Vitamin D Deficiency in Women with Breast Cancer: A Correlation with Osteoporosis? A Machine Learning Approach with Multiple Factor Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Outcome Measures
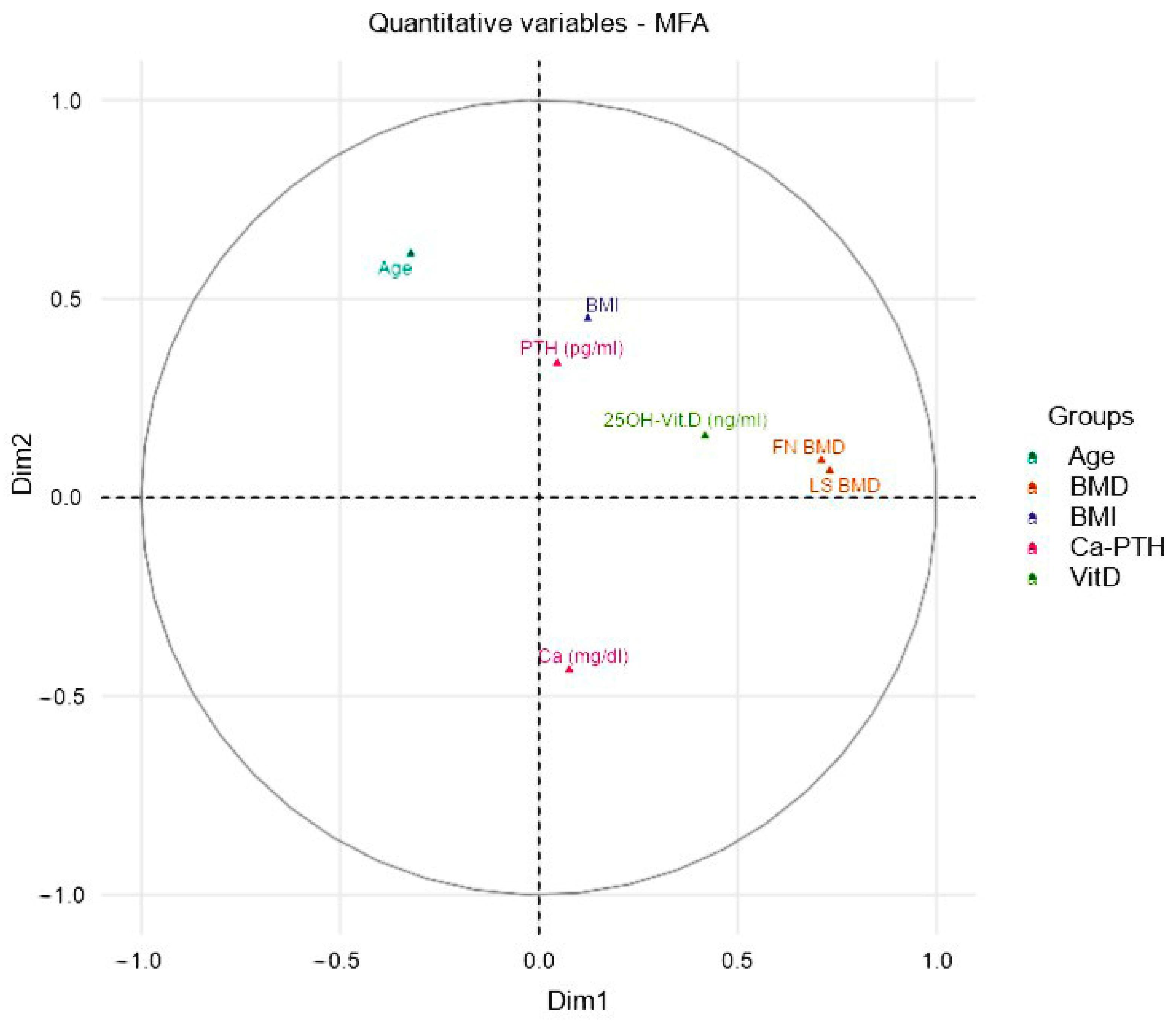
2.3. Multiple Factor Analysis
2.4. Data Management and Statistical Analysis
3. Results
3.1. Machine Learning Results
3.2. K-Means Clustering Model Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Iacoviello, L.; Bonaccio, M.; de Gaetano, G.; Donati, M.B. Epidemiology of breast cancer, a paradigm of the “common soil” hypothesis. Semin. Cancer Biol. 2021, 72, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Lima, S.M.; Kehm, R.D.; Terry, M.B. Global breast cancer incidence and mortality trends by region, age-groups, and fertility patterns. EClinicalMedicine 2021, 38, 100985. [Google Scholar] [CrossRef] [PubMed]
- Montagnese, C.; Porciello, G.; Vitale, S.; Palumbo, E.; Crispo, A.; Grimaldi, M.; Calabrese, I.; Pica, R.; Prete, M.; Falzone, L.; et al. Quality of Life in Women Diagnosed with Breast Cancer after a 12-Month Treatment of Lifestyle Modifications. Nutrients 2020, 13, 136. [Google Scholar] [CrossRef] [PubMed]
- Paolucci, T.; Saggino, A.; Agostini, F.; Paoloni, M.; Bernetti, A.; Mangone, M.; Santilli, V.; Saggini, R.; Tommasi, M. The influence of rehabilitation on quality of life in breast cancer survivors: A clinical study. Int. J. Environ. Res. Public Health 2021, 18, 8585. [Google Scholar] [CrossRef] [PubMed]
- Waqas, K.; Lima Ferreira, J.; Tsourdi, E.; Body, J.J.; Hadji, P.; Zillikens, M.C. Updated guidance on the management of cancer treatment-induced bone loss (CTIBL) in pre- and postmenopausal women with early-stage breast cancer. J. Bone Oncol. 2021, 28, 100355. [Google Scholar] [CrossRef] [PubMed]
- Trémollieres, F.A. Screening for osteoporosis after breast cancer: For whom, why and when. Maturitas 2014, 79, 343–348. [Google Scholar] [CrossRef]
- Scaturro, D.; de Sire, A.; Terrana, P.; Curci, C.; Vitagliani, F.; Falco, V.; Cuntrera, D.; Iolascon, G.; Mauro, G.L. Early Denosumab for the prevention of osteoporotic fractures in breast cancer women undergoing aromatase inhibitors: A case-control retrospective study. J. Back Musculoskelet. Rehabil. 2022, 35, 207–212. [Google Scholar] [CrossRef]
- Shapiro, C.L. Osteoporosis: A long-term and late-effect of breast cancer treatments. Cancers 2020, 12, 3094. [Google Scholar] [CrossRef]
- De Sire, A.; Ferrillo, M.; Gennari, A.; Cisari, C.; Pasqua, S.; Foglio Bonda, P.L.; Invernizzi, M.; Migliario, M. Bone health, vitamin d status and oral hygiene screening in breast cancer women before starting osteoporosis treatment: A cross-sectional study. J. Biol. Regul. Homeost. Agents 2021, 35, 287–292. [Google Scholar] [CrossRef]
- Shapiro, C.L. Management of osteoporosis in women with breast cancer. Breast Cancer Manag. 2020, 9, BMT40. [Google Scholar] [CrossRef]
- Hadji, P. Cancer Treatment-Induced Bone Loss in women with breast cancer. Bonekey Rep. 2015, 4, 692. [Google Scholar] [CrossRef]
- De Sire, A.; Lippi, L.; Venetis, K.; Morganti, S.; Sajjadi, E.; Curci, C.; Ammendolia, A.; Criscitiello, C.; Fusco, N.; Invernizzi, M. Efficacy of Antiresorptive Drugs on Bone Mineral Density in Post-Menopausal Women with Early Breast Cancer Receiving Adjuvant Aromatase Inhibitors: A Systematic Review of Randomized Controlled Trials. Front. Oncol. 2022, 11, 829875. [Google Scholar] [CrossRef]
- McNeish, B.L.; Richardson, J.K.; Bell, S.G.; Whitney, D.G. Chemotherapy-induced peripheral neuropathy increases nontraumatic fracture risk in breast cancer survivors. JBMR Plus 2021, 5, e10519. [Google Scholar] [CrossRef]
