Gut Microbiome and Serum Metabolome Profiles of Capsaicin with Cognitive Benefits in APP/PS1 Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Gavage Protocol
2.3. Histology
2.4. Behavioral Tests
2.5. Fecal Microbiome Preprocessing and Data Analysis
2.6. Serum Metabolomics Analyses
2.7. Statistics
3. Results
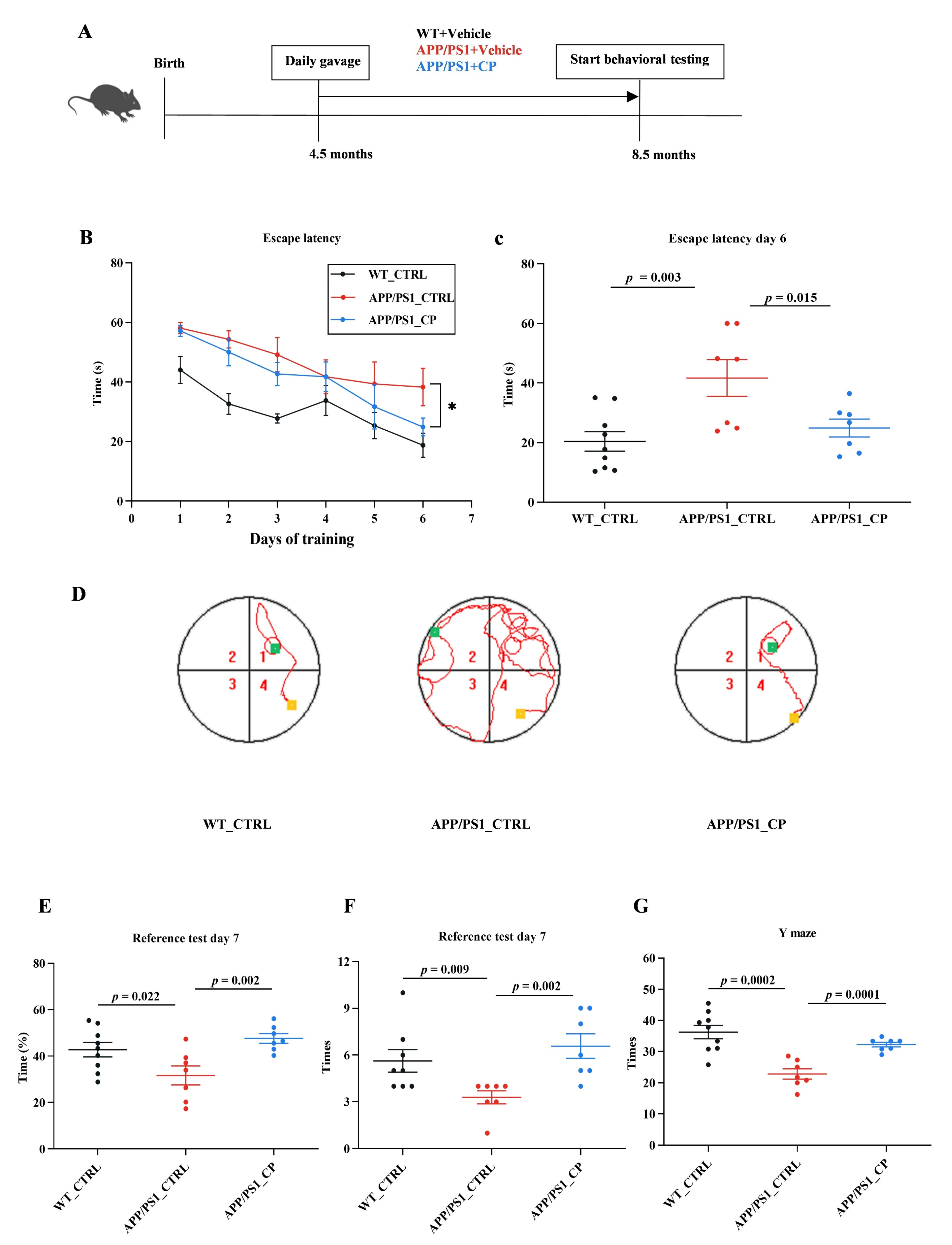
3.1. Capsaicin Improved Cognitive Deficts in APP/PS1 Mice
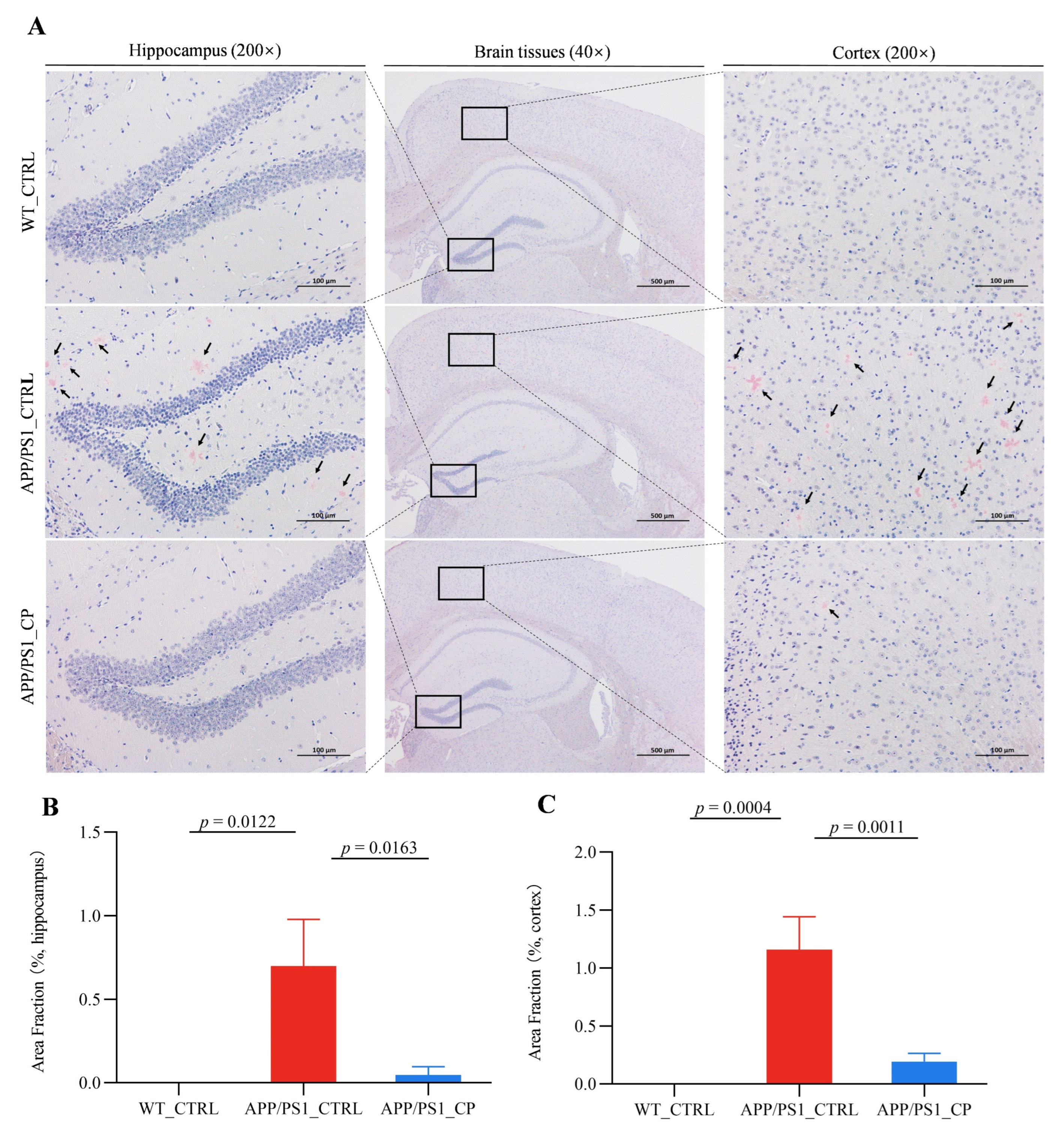
3.2. Capsaicin Reduced the Deposit of Amyloid Plaque in the Hippocampus and Cortex of APP/PS1 Mice
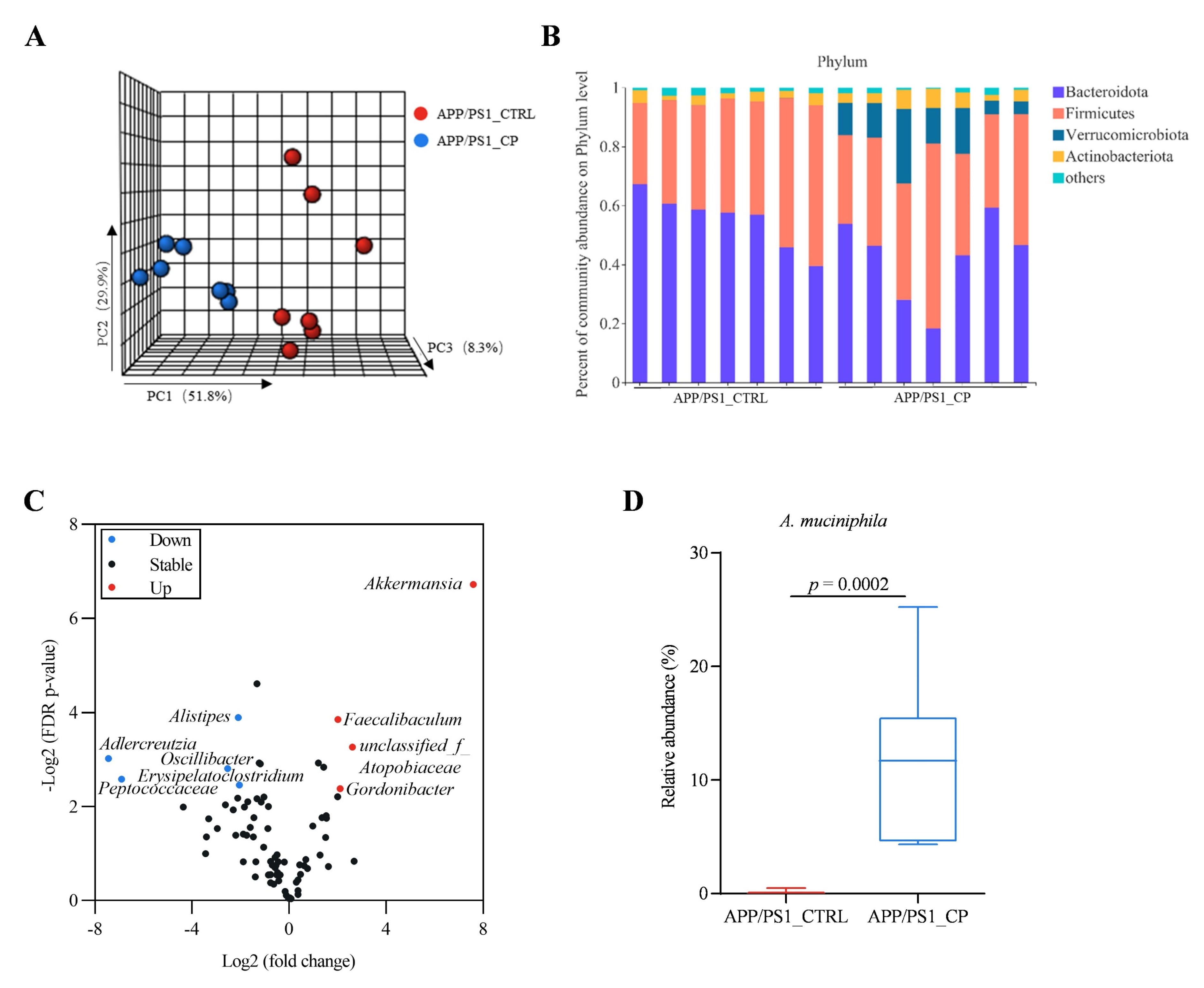
3.3. Capsaicin Increased the Relative Abundance of Phylum Verrucomicrobiata, Genus Akkermansia, and Species A. Muciniphila in APP/PS1 Mice
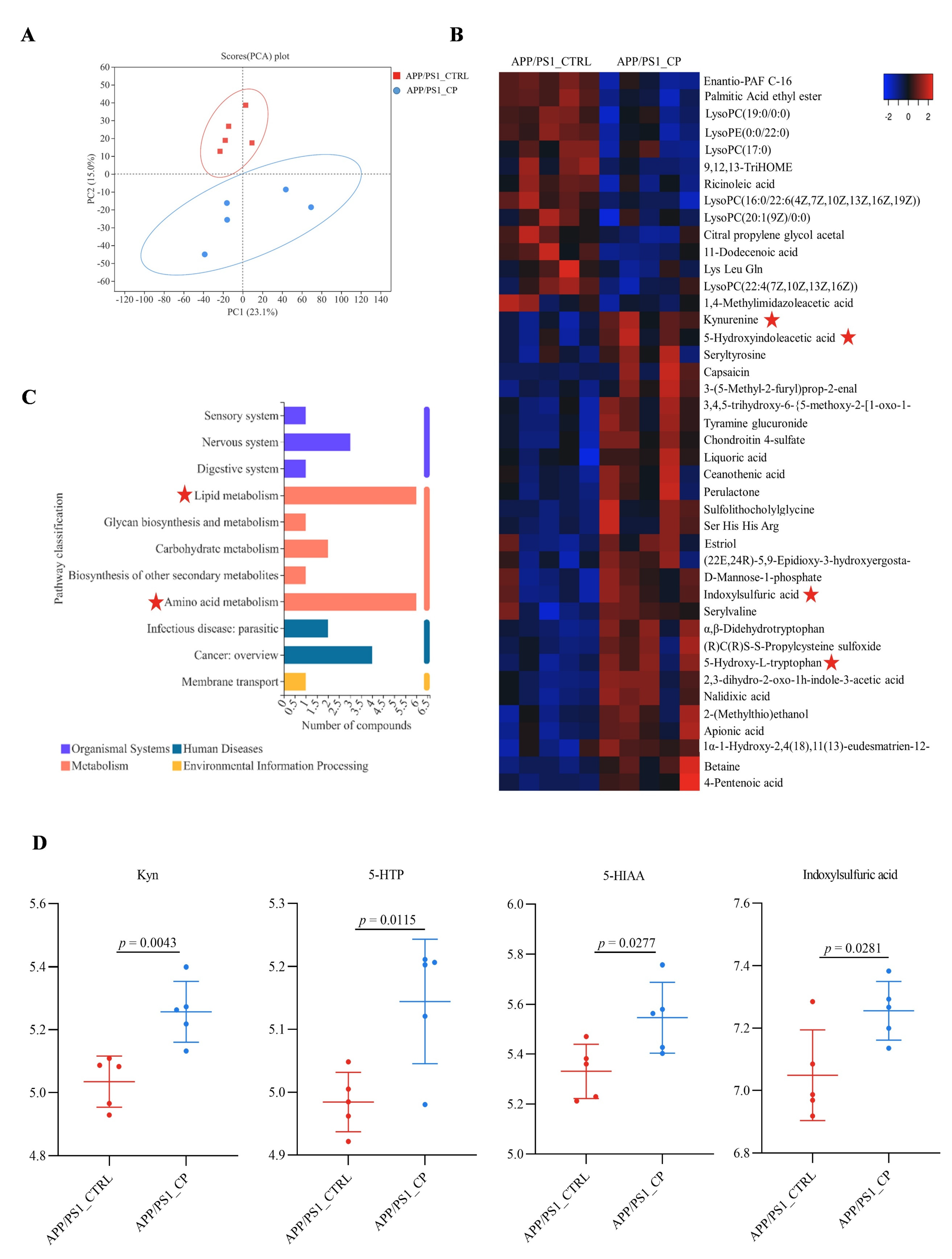
3.4. Capsaicin Upregulated Tryptophan Metabolism and Downregulated Lipid Metabolism in APP/PS1 Mice
3.5. The Correlation between Gut Microbiota and Serum Metabolites
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Leidal, A.M.; Levine, B.; Debnath, J. Autophagy and the cell biology of age-related disease. Nat. Cell Biol. 2018, 20, 1338–1348. [Google Scholar] [CrossRef] [PubMed]
- Menzies, F.M.; Fleming, A.; Rubinsztein, D.C. Compromised autophagy and neurodegenerative diseases. Nat. Rev. Neurosci. 2015, 16, 345–357. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.Y.; Wang, W.; Wu, J.J.; Liu, L.; Theodoratou, E.; Car, J.; Middleton, L.; Russ, T.C.; Deary, I.J.; Campbell, H.; et al. Epidemiology of Alzheimer’s disease and other forms of dementia in China, 1990–2010: A systematic review and analysis. Lancet 2013, 381, 2016–2023. [Google Scholar] [CrossRef] [PubMed]
- Angelucci, F.; Cechova, K.; Amlerova, J.; Hort, J. Antibiotics, gut microbiota, and Alzheimer’s disease. J. Neuroinflamm. 2019, 16, 108–128. [Google Scholar] [CrossRef]
