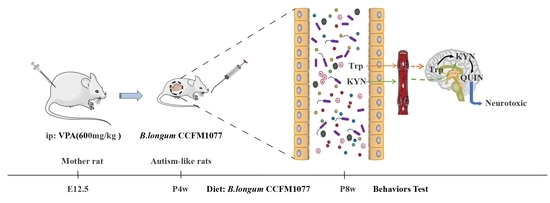
Bifidobacterium longum CCFM1077 Ameliorated Neurotransmitter Disorder and Neuroinflammation Closely Linked to Regulation in the Kynurenine Pathway of Autistic-like Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Experiments
2.2. Behavioural Tests
2.3. Neuroendocrine Secretion Analysis
2.4. Immunohistochemical Analysis
2.5. Gut Microbiota Analysis
2.6. Statistical Analyses
3. Results
3.1. B. longum CCFM1077 Could Alleviate Autistic-like Behaviors
3.2. Effect of B. longum CCFM1077 on KP Metabolism Was Related to Autistic-like Behaviours
3.3. Regulation Effect of B. longum CCFM1077 on the KP Metabolism Was Related to Gut Microbiome Enhancement
3.4. B. longum CCFM1077 Could Likely Regulate Neurotransmitters Levels by Promoting the KP Metabolism
3.5. B. longum CCFM1077 Could Ameliorate the Microglial Activity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cheroni, C.; Caporale, N.; Testa, G. Autism spectrum disorder at the crossroad between genes and environment: Contributions, convergences, and interactions in ASD developmental pathophysiology. Mol. Autism 2020, 11, 69. [Google Scholar] [CrossRef] [PubMed]
- Ng, Q.X.; Loke, W.; Venkatanarayanan, N.; Lim, D.Y.; Soh, A.Y.S.; Yeo, W.S. A Systematic Review of the Role of Prebiotics and Probiotics in Autism Spectrum Disorders. Medicina 2019, 55, 129. [Google Scholar] [CrossRef] [Green Version]
- Sharon, G.; Sampson, T.R.; Geschwind, D.H.; Mazmanian, S.K. The Central Nervous System and the Gut Microbiome. Cell 2016, 167, 915–932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srikantha, P.; Mohajeri, M.H. The Possible Role of the Microbiota-Gut-Brain-Axis in Autism Spectrum Disorder. Int. J. Mol. Sci. 2019, 20, 2115. [Google Scholar] [CrossRef] [Green Version]
- Lim, C.K.; Essa, M.M.; de Paula Martins, R.; Lovejoy, D.B.; Bilgin, A.A.; Waly, M.I.; Al-Farsi, Y.M.; Al-Sharbati, M.; Al-Shaffae, M.A.; Guillemin, G.J. Altered kynurenine pathway metabolism in autism: Implication for immune-induced glutamatergic activity. Autism Res. 2016, 9, 621–631. [Google Scholar] [CrossRef] [PubMed]
- Gevi, F.; Zolla, L.; Gabriele, S.; Persico, A.M. Urinary metabolomics of young Italian autistic children supports abnormal tryptophan and purine metabolism. Mol. Autism 2016, 7, 47. [Google Scholar] [CrossRef] [Green Version]
- Cascio, L.; Chen, C.F.; Pauly, R.; Srikanth, S.; Jones, K.; Skinner, C.D.; Stevenson, R.E.; Schwartz, C.E.; Boccuto, L. Abnormalities in the genes that encode Large Amino Acid Transporters increase the risk of Autism Spectrum Disorder. Mol. Genet. Genom. Med. 2020, 8, e1036. [Google Scholar] [CrossRef] [Green Version]
- Haslinger, D.; Waltes, R.; Yousaf, A.; Lindlar, S.; Schneider, I.; Lim, C.K.; Tsai, M.M.; Garvalov, B.K.; Acker-Palmer, A.; Krezdorn, N.; et al. Loss of the Chr16p11.2 ASD candidate gene QPRT leads to aberrant neuronal differentiation in the SH-SY5Y neuronal cell model. Mol. Autism 2018, 9, 56. [Google Scholar] [CrossRef] [Green Version]
- Burnashev, N.; Szepetowski, P. NMDA receptor subunit mutations in neurodevelopmental disorders. Curr. Opin. Pharmacol. 2015, 20, 73–82. [Google Scholar] [CrossRef]
- Mithaiwala, M.N.; Santana-Coelho, D.; Porter, G.A.; O’Connor, J.C. Neuroinflammation and the Kynurenine Pathway in CNS Disease: Molecular Mechanisms and Therapeutic Implications. Cells 2021, 10, 1548. [Google Scholar] [CrossRef]