- De Matos, L.V.; Fernandes, L.; Neves, M.T.; Alves, F.; Baleiras, M.; Ferreira, A.; Cotovio, P.G.; Domingues, T.D.; Malheiro, M.; Plácido, A.; et al. From theory to practice: Bone health in women with early breast cancer treated with aromatase inhibitors. Curr. Oncol. 2021, 28, 104. [Google Scholar] [CrossRef]
- Stumpf, U.; Kostev, K.; Siebenbürger, G.; Böcker, W.; Hadji, P. Influence of chemotherapy and endocrine treatment on fractures in postmenopausal women with breast cancer—A retrospective cohort study. J. Bone Oncol. 2020, 22, 100292. [Google Scholar] [CrossRef]
- Diana, A.; Carlino, F.; Giunta, E.F.; Franzese, E.; Guerrera, L.P.; Di Lauro, V.; Ciardiello, F.; Daniele, B.; Orditura, M. Cancer Treatment–Induced Bone Loss (CTIBL): State of the Art and Proper Management in Breast Cancer Patients on Endocrine Therapy. Curr. Treat. Options Oncol. 2021, 22, 45. [Google Scholar] [CrossRef]
- Sharifi, F.; Sharifi, N. The effect of educational intervention on lifestyle modification associated with osteoporosis in female students. Iran. J. Obstet. Gynecol. Infertil. 2017, 20, 36–43. [Google Scholar] [CrossRef]
- Mahadik, A.B.; Giri, A.B.; Bhambre, A.S.; Mundhe, S.A.; Ramdas, D. Shinde Osteoporosis and lifestyle medicine. GSC Biol. Pharm. Sci. 2021, 14, 155–165. [Google Scholar] [CrossRef]
- Agostini, D.; Zeppa, S.D.; Lucertini, F.; Annibalini, G.; Gervasi, M.; Marini, C.F.; Piccoli, G.; Stocchi, V.; Barbieri, E.; Sestili, P. Muscle and bone health in postmenopausal women: Role of protein and vitamin d supplementation combined with exercise training. Nutrients 2018, 10, 1103. [Google Scholar] [CrossRef] [Green Version]
- LeBoff, M.S.; Murata, E.M.; Cook, N.R.; Cawthon, P.; Chou, S.H.; Kotler, G.; Bubes, V.; Buring, J.E.; Manson, J.A.E. VITamin D and OmegA-3 TriaL (VITAL): Effects of Vitamin D Supplements on Risk of Falls in the US Population. J. Clin. Endocrinol. Metab. 2020, 105, 2929–2938. [Google Scholar] [CrossRef]
- Eleni, A.; Panagiotis, P. A systematic review and meta-analysis of vitamin D and calcium in preventing osteoporotic fractures. Clin. Rheumatol. 2020, 39, 3571–3579. [Google Scholar] [CrossRef] [PubMed]
- Hage, M.P.; El-Hajj Fuleihan, G. Bone and mineral metabolism in patients undergoing Roux-en-Y gastric bypass. Osteoporos. Int. 2014, 25, 423–439. [Google Scholar] [CrossRef] [PubMed]
- Van Poznak, C.H. Bone Health in Adults Treated with Endocrine Therapy for Early Breast or Prostate Cancer. Am. Soc. Clin. Oncol. Educ. B. 2015, 35, e567–e574. [Google Scholar] [CrossRef] [PubMed]
- Yao, P.; Bennett, D.; Mafham, M.; Lin, X.; Chen, Z.; Armitage, J.; Clarke, R. Vitamin D and Calcium for the Prevention of Fracture: A Systematic Review and Meta-analysis. JAMA Netw. Open 2019, 2, e1917789. [Google Scholar] [CrossRef] [PubMed]
- McCabe, M.P.; Smyth, M.P.; Richardson, D.R. Current concept review: Vitamin D and stress fractures. Foot Ankle Int. 2012, 33, 526–533. [Google Scholar] [CrossRef]
- Ciebiera, M.; Ali, M.; Prince, L.; Zgliczyński, S.; Jakiel, G.; Al-Hendy, A. The Significance of Measuring Vitamin D Serum Levels in Women with Uterine Fibroids. Reprod. Sci. 2021, 28, 2098–2109. [Google Scholar] [CrossRef]
- Tuckey, R.C.; Cheng, C.Y.S.; Slominski, A.T. The serum vitamin D metabolome: What we know and what is still to discover. J. Steroid Biochem. Mol. Biol. 2019, 186, 4–21. [Google Scholar] [CrossRef]
- Bislev, L.S.; Sundekilde, U.K.; Kilic, E.; Dalsgaard, T.K.; Rejnmark, L.; Bertram, H.C. Circulating levels of muscle-related metabolites increase in response to a daily moderately high dose of a vitamin d3 supplement in women with vitamin d insufficiency—secondary analysis of a randomized placebo-controlled trial. Nutrients 2020, 12, 1310. [Google Scholar] [CrossRef]