- Qian, X.H.; Song, X.X.; Liu, X.L.; Chen, S.D.; Tang, H.D. Inflammatory pathways in Alzheimer’s disease mediated by gut microbiota. Ageing Res. Rev. 2021, 68, 1013–1017. [Google Scholar]
- Heijtza, R.D.; Wang, S.G.; Anuar, F.; Qian, Y.; Bjorkholm, B.; Samuelsson, A.; Hibberd, M.L.; Forssberg, H.; Pettersson, S. Normal gut microbiota modulates brain development and behavior. Proc. Natl. Acad. Sci. USA 2011, 108, 3047–3052. [Google Scholar] [CrossRef]
- Erny, D.; Dokalis, N.; Mezo, C.; Castoldi, A.; Mossad, O.; Staszewski, O.; Frosch, M.; Villa, M.; Fuchs, V.; Mayer, A.; et al. Microbiota-derived acetate enables the metabolic fitness of the brain innate immune system during health and disease. Cell Metab. 2021, 33, 2260–2302. [Google Scholar] [CrossRef]
- Dodiya, H.B.; Kuntz, T.; Shaik, S.M.; Baufeld, C.; Leibowitz, J.; Zhang, X.L.; Gottel, N.; Zhang, X.Q.; Butovsky, O.; Gilbert, J.A.; et al. Sex-specific effects of microbiome perturbations on cerebral A beta amyloidosis and microglia phenotypes. J. Exp. Med. 2019, 216, 1542–1560. [Google Scholar] [CrossRef]
- Wang, X.Y.; Sun, G.Q.; Feng, T.; Zhang, J.; Huang, X.; Wang, T.; Xie, Z.Q.; Chu, X.K.; Yang, J.; Wang, H.; et al. Sodium oligomannate therapeutically remodels gut microbiota and suppresses gut bacterial amino acids-shaped neuroinflammation to inhibit Alzheimer’s disease progression. Cell Res. 2019, 29, 787–803. [Google Scholar] [CrossRef]
- Nagpal, R.; Neth, B.J.; Wang, S.; Craft, S.; Yadav, H. Modified Mediterranean-ketogenic diet modulates gut microbiome and short-chain fatty acids in association with Alzheimer’s disease markers in subjects with mild cognitive impairment. EBioMedicine 2019, 47, 529–542. [Google Scholar] [CrossRef]
- Zmora, N.; Suez, J.; Elinav, E. You are what you eat: Diet, health and the gut microbiota. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 35–56. [Google Scholar] [CrossRef]