- Savino, R.; Carotenuto, M.; Polito, A.N.; Di Noia, S.; Albenzio, M.; Scarinci, A.; Ambrosi, A.; Sessa, F.; Tartaglia, N.; Messina, G. Analyzing the Potential Biological Determinants of Autism Spectrum Disorder: From Neuroinflammation to the Kynurenine Pathway. Brain Sci. 2020, 10, 631. [Google Scholar] [CrossRef]
- Kennedy, P.J.; Cryan, J.F.; Dinan, T.G.; Clarke, G. Kynurenine pathway metabolism and the microbiota-gut-brain axis. Neuropharmacology 2017, 112, 399–412. [Google Scholar] [CrossRef] [PubMed]
- Desbonnet, L.; Clarke, G.; Traplin, A.; O’Sullivan, O.; Crispie, F.; Moloney, R.D.; Cotter, P.D.; Dinan, T.G.; Cryan, J.F. Gut microbiota depletion from early adolescence in mice: Implications for brain and behaviour. Brain Behav. Immun. 2015, 48, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Clarke, G.; Grenham, S.; Scully, P.; Fitzgerald, P.; Moloney, R.D.; Shanahan, F.; Dinan, T.G.; Cryan, J.F. The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Mol. Psychiatry 2013, 18, 666–673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El Aidy, S.; Kunze, W.; Bienenstock, J.; Kleerebezem, M. The microbiota and the gut-brain axis: Insights from the temporal and spatial mucosal alterations during colonisation of the germfree mouse intestine. Benef. Microbes 2012, 3, 251–259. [Google Scholar] [CrossRef]
- Purton, T.; Staskova, L.; Lane, M.M.; Dawson, S.L.; West, M.; Firth, J.; Clarke, G.; Cryan, J.F.; Berk, M.; O’Neil, A.; et al. Prebiotic and probiotic supplementation and the tryptophan-kynurenine pathway: A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2021, 123, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Rudzki, L.; Ostrowska, L.; Pawlak, D.; Małus, A.; Pawlak, K.; Waszkiewicz, N.; Szulc, A. Probiotic Lactobacillus Plantarum 299v decreases kynurenine concentration and improves cognitive functions in patients with major depression: A double-blind, randomized, placebo-controlled study. Psychoneuroendocrinology 2019, 100, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Desbonnet, L.; Garrett, L.; Clarke, G.; Bienenstock, J.; Dinan, T.G. The probiotic Bifidobacteria infantis: An assessment of potential antidepressant properties in the rat. J. Psychiatr. Res. 2008, 43, 164–174. [Google Scholar] [CrossRef]
- Valladares, R.; Bojilova, L.; Potts, A.H.; Cameron, E.; Gardner, C.; Lorca, G.; Gonzalez, C.F. Lactobacillus johnsonii inhibits indoleamine 2,3-dioxygenase and alters tryptophan metabolite levels in BioBreeding rats. FASEB J. 2013, 27, 1711–1720. [Google Scholar] [CrossRef]
- Matsuo, K.; Yabuki, Y.; Fukunaga, K. Improvement of Social Interaction and Cognition by Oxytocin for Autism-like Behaviors in Valproic Acid-Exposed Rats. Biol. Psychiatry 2017, 81, S200–S201. [Google Scholar] [CrossRef]
- Simmons, D.H.; Titley, H.K.; Hansel, C.; Mason, P. Behavioral Tests for Mouse Models of Autism: An Argument for the Inclusion of Cerebellum-Controlled Motor Behaviors. Neuroscience 2021, 462, 303–319. [Google Scholar] [CrossRef] [PubMed]
