, 45, 281–284. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Ning, M.; Feng, J.; Chen, J.; Dai, Y.; Hu, Y.; Zhou, Y.H. Hepatitis B Viral Markers in the Human Milk of HBsAg-Positive Mothers: An Observational Study. J. Hum. Lact. 2021, 8903344211043066. [Google Scholar] [CrossRef]
- Hill, J.B.; Sheffield, J.S.; Kim, M.J.; Alexander, J.M.; Sercely, B.; Wendel, G.D. Risk of hepatitis B transmission in breast-fed infants of chronic hepatitis B carriers. Obstet Gynecol. 2002, 99, 1049–1052. [Google Scholar] [CrossRef]
- Beasley, R.P.; Stevens, C.E.; Shiao, I.S.; Meng, H.C. Evidence against breast-feeding as a mechanism for vertical transmission of hepatitis B. Lancet 1975, 2, 740–741. [Google Scholar] [CrossRef]
- Derso, A.; Boxall, E.H.; Tarlow, M.J.; Flewett, T.H. Transmission of HBsAg from mother to infant in four ethnic groups. Br. Med. J. 1978, 1, 949–952. [Google Scholar] [CrossRef] [Green Version]
- Ballard, O.; Morrow, A.L. Human milk composition: Nutrients and bioactive factors. Pediatr. Clin. N. Am. 2013, 60, 49–74. [Google Scholar] [CrossRef] [Green Version]
- Frank, N.M.; Lynch, K.F.; Uusitalo, U.; Yang, J.; Lonnrot, M.; Virtanen, S.M.; Hyoty, H.; Norris, J.M.; Group, T.S. The relationship between breastfeeding and reported respiratory and gastrointestinal infection rates in young children. BMC Pediatr. 2019, 19, 339. [Google Scholar] [CrossRef] [PubMed]
- Clarke, N.M.; May, J.T. Effect of antimicrobial factors in human milk on rhinoviruses and milk-borne cytomegalovirus in vitro. J. Med. Microbiol. 2000, 49, 719–723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Habte, H.H.; Kotwal, G.J.; Lotz, Z.E.; Tyler, M.G.; Abrahams, M.; Rodriques, J.; Kahn, D.; Mall, A.S. Antiviral activity of purified human breast milk mucin. Neonatology 2007, 92, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Isaacs, C.E.; Kashyap, S.; Heird, W.C.; Thormar, H. Antiviral and antibacterial lipids in human milk and infant formula feeds. Arch. Dis. Child. 1990, 65, 861–864. [Google Scholar] [CrossRef] [Green Version]
- Gregory, K.E.; Walker, W.A. Immunologic Factors in Human Milk and Disease Prevention in the Preterm Infant. Curr. Pediatr. Rep. 2013, 1, 222–228. [Google Scholar] [CrossRef] [Green Version]
- Berlutti, F.; Pantanella, F.; Natalizi, T.; Frioni, A.; Paesano, R.; Polimeni, A.; Valenti, P. Antiviral properties of lactoferrin--a natural immunity molecule. Molecules 2011, 16, 6992–7018. [Google Scholar] [CrossRef] [Green Version]
- Kell, D.B.; Heyden, E.L.; Pretorius, E. The Biology of Lactoferrin, an Iron-Binding Protein That Can Help Defend Against Viruses and Bacteria. Front. Immunol. 2020, 11, 1221. [Google Scholar] [CrossRef]
- Dalmastri, C.; Valenti, P.; Visca, P.; Vittorioso, P.; Orsi, N. Enhanced antimicrobial activity of lactoferrin by binding to the bacterial surface. Microbiologica 1988, 11, 225–230. [Google Scholar]
- He, J.; Furmanski, P. Sequence specificity and transcriptional activation in the binding of lactoferrin to DNA. Nature 1995, 373, 721–724. [Google Scholar] [CrossRef]
- Fan, H.; Hong, B.; Luo, Y.; Peng, Q.; Wang, L.; Jin, X.; Chen, Y.; Hu, Y.; Shi, Y.; Li, T.; et al. The effect of whey protein on viral infection and replication of SARS-CoV-2 and pangolin coronavirus in vitro. Signal Transduct. Target. Ther. 2020, 5, 275. [Google Scholar] [CrossRef]
- Lai, X.; Yu, Y.; Xian, W.; Ye, F.; Ju, X.; Luo, Y.; Dong, H.; Zhou, Y.H.; Tan, W.; Zhuang, H.; et al. Identified human breastmilk compositions effectively inhibit SARS-CoV-2 and variants infection and replication. iScience 2022, 25, 104136. [Google Scholar] [CrossRef] [PubMed]
- Michailidis, E.; Pabon, J.; Xiang, K.; Park, P.; Ramanan, V.; Hoffmann, H.H.; Schneider, W.M.; Bhatia, S.N.; de Jong, Y.P.; Shlomai, A.; et al. A robust cell culture system supporting the complete life cycle of hepatitis B virus. Sci. Rep. 2017, 7, 16616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masson, P.L.; Heremans, J.F. Lactoferrin in milk from different species. Comp. Biochem. Physiol. B 1971, 39, 119–129. [Google Scholar] [CrossRef]