- Yuan, J.; Zhang, Z.X.; Wen, H.B.; Hong, X.; Hong, Z.; Qu, Q.M.; Tang, M.; Wu, J.X.; Xu, Q.; Li, H.; et al. Incidence of dementia and subtypes: A cohort study in four regions in China. Alzheimers Dement. 2016, 12, 262–271. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.H.; Bu, X.L.; Wang, J.; Zhang, T.; Xiang, Y.; Shen, L.L.; Wang, Q.H.; Deng, B.; Wang, X.; Zhu, C.; et al. The Associations between a Capsaicin-Rich Diet and Blood Amyloid-beta Levels and Cognitive Function. J. Alzheimers Dis. 2016, 52, 1081–1088. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Sun, B.L.; Xiang, Y.; Tian, D.Y.; Zhu, C.; Li, W.W.; Liu, Y.H.; Bu, X.L.; Shen, L.L.; Jin, W.S.; et al. Capsaicin consumption reduces brain amyloid-beta generation and attenuates Alzheimer’s disease-type pathology and cognitive deficits in APP/PS1 mice. Transl. Psychiat. 2020, 10, 230–242. [Google Scholar] [CrossRef]
- Du, Y.H.; Fu, M.; Huang, Z.L.; Tian, X.; Li, J.J.; Pang, Y.Y.; Song, W.H.; Wang, Y.T.; Dong, Z.F. TRPV1 activation alleviates cognitive and synaptic plasticity impairments through inhibiting AMPAR endocytosis in APP23/PS45 mouse model of Alzheimer’s disease. Aging Cell 2020, 19, e13113. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.; Wang, B.; Kaliannan, K.; Wang, X.; Lang, H.; Hui, S.; Huang, L.; Zhang, Y.; Zhou, M.; Chen, M.; et al. Gut Microbiota Mediates the Protective Effects of Dietary Capsaicin against Chronic Low-Grade Inflammation and Associated Obesity Induced by High-Fat Diet. MBio 2017, 8, e00470-17. [Google Scholar] [CrossRef]
- Hui, S.C.; Liu, Y.; Chen, M.T.; Wang, X.L.; Lang, H.D.; Zhou, M.; Yi, L.; Mi, M.T. Capsaicin Improves Glucose Tolerance and Insulin Sensitivity Through Modulation of the Gut Microbiota-Bile Acid-FXR Axis in Type 2 Diabetic db/db Mice. Mol. Nutr. Food Res. 2019, 63, e1900608. [Google Scholar] [CrossRef] [PubMed]