- Chiodini, I.; Gatti, D.; Soranna, D.; Merlotti, D.; Mingiano, C.; Fassio, A.; Adami, G.; Falchetti, A.; Eller-Vainicher, C.; Rossini, M.; et al. Vitamin D Status and SARS-CoV-2 Infection and COVID-19 Clinical Outcomes. Front Public Health. 2021, 9, 736665. [Google Scholar] [CrossRef]
- Gallelli, L.; Mannino, G.C.; Luciani, F.; de Sire, A.; Mancuso, E.; Gangemi, P.; Cosco, L.; Monea, G.; Averta, C.; Minchella, P.; et al. Vitamin D Serum Levels in Subjects Tested for SARS-CoV-2: What Are the Differences among Acute, Healed, and Negative COVID-19 Patients? A Multicenter Real-Practice Study. Nutrients 2021, 13, 3932. [Google Scholar] [CrossRef]
- Gallelli, L.; Michniewicz, A.; Cione, E.; Squillace, A.; Colosimo, M.; Pelaia, C.; Fazio, A.; Zampogna, S.; Peltrone, F.; Iannacchero, R.; et al. 25-hydroxy vitamin D detection using different analytic methods in patients with migraine. J. Clin. Med. 2019, 8, 895. [Google Scholar] [CrossRef] [Green Version]
- Pignolo, A.; Mastrilli, S.; Davì, C.; Arnao, V.; Aridon, P.; Dos Santos Mendes, F.A.; Gagliardo, C.; D’Amelio, M. Vitamin D and Parkinson’s Disease. Nutrients 2022, 14, 1220. [Google Scholar] [CrossRef]
- Bertoldo, E.; Adami, G.; Rossini, M.; Giollo, A.; Orsolini, G.; Viapiana, O.; Gatti, D.; Fassio, A. The Emerging Roles of Endocrine Hormones in Different Arthritic Disorders. Front Endocrinol. 2021, 12, 620920. [Google Scholar] [CrossRef]
- Ferrillo, M.; Migliario, M.; Roccuzzo, A.; Molinero-Mourelle, P.; Falcicchio, G.; Umano, G.R.; Pezzotti, F.; Foglio Bonda, P.L.; Calafiore, D.; de Sire, A. Periodontal Disease and Vitamin D Deficiency in Pregnant Women: Which Correlation with Preterm and Low-Weight Birth? J. Clin. Med. 2021, 10, 4578. [Google Scholar] [CrossRef]
- Kling, J.M.; Clarke, B.L.; Sandhu, N.P. Osteoporosis prevention, screening, and treatment: A review. J. Women’s Health 2014, 23, 563–572. [Google Scholar] [CrossRef]
- Benarba, B.; Gouri, A. Role of vitamin D in breast cancer prevention and therapy: Recent findings. J. Med. 2020, 21, 46–50. [Google Scholar] [CrossRef]
- Datta, M.; Schwartz, G.G. Calcium and vitamin D supplementation and loss of bone mineral density in women undergoing breast cancer therapy. Crit. Rev. Oncol. Hematol. 2013, 88, 613–624. [Google Scholar] [CrossRef] [Green Version]
- Hossain, S.; Beydoun, M.A.; Beydoun, H.A.; Chen, X.; Zonderman, A.B.; Wood, R.J. Vitamin D and breast cancer: A systematic review and meta-analysis of observational studies. Clin. Nutr. ESPEN 2019, 30, 170–184. [Google Scholar] [CrossRef]
- Al-Azhri, J.; Zhang, Y.; Bshara, W.; Zirpoli, G.; McCann, S.E.; Khoury, T.; Morrison, C.D.; Edge, S.B.; Ambrosone, C.B.; Yao, S. Tumor expression of vitamin D receptor and breast cancer histopathological characteristics and prognosis. Clin. Cancer Res. 2017, 23, 97–103. [Google Scholar] [CrossRef] [Green Version]
- Goodwin, P.J.; Ennis, M.; Pritchard, K.I.; Koo, J.; Hood, N. Prognostic effects of 25-hydroxyvitamin D levels in early breast cancer. J. Clin. Oncol. 2009, 27, 3757–3763. [Google Scholar] [CrossRef]
- Chlebowski, R.T. Vitamin D and Breast Cancer Incidence and Outcome. Anticancer. Agents Med. Chem. 2012, 13, 98–106. [Google Scholar] [CrossRef]
- Huss, L.; Butt, S.T.; Borgquist, S.; Elebro, K.; Sandsveden, M.; Rosendahl, A.; Manjer, J. Vitamin D receptor expression in invasive breast tumors and breast cancer survival. Breast Cancer Res. 2019, 21, 84. [Google Scholar] [CrossRef] [PubMed]
- Deluca, G.C.; Kimball, S.M.; Kolasinski, J.; Ramagopalan, S.V.; Ebers, G.C. Review: The role of vitamin D in nervous system health and disease. Neuropathol. Appl. Neurobiol. 2013, 39, 458–484. [Google Scholar] [CrossRef] [PubMed]