- Cheng, J.B.; Wang, J.Q.; Bu, D.P.; Liu, G.L.; Zhang, C.G.; Wei, H.Y.; Zhou, L.Y.; Wang, J.Z. Factors affecting the lactoferrin concentration in bovine milk. J. Dairy Sci. 2008, 91, 970–976. [Google Scholar] [CrossRef]
- Hara, K.; Ikeda, M.; Saito, S.; Matsumoto, S.; Numata, K.; Kato, N.; Tanaka, K.; Sekihara, H. Lactoferrin inhibits hepatitis B virus infection in cultured human hepatocytes. Hepatol. Res. 2002, 24, 228–235. [Google Scholar] [CrossRef]
- Florian, P.E.; Macovei, A.; Lazar, C.; Milac, A.L.; Sokolowska, I.; Darie, C.C.; Evans, R.W.; Roseanu, A.; Branza-Nichita, N. Characterization of the anti-HBV activity of HLP1-23, a human lactoferrin-derived peptide. J. Med. Virol. 2013, 85, 780–788. [Google Scholar] [CrossRef]
- Li, S.; Zhou, H.; Huang, G.; Liu, N. Inhibition of HBV infection by bovine lactoferrin and iron-, zinc-saturated lactoferrin. Med. Microbiol. Immunol. 2009, 198, 19–25. [Google Scholar] [CrossRef]
- Chen, X.; Chen, J.; Wen, J.; Xu, C.; Zhang, S.; Zhou, Y.H.; Hu, Y. Breastfeeding is not a risk factor for mother-to-child transmission of hepatitis B virus. PLoS ONE 2013, 8, e55303. [Google Scholar] [CrossRef] [Green Version]
- European Association for the Study of the Liver (EASL). EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J. Hepatol. 2017, 67, 370–398. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization (WHO). Prevention of Mother-to-Child Transmission of Hepatitis B Virus: Guidelines on Antiviral Prophylaxis in Pregnancy. In WHO Guidelines Approved by the Guidelines Review Committee; WHO: Geneva, Switzerland, 2020. [Google Scholar]
- Terrault, N.A.; Lok, A.S.F.; McMahon, B.J.; Chang, K.M.; Hwang, J.P.; Jonas, M.M.; Brown, R.S., Jr.; Bzowej, N.H.; Wong, J.B. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology 2018, 67, 1560–1599. [Google Scholar] [CrossRef]
- Zhou, Y.H.; Hu, Y.; Liu, X.; Yang, H. Chinese Society of Obstetrics and Gynecology (CSOG); Maternal-Fetal Medicine (MFM) Committee; Guideline: Management of Hepatitis B During Pregnancy and Prevention of Mother-to-Child Transmission of Hepatitis B Virus. Matern. Fetal Med. 2021, 3, 7–17. [Google Scholar] [CrossRef]
- Kumar, M.; Abbas, Z.; Azami, M.; Belopolskaya, M.; Dokmeci, A.K.; Ghazinyan, H.; Jia, J.; Jindal, A.; Lee, H.C.; Lei, W.; et al. Asian Pacific association for the study of liver (APASL) guidelines: Hepatitis B virus in pregnancy. Hepatol. Int. 2022, 1–43. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.; Salem, S.B.; Cohen, S.M. Evaluation and management of hepatitis B in pregnancy: A survey of current practices. Gastroenterol. Hepatol. 2010, 6, 570–578. [Google Scholar]
- Hu, Y.; Dai, X.; Zhou, Y.H.; Yang, H. A knowledge survey of obstetrics and gynecology staff on the prevention of mother-to-child transmission of hepatitis B virus. J. Infect. Dev. Ctries. 2013, 7, 391–397. [Google Scholar] [CrossRef] [Green Version]
- Tong Leung, V.K.; Lao, T.T.; Suen, S.S.; Chan, O.K.; Singh Sahota, D.; Lau, T.K.; Yeung Leung, T. Breastfeeding initiation: Is this influenced by maternal hepatitis B infection? J. Matern.-Fetal Neonatal Med. 2012, 25, 2390–2394. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, Y.; Xiang, K.; Liu, J.; Song, J.; Feng, J.; Chen, J.; Dai, Y.; Hu, Y.; Zhuang, H.; Zhou, Y. Inhibition of In Vitro Infection of Hepatitis B Virus by Human Breastmilk. Nutrients 2022, 14, 1561. https://doi.org/10.3390/nu14081561
Luo Y, Xiang K, Liu J, Song J, Feng J, Chen J, Dai Y, Hu Y, Zhuang H, Zhou Y. Inhibition of In Vitro Infection of Hepatitis B Virus by Human Breastmilk. Nutrients. 2022; 14(8):1561. https://doi.org/10.3390/nu14081561
Chicago/Turabian StyleLuo, Yuqian, Kuanhui Xiang, Jingli Liu, Ji Song, Jing Feng, Jie Chen, Yimin Dai, Yali Hu, Hui Zhuang, and Yihua Zhou. 2022. "Inhibition of In Vitro Infection of Hepatitis B Virus by Human Breastmilk" Nutrients 14, no. 8: 1561. https://doi.org/10.3390/nu14081561
APA StyleLuo, Y., Xiang, K., Liu, J., Song, J., Feng, J., Chen, J., Dai, Y., Hu, Y., Zhuang, H., & Zhou, Y. (2022). Inhibition of In Vitro Infection of Hepatitis B Virus by Human Breastmilk. Nutrients, 14(8), 1561. https://doi.org/10.3390/nu14081561