- Jankowsky, J.L.; Fadale, D.J.; Anderson, J.; Xu, G.M.; Gonzales, V.; Jenkins, N.A.; Copeland, N.G.; Lee, M.K.; Younkin, L.H.; Wagner, S.L.; et al. Mutant presenilins specifically elevate the levels of the 42 residue beta-amyloid peptide in vivo evidence for augmentation of a 42-specific gamma secretase. Hum. Mol. Genet. 2004, 13, 159–170. [Google Scholar] [CrossRef]
- Garcia-Alloza, M.; Robbins, E.M.; Zhang-Nunes, S.X.; Purcell, S.M.; Betensky, R.A.; Raju, S.; Prada, C.; Greenberg, S.M.; Bacskai, B.J.; Frosch, M.P. Characterization of amyloid deposition in the APPswe/PS1dE9 mouse model of Alzheimer disease. Neurobiol. Dis. 2006, 24, 516–524. [Google Scholar] [CrossRef]
- Dong, S.Q.; Huang, X.C.; Zhen, J.; Van Halm-Lutterodt, N.; Wang, J.J.; Zhou, C.; Yuan, L.H. Dietary Vitamin E Status Dictates Oxidative Stress Outcomes by Modulating Effects of Fish Oil Supplementation in Alzheimer Disease Model APP(swe)/PS1(dE9) Mice. Mol. Neurobiol. 2018, 55, 9204–9219. [Google Scholar] [CrossRef]
- Lonnemann, N.; Hosseini, S.; Marchetti, C.; Skouras, D.B.; Stefanoni, D.; D’Alessandro, A.; Dinarello, C.A.; Korte, M. The NLRP3 inflammasome inhibitor OLT1177 rescues cognitive impairment in a mouse model of Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2020, 117, 32145–32154. [Google Scholar] [CrossRef] [PubMed]
- Cano, A.; Ettcheto, M.; Chang, J.H.; Barroso, E.; Espina, M.; Kuhne, B.A.; Barenys, M.; Auladell, C.; Folch, J.; Souto, E.B.; et al. Dual-drug loaded nanoparticles of Epigallocatechin-3-gallate (EGCG)/Ascorbic acid enhance therapeutic efficacy of EGCG in a APPswe/PS1dE9 Alzheimer’s disease mice model. J. Control. Release 2019, 301, 62–75. [Google Scholar] [CrossRef]