- Carlberg, C.; Muñoz, A. An update on vitamin D signaling and cancer. Semin. Cancer Biol. 2022, 79, 217–230. [Google Scholar] [CrossRef] [PubMed]
- Murray, A.; Madden, S.F.; Synnott, N.C.; Klinger, R.; O’Connor, D.; O’Donovan, N.; Gallagher, W.; Crown, J.; Duffy, M.J. Vitamin D receptor as a target for breast cancer therapy. Endocr. Relat. Cancer 2017, 24, 181–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jenkinson, C. The vitamin D metabolome: An update on analysis and function. Cell Biochem. Funct. 2019, 37, 408–423. [Google Scholar] [CrossRef] [PubMed]
- Bernhardt, S.M.; Borges, V.F.; Schedin, P. Vitamin d as a potential preventive agent for young women’s breast cancer. Cancer Prev. Res. 2021, 14, 825–838. [Google Scholar] [CrossRef] [PubMed]
- Back, J.; Lee, Y.K.; Nizami, S.; Soung, D.Y.; Song, L.; Lee, F.Y. Dissecting out multiple mechanisms of breast cancer-induced bone destruction: Molecular Evidences. J. Orthop. Res. 2017, 35. Available online: http://www.ors.org/Transactions/63/2249.pdf (accessed on 30 March 2022).
- Young, A.; Bu, W.; Jiang, W.; Ku, A.; Kapali, J.; Dhamne, S.; Qin, L.; Hilsenbeck, S.G.; Du, Y.C.N.; Li, Y. Targeting the Pro-survival Protein BCL-2 to Prevent Breast Cancer. Cancer Prev. Res. 2022, 15, 3–10. [Google Scholar] [CrossRef]
- Wang, W.T.; Liang, J.H.; Wang, L.; Zhu, H.Y.; Xia, Y.; Fan, L.; Li, J.Y.; Xu, W. The prognostic value of 25-hydroxy vitamin D deficiency and its interaction with c-Myc expression in diffuse large B cell lymphoma. Ann. Hematol. 2020, 99, 2377–2384. [Google Scholar] [CrossRef]
- Holder, L.B.; Haque, M.M.; Skinner, M.K. Machine learning for epigenetics and future medical applications. Epigenetics 2017, 12, 505–514. [Google Scholar] [CrossRef] [Green Version]
- Banegas-Luna, A.J.; Peña-García, J.; Iftene, A.; Guadagni, F.; Ferroni, P.; Scarpato, N.; Zanzotto, F.M.; Bueno-Crespo, A.; Pérez-Sánchez, H. Towards the interpretability of machine learning predictions for medical applications targeting personalised therapies: A cancer case survey. Int. J. Mol. Sci. 2021, 22, 4394. [Google Scholar] [CrossRef]
- Cuocolo, R.; Caruso, M.; Perillo, T.; Ugga, L.; Petretta, M. Machine Learning in oncology: A clinical appraisal. Cancer Lett. 2020, 481, 55–62. [Google Scholar] [CrossRef]
- Liu, H.; Guan, J.; Li, H.; Bao, Z.; Wang, Q.; Luo, X.; Xue, H. Predicting the Disease Genes of Multiple Sclerosis Based on Network Representation Learning. Front. Genet. 2020, 11, 328. [Google Scholar] [CrossRef] [Green Version]
- Pinto, M.; Marotta, N.; Caracò, C.; Simeone, E.; Ammendolia, A.; de Sire, A. Quality of Life Predictors in Patients With Melanoma: A Machine Learning Approach. Front. Oncol. 2022, 12, 843611. [Google Scholar] [CrossRef]
- Bécue-Bertaut, M.; Pagès, J. Multiple factor analysis and clustering of a mixture of quantitative, categorical and frequency data. Comput. Stat. Data Anal. 2008, 52, 3255–3268. [Google Scholar] [CrossRef]
- Abdi, H.; Williams, L.J.; Valentin, D. Multiple factor analysis: Principal component analysis for multitable and multiblock data sets. Wiley Interdiscip. Rev. Comput. Stat. 2013, 5, 149–179. [Google Scholar] [CrossRef]
- Fugere, T.; Chen, Z.J.; Makhoul, I. Practical vitamin D supplementation using machine learning. J. Bone Metab. 2020, 27, 111–117. [Google Scholar] [CrossRef]
- Escala-Garcia, M.; Morra, A.; Canisius, S.; Chang-Claude, J.; Kar, S.; Zheng, W.; Bojesen, S.E.; Easton, D.; Pharoah, P.D.P.; Schmidt, M.K. Breast cancer risk factors and their effects on survival: A Mendelian randomisation study. BMC Med. 2020, 18, 327. [Google Scholar] [CrossRef]