- Cleal, M.; Fontana, B.D.; Ranson, D.C.; McBride, S.D.; Swinny, J.D.; Redhead, E.S.; Parker, M.O. The Free-movement pattern Y-maze: A cross-species measure of working memory and executive function. Behav. Res. Methods 2021, 53, 536–557. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Mendez, M.E.; Castro-Sánchez, L.A.; Dagnino-Acosta, A.; Aguilar-Martínez, I.; Pérez-Burgos, A.; Vázquez-Jiménez, C.; Moreno-Galindo, E.G.; Álvarez-Cervera, F.J.; Góngora-Alfaro, J.L.; Navarro-Polanco, R.A.; et al. Capsaicin produces antidepressant-like effects in the forced swimming test and enhances the response of a sub-effective dose of amitriptyline in rats. Physiol. Behav. 2018, 195, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Lefèvre, A.; Mavel, S.; Nadal-Desbarats, L.; Galineau, L.; Attucci, S.; Dufour, D.; Sokol, H.; Emond, P. Validation of a global quantitative analysis methodology of tryptophan metabolites in mice using LC-MS. Talanta 2019, 195, 593–598. [Google Scholar] [CrossRef]
- Wang, L.S.; Zhang, M.D.; Tao, X.; Zhou, Y.F.; Liu, X.M.; Pan, R.L.; Liao, Y.H.; Chang, Q. LC-MS/MS-based quantification of tryptophan metabolites and neurotransmitters in the serum and brain of mice. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2019, 1112, 24–32. [Google Scholar] [CrossRef]
- Zhu, L.; Gu, P.; Shen, H. Protective effects of berberine hydrochloride on DSS-induced ulcerative colitis in rats. Int. Immunopharmacol. 2019, 68, 242–251. [Google Scholar] [CrossRef]
- Mao, B.; Gu, J.; Li, D.; Cui, S.; Zhao, J.; Zhang, H.; Chen, W. Effects of Different Doses of Fructooligosaccharides (FOS) on the Composition of Mice Fecal Microbiota, Especially the Bifidobacterium Composition. Nutrients 2018, 10, 1105. [Google Scholar] [CrossRef] [Green Version]
- Gao, K.; Mu, C.L.; Farzi, A.; Zhu, W.Y. Tryptophan Metabolism: A Link between the Gut Microbiota and Brain. Adv. Nutr. 2020, 11, 709–723. [Google Scholar] [CrossRef]
- Tian, P.; Chen, Y.; Zhu, H.; Wang, L.; Qian, X.; Zou, R.; Zhao, J.; Zhang, H.; Qian, L.; Wang, Q.; et al. Bifidobacterium breve CCFM1025 attenuates major depression disorder via regulating gut microbiome and tryptophan metabolism: A randomized clinical trial. Brain Behav. Immun. 2022, 100, 233–241. [Google Scholar] [CrossRef]
- Le Floc’h, N.; Otten, W.; Merlot, E. Tryptophan metabolism, from nutrition to potential therapeutic applications. Amino Acids 2011, 41, 1195–1205. [Google Scholar] [CrossRef]
- McDougle, C.J.; Naylor, S.T.; Cohen, D.J.; Aghajanian, G.K.; Heninger, G.R.; Price, L.H. Effects of tryptophan depletion in drug-free adults with autistic disorder. Arch. Gen. Psychiatry 1996, 53, 993–1000. [Google Scholar] [CrossRef] [PubMed]
- Notarangelo, F.M.; Pocivavsek, A. Elevated kynurenine pathway metabolism during neurodevelopment: Implications for brain and behavior. Neuropharmacology 2017, 112, 275–285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boyd, B.A.; McDonough, S.G.; Bodfish, J.W. Evidence-based behavioral interventions for repetitive behaviors in autism. J. Autism Dev. Disord. 2012, 42, 1236–1248. [Google Scholar] [CrossRef] [PubMed]
- Hamed, N.O.; Al-Ayadhi, L.; Osman, M.A.; Elkhawad, A.O.; Qasem, H.; Al-Marshoud, M.; Merghani, N.M.; El-Ansary, A. Understanding the roles of glutamine synthetase, glutaminase, and glutamate decarboxylase autoantibodies in imbalanced excitatory/inhibitory neurotransmission as etiological mechanisms of autism. Psychiatry Clin. Neurosci. 2018, 72, 362–373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quaak, I.; Brouns, M.R.; Van de Bor, M. The dynamics of autism spectrum disorders: How neurotoxic compounds and neurotransmitters interact. Int. J. Environ. Res. Public Health 2013, 10, 3384–3408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rubenstein, J.L.; Merzenich, M.M. Model of autism: Increased ratio of excitation/inhibition in key neural systems. Genes Brain Behav. 2003, 2, 255–267. [Google Scholar] [CrossRef]