- Vorhees, C.V.; Williams, M.T. Morris water maze: Procedures for assessing spatial and related forms of learning and memory. Nat. Protoc. 2006, 1, 848–858. [Google Scholar] [CrossRef] [PubMed]
- Bloom, G.S. Amyloid-beta and Tau the Trigger and Bullet in Alzheimer Disease Pathogenesis. JAMA Neurol. 2014, 71, 505–508. [Google Scholar] [CrossRef] [PubMed]
- Harach, T.; Marungruang, N.; Duthilleul, N.; Cheatham, V.; Mc Coy, K.D.; Frisoni, G.; Neher, J.J.; Fak, F.; Jucker, M.; Lasser, T.; et al. Reduction of Abeta amyloid pathology in APPPS1 transgenic mice in the absence of gut microbiota. Sci. Rep. 2017, 7, 41802. [Google Scholar] [CrossRef]
- Ou, Z.H.; Deng, L.L.; Lu, Z.; Wu, F.F.; Liu, W.T.; Huang, D.Q.; Peng, Y.Z. Protective effects of Akkermansia muciniphila on cognitive deficits and amyloid pathology in a mouse model of Alzheimer’s disease. Nutr. Diabetes 2020, 10, 12–22. [Google Scholar] [CrossRef]
- Wang, X.F.; Yang, S.T.; Li, S.H.; Zhao, L.; Hao, Y.L.; Qin, J.J.; Zhang, L.; Zhang, C.Y.; Bian, W.J.; Zuo, L.; et al. Aberrant gut microbiota alters host metabolome and impacts renal failure in humans and rodents. Gut 2020, 69, 2131–2142. [Google Scholar] [CrossRef] [PubMed]
- Agus, A.; Planchais, J.; Sokol, H. Gut Microbiota Regulation of Tryptophan Metabolism in Health and Disease. Cell Host Microbe 2018, 23, 716–724. [Google Scholar] [CrossRef]
- Cryan, J.F.; O’Riordan, K.J.; Sandhu, K.; Peterson, V.; Dinan, T.G. The gut microbiome in neurological disorders. Lancet Neurol. 2020, 19, 179–194. [Google Scholar] [CrossRef]
- Cryan, J.F.; O’Riordan, K.J.; Cowan, C.S.M.; Sandhu, K.V.; Bastiaanssen, T.F.S.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V.; et al. The Microbiota-Gut-Brain Axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef]