- Li, H.; Giger, M.L. Artificial intelligence and interpretations in breast cancer imaging. In Artificial Intelligence in Medicine; Academic Press: Cambridge, MA, USA, 2021; pp. 291–308. [Google Scholar] [CrossRef]
- Malherbe, K. Tumor Microenvironment and the Role of Artificial Intelligence in Breast Cancer Detection and Prognosis. Am. J. Pathol. 2021, 191, 1364–1373. [Google Scholar] [CrossRef]
- Asparouhov, T.; Muthén, B. Multiple-Group Factor Analysis Alignment. Struct. Equ. Model. 2014, 21, 495–508. [Google Scholar] [CrossRef]
- Juhász, F. On the Theoretical Backgrounds of Cluster Analysis Based on the Eigenvalue Problem of the Association Matrix. Statistics 1989, 20, 573–581. [Google Scholar] [CrossRef]
- Allefeld, C.; Müller, M.; Kurths, J. Eigenvalue decomposition as a generalized synchronization cluster analysis. Int. J. Bifurc. Chaos 2007, 17, 3493–3497. [Google Scholar] [CrossRef] [Green Version]
- Ferro, S.; Bottigliengo, D.; Gregori, D.; Fabricio, A.S.C.; Gion, M.; Baldi, I. Phenomapping of patients with primary breast cancer using machine learning-based unsupervised cluster analysis. J. Pers. Med. 2021, 11, 272. [Google Scholar] [CrossRef] [PubMed]
- Lê, S.; Josse, J.; Husson, F. FactoMineR: An R package for multivariate analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Irnawati, I.; Riswanto, F.D.O.; Riyanto, S.; Martono, S.; Rohman, A. The use of software packages of R factoextra and FactoMineR and their application in principal component analysis for authentication of oils. Indones. J. Chemom. Pharm. Anal. 2020, 1. [Google Scholar] [CrossRef]
- Tibshirani, R.; Walther, G.; Hastie, T. Estimating the number of clusters in a data set via the gap statistic. J. R. Stat. Soc. Ser. B Stat. Methodol. 2001, 63, 411–423. [Google Scholar] [CrossRef]
- Semjon, B.; Marcinčáková, D.; Koréneková, B.; Bartkovský, M.; Nagy, J.; Turek, P.; Marcinčák, S. Multiple factorial analysis of physicochemical and organoleptic properties of breast and thigh meat of broilers fed a diet supplemented with humic substances. Poult. Sci. 2020, 99, 1750–1760. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, H.; Li, G.H.Y.; Long, M.T.; Cheung, C.L.; Vasan, R.S.; Hsu, Y.H.; Kiel, D.P.; Liu, C.T. Metabolomics Insights into Osteoporosis Through Association With Bone Mineral Density. J. Bone Miner. Res. 2021, 36, 729–738. [Google Scholar] [CrossRef]
- Shevroja, E.; Cafarelli, F.P.; Guglielmi, G.; Hans, D. DXA parameters, Trabecular Bone Score (TBS) and Bone Mineral Density (BMD), in fracture risk prediction in endocrine-mediated secondary osteoporosis. Endocrine 2021, 74, 20–28. [Google Scholar] [CrossRef]
- Drudge-Coates, L.; van Muilekom, E.; de la Torre-Montero, J.C.; Leonard, K.; van Oostwaard, M.; Niepel, D.; Jensen, B.T. Management of bone health in patients with cancer: A survey of specialist nurses. Support. Care Cancer 2020, 28, 1151–1162. [Google Scholar] [CrossRef] [Green Version]
- Fukumoto, S.; Matsumoto, T. Cancer treatment-induced bone loss (CTIBL). Jpn. J. Cancer Chemother. 2018, 45, 1685–1689. [Google Scholar] [CrossRef]
- Allison, R.J.; Farooq, A.; Cherif, A.; Hamilton, B.; Close, G.L.; Wilson, M.G. Why don’t serum Vitamin D concentrations associate with BMD by DXA? A case of being â “bound” to the wrong assay? Implications for Vitamin D screening. Br. J. Sports Med. 2018, 52, 522–526. [Google Scholar] [CrossRef]
- Gradishar, W.J.; Anderson, B.O.; Abraham, J.; Aft, R.; Agnese, D.; Allison, K.H.; Blair, S.L.; Burstein, H.J.; Dang, C.; Elias, A.D.; et al. Breast cancer, version 3.2020. JNCCN J. Natl. Compr. Cancer Netw. 2020, 18, 452–478. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Liu, R.; Zhu, H.; Zhou, D.; Mei, Q.; Xu, G. Vitamin D receptor Fok i polymorphism is associated with low bone mineral density in postmenopausal women: A meta-analysis focused on populations in Asian countries. Eur. J. Obstet. Gynecol. Reprod. Biol. 2013, 169, 380–386. [Google Scholar] [CrossRef]