- Tavares, R.G.; Schmidt, A.P.; Tasca, C.I.; Souza, D.O. Quinolinic acid-induced seizures stimulate glutamate uptake into synaptic vesicles from rat brain: Effects prevented by guanine-based purines. Neurochem. Res. 2008, 33, 97–102. [Google Scholar] [CrossRef]
- Lugo-Huitrón, R.; Ugalde Muñiz, P.; Pineda, B.; Pedraza-Chaverrí, J.; Ríos, C.; Pérez-de la Cruz, V. Quinolinic acid: An endogenous neurotoxin with multiple targets. Oxidative Med. Cell. Longev. 2013, 2013, 104024. [Google Scholar] [CrossRef] [Green Version]
- Rahman, A.; Rao, M.S.; Khan, K.M. Intraventricular infusion of quinolinic acid impairs spatial learning and memory in young rats: A novel mechanism of lead-induced neurotoxicity. J. Neuroinflamm. 2018, 15, 263. [Google Scholar] [CrossRef]
- Lovelace, M.D.; Varney, B.; Sundaram, G.; Franco, N.F.; Ng, M.L.; Pai, S.; Lim, C.K.; Guillemin, G.J.; Brew, B.J. Current Evidence for a Role of the Kynurenine Pathway of Tryptophan Metabolism in Multiple Sclerosis. Front. Immunol. 2016, 7, 246. [Google Scholar] [CrossRef] [Green Version]
- Lim, C.K.; Bilgin, A.; Lovejoy, D.B.; Tan, V.; Bustamante, S.; Taylor, B.V.; Bessede, A.; Brew, B.J.; Guillemin, G.J. Kynurenine pathway metabolomics predicts and provides mechanistic insight into multiple sclerosis progression. Sci. Rep. 2017, 7, 41473. [Google Scholar] [CrossRef] [PubMed]
- Hardan, A.Y.; Hendren, R.L.; Aman, M.G.; Robb, A.; Melmed, R.D.; Andersen, K.A.; Luchini, R.; Rahman, R.; Ali, S.; Jia, X.D.; et al. Efficacy and safety of memantine in children with autism spectrum disorder: Results from three phase 2 multicenter studies. Autism 2019, 23, 2096–2111. [Google Scholar] [CrossRef] [PubMed]
- Braidy, N.; Grant, R.; Adams, S.; Brew, B.J.; Guillemin, G.J. Mechanism for quinolinic acid cytotoxicity in human astrocytes and neurons. Neurotox. Res. 2009, 16, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Steiner, J.; Walter, M.; Gos, T.; Guillemin, G.J.; Bernstein, H.G.; Sarnyai, Z.; Mawrin, C.; Brisch, R.; Bielau, H.; zu Schwabedissen, L.M.; et al. Severe depression is associated with increased microglial quinolinic acid in subregions of the anterior cingulate gyrus: Evidence for an immune-modulated glutamatergic neurotransmission? J. Neuroinflamm. 2011, 8, 94. [Google Scholar] [CrossRef] [Green Version]
- Laskaris, L.E.; Di Biase, M.A.; Everall, I.; Chana, G.; Christopoulos, A.; Skafidas, E.; Cropley, V.L.; Pantelis, C. Microglial activation and progressive brain changes in schizophrenia. Br. J. Pharmacol. 2016, 173, 666–680. [Google Scholar] [CrossRef]
- Chien, Y.L.; Chen, Y.C.; Gau, S.S. Altered cingulate structures and the associations with social awareness deficits and CNTNAP2 gene in autism spectrum disorder. Neuroimage Clin. 2021, 31, 102729. [Google Scholar] [CrossRef]
- Guo, B.; Chen, J.; Chen, Q.; Ren, K.; Feng, D.; Mao, H.; Yao, H.; Yang, J.; Liu, H.; Liu, Y.; et al. Anterior cingulate cortex dysfunction underlies social deficits in Shank3 mutant mice. Nat. Neurosci. 2019, 22, 1223–1234. [Google Scholar] [CrossRef]