- Hu, X.; Wang, T.; Jin, F. Alzheimer’s disease and gut microbiota. Sci. China Life Sci. 2016, 59, 1006–1023. [Google Scholar] [CrossRef]
- Erny, D.; de Angelis, A.L.H.; Jaitin, D.; Wieghofer, P.; Staszewski, O.; David, E.; Keren-Shaul, H.; Mahlakoiv, T.; Jakobshagen, K.; Buch, T.; et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat. Neurosci. 2015, 18, 965–977. [Google Scholar] [CrossRef] [PubMed]
- Gareau, M.G.; Wine, E.; Rodrigues, D.M.; Cho, J.H.; Whary, M.T.; Philpott, D.J.; MacQueen, G.; Sherman, P.M. Bacterial infection causes stress-induced memory dysfunction in mice. Gut 2011, 60, 307–317. [Google Scholar] [CrossRef]
- Zarrinpar, A.; Chaix, A.; Xu, Z.J.Z.; Chang, M.W.; Marotz, C.A.; Saghatelian, A.; Knight, R.; Panda, S. Antibiotic-induced microbiome depletion alters metabolic homeostasis by affecting gut signaling and colonic metabolism. Nat. Commun. 2018, 9, 2872–2885. [Google Scholar] [CrossRef] [PubMed]
- Minter, M.R.; Zhang, C.; Leone, V.; Ringus, D.L.; Zhang, X.Q.; Oyler-Castrillo, P.; Musch, M.W.; Liao, F.; Ward, J.F.; Holtzman, D.M.; et al. Antibiotic-induced perturbations in gut microbial diversity influences neuro-inflammation and amyloidosis in a murine model of Alzheimer’s disease. Sci. Rep. 2016, 6, 30028. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.Y.; Hougen, H.; Vollmer, A.C.; Hiebert, S.M. Gut bacteria profiles of Mus musculus at the phylum and family levels are influenced by saturation of dietary fatty acids. Anaerobe 2012, 18, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.N.; Zhu, J.; Pan, W.S.; Shen, S.R.; Shan, W.G.; Das, U.N. Effects of Fish Oil with a High Content of n-3 Polyunsaturated Fatty Acids on Mouse Gut Microbiota. Arch. Med. Res. 2014, 45, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Cowan, T.E.; Palmnas, M.S.A.; Yang, J.; Bomhof, M.R.; Ardell, K.L.; Reimer, R.A.; Vogel, H.J.; Shearer, J. Chronic coffee consumption in the diet-induced obese rat: Impact on gut microbiota and serum metabolomics. J. Nutr. Biochem. 2014, 25, 489–495. [Google Scholar] [CrossRef]