- Annalora, A.J.; Jozic, M.; Marcus, C.B.; Iversen, P.L. Alternative splicing of the vitamin D receptor modulates target gene expression and promotes ligand-independent functions. Toxicol. Appl. Pharmacol. 2019, 364, 55–67. [Google Scholar] [CrossRef]
- Zhipeng Ai, J.Z.; Hong Liu, X.N. The Association between Vitamin D Receptor FokI Gene Polymorphism and Osteoporosis in Postmenopausal Women: A Meta-Analysis. J. Osteoporos. Phys. Act. 2015, 3, 2. [Google Scholar] [CrossRef] [Green Version]
- Yadav, U.; Kumar, P.; Rai, V. Vitamin D receptor (VDR) gene FokI, BsmI, ApaI, and TaqI polymorphisms and osteoporosis risk: A meta-analysis. Egypt. J. Med. Hum. Genet. 2020, 21, 15. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.; Peters, M.; Mueller, R.O. Correlational analysis of ordinal data: From Pearson’s r to Bayesian polychoric correlation. Asia Pacific Educ. Rev. 2010, 11, 459–466. [Google Scholar] [CrossRef]
- Adler, J.; Parmryd, I. Quantifying colocalization by correlation: The pearson correlation coefficient is superior to the Mander’s overlap coefficient. Cytom. Part A 2010, 77, 733–742. [Google Scholar] [CrossRef]
- Feng, W.; Zhu, Q.; Zhuang, J.; Yu, S. An expert recommendation algorithm based on Pearson correlation coefficient and FP-growth. Cluster Comput. 2019, 22, 7401–7412. [Google Scholar] [CrossRef]
- Visbal-Cadavid, D.; Mendoza-Mendoza, A.; Hoz-Dominguez, E.D. La Use of Factorial Analysis of Mixed Data (FAMD) and Hierarchical Cluster Analysis on Principal Component (HCPC) for Multivariate Analysis of Academic Performance of Industrial Engineering Programs. J. Southwest Jiaotong Univ. 2020, 55. [Google Scholar] [CrossRef]
- Shapiro, C.L.; Lacchetti, C.; Neuner, J. Management of osteoporosis in survivors of adult cancers with nonmetastatic disease: ASCO clinical practice guideline summary. J. Oncol. Pract. 2019, 15, 665–669. [Google Scholar] [CrossRef]
- Migliaccio, S.; de Sire, A.; Marocco, C.; Fornari, R.; Paoletta, M.; Greco, E.A.; Amar, I.D.; Moretti, A.; Ronzoni, S.; Gimigliano, F.; et al. Approach in aromatase inhibitors—Induced osteoporosis: Results from an Italian multicenter observational study. Clin. Cases Miner. Bone Metab. 2018, 15, 334–339. [Google Scholar]
- Iravani, M.; Lagerquist, M.; Ohlsson, C.; Savendahl, L. Regulation of bone growth via ligand-specific activation of estrogen receptor alpha. J. Endocrinol. 2017, 232, 403–410. [Google Scholar] [CrossRef]
- Li, P.; Sundh, D.; Ji, B.; Lappa, D.; Ye, L.; Nielsen, J.; Lorentzon, M. Metabolic Alterations in Older Women With Low Bone Mineral Density Supplemented With Lactobacillus reuteri. JBMR Plus 2021, 5, e10478. [Google Scholar] [CrossRef]
- Zhao, Q.; Shen, H.; Su, K.J.; Zhang, J.G.; Tian, Q.; Zhao, L.J.; Qiu, C.; Zhang, Q.; Garrett, T.J.; Liu, J.; et al. Metabolomic profiles associated with bone mineral density in US Caucasian women. Nutr. Metab. 2018, 15, 57. [Google Scholar] [CrossRef]
- Miyamoto, T.; Hirayama, A.; Sato, Y.; Koboyashi, T.; Katsuyama, E.; Kanagawa, H.; Miyamoto, H.; Mori, T.; Yoshida, S.; Fujie, A.; et al. A serum metabolomics-based profile in low bone mineral density postmenopausal women. Bone 2017, 95, 1–4. [Google Scholar] [CrossRef]
- Razzaq, A.; Sadia, B.; Raza, A.; Hameed, M.K.; Saleem, F. Metabolomics: A way forward for crop improvement. Metabolites 2019, 9, 303. [Google Scholar] [CrossRef] [Green Version]