- Juckel, G.; Manitz, M.P.; Freund, N.; Gatermann, S. Impact of Poly I:C induced maternal immune activation on offspring’s gut microbiome diversity—Implications for schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry 2021, 110, 110306. [Google Scholar] [CrossRef]
- Zavitsanou, K.; Lim, C.K.; Purves-Tyson, T.; Karl, T.; Kassiou, M.; Banister, S.D.; Guillemin, G.J.; Weickert, C.S. Effect of maternal immune activation on the kynurenine pathway in preadolescent rat offspring and on MK801-induced hyperlocomotion in adulthood: Amelioration by COX-2 inhibition. Brain Behav. Immun. 2014, 41, 173–181. [Google Scholar] [CrossRef]
- Fujigaki, H.; Yamamoto, Y.; Saito, K. L-Tryptophan-kynurenine pathway enzymes are therapeutic target for neuropsychiatric diseases: Focus on cell type differences. Neuropharmacology 2017, 112, 264–274. [Google Scholar] [CrossRef]
- Boccuto, L.; Chen, C.F.; Pittman, A.R.; Skinner, C.D.; McCartney, H.J.; Jones, K.; Bochner, B.R.; Stevenson, R.E.; Schwartz, C.E. Decreased tryptophan metabolism in patients with autism spectrum disorders. Mol. Autism 2013, 4, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lunke, S.; Maxwell, S.; Khurana, I.; Harikrishnan, K.N.; Okabe, J.; Al-Hasani, K.; El-Osta, A. Epigenetic evidence of an Ac/Dc axis by VPA and SAHA. Clin. Epigenet. 2021, 13, 58. [Google Scholar] [CrossRef] [PubMed]
- Bold, J.; Sakata-Haga, H.; Fukui, Y. Spinal nerve defects in mouse embryos prenatally exposed to valproic acid. Anat. Sci. Int. 2018, 93, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Hughes, A.; Greene, N.D.E.; Copp, A.J.; Galea, G.L. Valproic acid disrupts the biomechanics of late spinal neural tube closure in mouse embryos. Mech. Dev. 2018, 149, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Nagode, D.A.; Meng, X.; Winkowski, D.E.; Smith, E.; Khan-Tareen, H.; Kareddy, V.; Kao, J.P.Y.; Kanold, P.O. Abnormal Development of the Earliest Cortical Circuits in a Mouse Model of Autism Spectrum Disorder. Cell Rep. 2017, 18, 1100–1108. [Google Scholar] [CrossRef] [Green Version]
- Main, S.L.; Kulesza, R.J. Repeated prenatal exposure to valproic acid results in cerebellar hypoplasia and ataxia. Neuroscience 2017, 340, 34–47. [Google Scholar] [CrossRef]
- Maciejak, P.; Szyndler, J.; Turzyńska, D.; Sobolewska, A.; Kołosowska, K.; Lehner, M.; Płaźnik, A. The kynurenine pathway: A missing piece in the puzzle of valproate action? Neuroscience 2013, 234, 135–145. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kong, Q.; Chen, Q.; Mao, X.; Wang, G.; Zhao, J.; Zhang, H.; Chen, W. Bifidobacterium longum CCFM1077 Ameliorated Neurotransmitter Disorder and Neuroinflammation Closely Linked to Regulation in the Kynurenine Pathway of Autistic-like Rats. Nutrients 2022, 14, 1615. https://doi.org/10.3390/nu14081615
Kong Q, Chen Q, Mao X, Wang G, Zhao J, Zhang H, Chen W. Bifidobacterium longum CCFM1077 Ameliorated Neurotransmitter Disorder and Neuroinflammation Closely Linked to Regulation in the Kynurenine Pathway of Autistic-like Rats. Nutrients. 2022; 14(8):1615. https://doi.org/10.3390/nu14081615
Chicago/Turabian StyleKong, Qingmin, Qian Chen, Xuhua Mao, Gang Wang, Jianxin Zhao, Hao Zhang, and Wei Chen. 2022. "Bifidobacterium longum CCFM1077 Ameliorated Neurotransmitter Disorder and Neuroinflammation Closely Linked to Regulation in the Kynurenine Pathway of Autistic-like Rats" Nutrients 14, no. 8: 1615. https://doi.org/10.3390/nu14081615