- Moco, S.; Martin, F.P.J.; Rezzi, S. Metabolomics View on Gut Microbiome Modulation by Polyphenol-rich Foods. J. Proteome Res. 2012, 11, 4781–4790. [Google Scholar] [CrossRef]
- Del Chierico, F.; Vernocchi, P.; Dallapiccola, B.; Putignani, L. Mediterranean Diet and Health: Food Effects on Gut Microbiota and Disease Control. Int. J. Mol. Sci. 2014, 15, 11678–11699. [Google Scholar] [CrossRef]
- Hughes, T.F.; Andel, R.; Small, B.J.; Borenstein, A.R.; Mortimer, J.A.; Wolk, A.; Johansson, B.; Fratiglioni, L.; Pedersen, N.L.; Gatz, M. Midlife Fruit and Vegetable Consumption and Risk of Dementia in Later Life in Swedish Twins. Am. J. Geriat. Psychiat. 2010, 18, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.S.; Cho, C.H.; Yun, M.S.; Jang, S.J.; You, H.J.; Kim, J.H.; Han, D.; Cha, K.H.; Moon, S.H.; Lee, K.; et al. Akkermansia muciniphila secretes a glucagon-like peptide-1-inducing protein that improves glucose homeostasis and ameliorates metabolic disease in mice. Nat. Microbiol. 2021, 6, 563–573. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Q.X.; Feng, S.S.; Arjan, N.; Chen, W. A next generation probiotic, Akkermansia muciniphila. Crit. Rev. Food Sci. 2019, 59, 3227–3236. [Google Scholar] [CrossRef] [PubMed]
- Vogt, N.M.; Kerby, R.L.; Dill-McFarland, K.A.; Harding, S.J.; Merluzzi, A.P.; Johnson, S.C.; Carlsson, C.M.; Asthana, S.; Zetterberg, H.; Blennow, K.; et al. Gut microbiome alterations in Alzheimer’s disease. Sci. Rep. 2017, 7, 13537. [Google Scholar] [CrossRef]
- Ling, Z.X.; Zhu, M.L.; Yan, X.M.; Cheng, Y.W.; Shao, L.; Liu, X.; Jiang, R.L.; Wu, S.C. Structural and Functional Dysbiosis of Fecal Microbiota in Chinese Patients with Alzheimer’s Disease. Front. Cell Dev. Biol. 2021, 8, 634069. [Google Scholar] [CrossRef]