- Canuto, G.A.B.; Da Costa, J.L.; Da Cruz, P.L.R.; De Souza, A.R.L.; Faccio, A.T.; Klassen, A.; Rodrigues, K.T.; Tavares, M.F.M. Metabolômica: Definições, Estado-Da-Arte E Aplicações Representativas. Quim. Nova 2018, 41, 75–91. [Google Scholar] [CrossRef]
- Palacios-González, B.; Ramírez-Salazar, E.G.; Rivera-Paredez, B.; Quiterio, M.; Flores, Y.N.; Macias-Kauffer, L.; Moran-Ramos, S.; Denova-Gutiérrez, E.; Ibarra-González, I.; Vela-Amieva, M.; et al. A multi-omic analysis for low bone mineral density in postmenopausal women suggests a relationship between diet, metabolites, and microbiota. Microorganisms 2020, 8, 1630. [Google Scholar] [CrossRef]
- De Sire, A.; Marotta, N.; Marinaro, C.; Curci, C.; Invernizzi, M.; Ammendolia, A. Role of Physical Exercise and Nutraceuticals in Modulating Molecular Pathways of Osteoarthritis. Int. J. Mol. Sci. 2021, 22, 5722. [Google Scholar] [CrossRef]
- Casneuf, T.; Axel, A.E.; King, P.; Alvarez, J.D.; Werbeck, J.L.; Verhulst, T.; Verstraeten, K.; Hall, B.M.; Sasser, A.K. Interleukin-6 is a potential therapeutic target in interleukin-6 dependent, estrogen receptor-α- Positive breast cancer. Breast Cancer Targets Ther. 2016, 8, 13–27. [Google Scholar] [CrossRef] [Green Version]
- Iorio, G.C.; Ammendolia, A.; Marotta, N.; Ricardi, U.; de Sire, A. A bond between rheumatic diseases and cancer in the elderly: The interleukin-6 pathway. Int. J. Rheum. Dis. 2021, 24, 1317–1320. [Google Scholar] [CrossRef]
- Elnenaei, M.O.; Chandra, R.; Mangion, T.; Moniz, C. Genomic and metabolomic patterns segregate with responses to calcium and vitamin D supplementation. Br. J. Nutr. 2011, 105, 71–79. [Google Scholar] [CrossRef]
- Filip-Psurska, B.; Psurski, M.; Anisiewicz, A.; Libako, P.; Zbrojewicz, E.; Maciejewska, M.; Chodyński, M.; Kutner, A.; Wietrzyk, J. Vitamin d compounds pri-2191 and pri-2205 enhance anastrozole activity in human breast cancer models. Int. J. Mol. Sci. 2021, 22, 2781. [Google Scholar] [CrossRef]
- Silva-Sousa, A.C.; Mazzi-Chaves, J.F.; Freitas, J.V.; Salles, A.G.; Segato, R.A.B.; da Silva, L.A.B.; Antunes, L.A.A.; Antunes, L.S.; Baratto-Filho, F.; Sousa-Neto, M.D.; et al. Association between estrogen, vitamin D and microrna17 gene polymorphisms and periapical lesions. Braz. Dent. J. 2020, 31, 19–24. [Google Scholar] [CrossRef]
- Nepal, A.K.; van Essen, H.W.; van der Veen, A.J.; van Wieringen, W.N.; Stavenuiter, A.W.D.; Cayami, F.K.; Pals, G.; Micha, D.; Vanderschueren, D.; Lips, P.; et al. Mechanical stress regulates bone regulatory gene expression independent of estrogen and vitamin D deficiency in rats. J. Orthop. Res. 2021, 39, 42–52. [Google Scholar] [CrossRef]
- Paolino, S.; Pacini, G.; Schenone, C.; Patanè, M.; Sulli, A.; Sukkar, S.G.; Lercara, A.; Pizzorni, C.; Gotelli, E.; Cattelan, F.; et al. Nutritional status and bone microarchitecture in a cohort of systemic sclerosis patients. Nutrients 2020, 12, 1632. [Google Scholar] [CrossRef]
- Ozaki, D.; Kubota, R.; Maeno, T.; Abdelhakim, M.; Hitosugi, N. Association between gut microbiota, bone metabolism, and fracture risk in postmenopausal Japanese women. Osteoporos. Int. 2021, 32, 145–156. [Google Scholar] [CrossRef]
- Van Cromphaut, S.J.; Rummens, K.; Stockmans, I.; Van Herck, E.; Dijcks, F.A.; Ederveen, A.G.H.; Carmeliet, P.; Verhaeghe, J.; Bouillon, R.; Carmeliet, G. Intestinal Calcium Transporter Genes Are Upregulated by Estrogens and the Reproductive Cycle Through Vitamin D Receptor-Independent Mechanisms. J. Bone Miner. Res. 2003, 18, 1725–1736. [Google Scholar] [CrossRef] [PubMed]
- Singh, T.; Adams, B.D. The regulatory role of miRNAs on VDR in breast cancer. Transcription 2017, 8, 232–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Mean age (years) | 67.29 | ± | 8.16 |
| BMI (kg/m2) | 24.4 | ± | 4.24 |
| Smokers (n, %) | 17 | (31.48) | |
| Grade 1 (n, %) | 9 | (16.66) | |
| Grade 2 (n, %) | 34 | (62.96) | |
| Grade 3 (n, %) | 11 | (20.37) | |
| Surgery | |||
| Quadrantectomy (n, %) | 40 | (72.07) | |
| Nodulectomy (n, %) | 1 | (1.85) | |
| Lumpectomy (n, %) | 3 | (5.55) | |
| Mastectomy (n, %) | 10 | (18.51) | |
| Radiotherapy (n, %) | 43 | (79.62) | |
| Family history for osteoporotic fracture (n, %) | 10 | (18.51) | |
| FN BMD (g/cm2) | 0.744 | ± | 0.10 |
| FN T-score | −1.8 | ± | 0.88 |
| FN Z-score | −0.4 | ± | 0.82 |
| LS BMD (g/cm2) | 0.930 | ± | 0.17 |
| LS T-score | −1.9 | ± | 1.25 |
| LS Z-score | −0.4 | ± | 1.23 |
| Osteopenia (n, %) | 23 | (42.59) | |
| Osteoporosis (n, %) | 28 | (51.85) | |
| [25OH-Vit.D] (ng/mL) | 19.7 | ± | 7.20 |
| [25OH-Vit.D] <10 ng/mL (n, %) | 6 | (11.11) | |
| [25OH-Vit.D] <20 ng/mL (n, %) | 27 | (50.00) | |
| [25OH-Vit.D] <30 ng/mL (n, %) | 52 | (96.29) | |
| Calcemia (mg/dL) | 9.3 | ± | 0.48 |
| PTH (pg/mL) | 44.7 | ± | 12.94 |
| Overall (n = 54) | [25(OH)vit.D] ≤9.9 ng/mL (n = 6) | [25(OH)vit.D] = 10–19.99 ng/mL (n = 21) | [25(OH)vit.D] = 20–29 ng/mL (n = 25) | [25(OH)vit.D] ≥30 ng/mL (n = 2) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Osteopenia (n, %) | 23 | (43) | 1 | (2) | 12 | (22) | 8 | (15) | 2 | (4) | |||||
| Osteoporosis (n, %) | 28 | (52) | 5 | (9) | 8 | (15) | 15 | (28) | 0 | (0) | |||||
| FN BMD (g/cm2) | 0.744 | ± | 0.10 | 0.737 | ± | 0.12 | 0.720 | ± | 0.12 | 0.762 | ± | 0.08 | 0.798 | ± | 0.01 |
| FN T-score | −1.8 | ± | 0.88 | −2.3 | ± | 0.54 | −2.1 | ± | 0.97 | −1.7 | ± | 0.87 | −1.5 | ± | 0.45 |
| FN Z-score | −0.4 | ± | 0.82 | −1.2 | ± | 1.04 | −0.5 | ± | 0.75 | −0.2 | ± | 0.80 | −0.4 | ± | 0.35 |
| LS BMD (g/cm2) | 0.930 | ± | 0.17 | 0.740 | ± | 0.22 | 0.938 | ± | 0.17 | 0.967 | ± | 0.15 | 0.965 | ± | 0.30 |
| LS T-score | −1.9 | ± | 1.25 | −2.4 | ± | 0.79 | −1.96 | ± | 1.41 | −1.7 | ± | 1.23 | −1.6 | ± | 0.50 |
| LS Z-score | −0.4 | ± | 1.23 | −1.7 | ± | 0.83 | −0.18 | ± | 1.21 | −0.1 | ± | 1.19 | −0.3 | ± | 0.01 |
| 25OH-Vit.D T0 (ng/mL) | 19.7 | ± | 7.20 | 7.1 | ± | 1.96 | 15.6 | ± | 2.83 | 25.1 | ± | 2.73 | 32.4 | ± | 0.07 |
| Calcemia (mg/dL) | 9.3 | ± | 0.48 | 9.2 | ± | 0.41 | 9.2 | ± | 0.52 | 9.3 | ± | 0.52 | 8.9 | ± | 0.35 |
| PTH (pg/mL) | 44.7 | ± | 12.94 | 47.1 | ± | 12.01 | 51.4 | ± | 15.06 | 39.7 | ± | 10.16 | 39.5 | ± | 6.36 |
| r | p Value | |
|---|---|---|
| LS BMD (g/cm2) | 0.30 | 0.025 * |
| FN BMD (g/cm2) | 0.14 | 0.300 |
| Age (years) | −0.01 | 0.935 |
| BMI (kg/m2) | 0.06 | 0.654 |
| Calcemia (mg/dL) | 0.01 | 0.924 |
| PTH (pg/mL) | −0.22 | 0.092 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Sire, A.; Gallelli, L.; Marotta, N.; Lippi, L.; Fusco, N.; Calafiore, D.; Cione, E.; Muraca, L.; Maconi, A.; De Sarro, G.; et al. Vitamin D Deficiency in Women with Breast Cancer: A Correlation with Osteoporosis? A Machine Learning Approach with Multiple Factor Analysis. Nutrients 2022, 14, 1586. https://doi.org/10.3390/nu14081586
de Sire A, Gallelli L, Marotta N, Lippi L, Fusco N, Calafiore D, Cione E, Muraca L, Maconi A, De Sarro G, et al. Vitamin D Deficiency in Women with Breast Cancer: A Correlation with Osteoporosis? A Machine Learning Approach with Multiple Factor Analysis. Nutrients. 2022; 14(8):1586. https://doi.org/10.3390/nu14081586
Chicago/Turabian Stylede Sire, Alessandro, Luca Gallelli, Nicola Marotta, Lorenzo Lippi, Nicola Fusco, Dario Calafiore, Erika Cione, Lucia Muraca, Antonio Maconi, Giovambattista De Sarro, and et al. 2022. "Vitamin D Deficiency in Women with Breast Cancer: A Correlation with Osteoporosis? A Machine Learning Approach with Multiple Factor Analysis" Nutrients 14, no. 8: 1586. https://doi.org/10.3390/nu14081586