- Rosario, D.; Bidkhori, G.; Lee, S.; Bedarf, J.; Hildebrand, F.; Le Chatelier, E.; Uhlen, M.; Ehrlich, S.D.; Proctor, G.; Wullner, U.; et al. Systematic analysis of gut microbiome reveals the role of bacterial folate and homocysteine metabolism in Parkinson’s disease. Cell Rep. 2021, 34, 108807. [Google Scholar] [CrossRef]
- Platten, M.; Nollen, E.A.A.; Rohrig, U.F.; Fallarino, F.; Opitz, C.A. Tryptophan metabolism as a common therapeutic target in cancer, neurodegeneration and beyond. Nat. Rev. Drug Discov. 2019, 18, 379–401. [Google Scholar] [CrossRef]
- Aaldijk, E.; Vermeiren, Y. The role of serotonin within the microbiota-gut-brain axis in the development of Alzheimer’s disease: A narrative review. Ageing Res. Rev. 2022, 75, 101556. [Google Scholar] [CrossRef]
- Krautkramer, K.A.; Fan, J.; Backhed, F. Gut microbial metabolites as multi-kingdom intermediates. Nat. Rev. Microbiol. 2021, 19, 77–94. [Google Scholar] [CrossRef]
- Fekete, K.; Gyorei, E.; Lohner, S.; Verduci, E.; Agostoni, C.; Decsi, T. Long-chain polyunsaturated fatty acid status in obesity: A systematic review and meta-analysis. Obes. Rev. 2015, 16, 488–497. [Google Scholar] [CrossRef]
- Ebrahimpour, S.; Zakeri, M.; Esmaeili, A. Review Crosstalk between obesity, diabetes, and alzheimer’s disease: Introducing quercetin as an effective triple herbal medicine. Ageing Res. Rev. 2020, 62, 101095. [Google Scholar] [CrossRef] [PubMed]
- Baskaran, P.; Krishnan, V.; Ren, J.; Thyagarajan, B. Capsaicin induces browning of white adipose tissue and counters obesity by activating TRPV1 channel-dependent mechanisms. Br. J. Pharmacol. 2016, 173, 2369–2389. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.W.; Tang, C.; Tang, Y.; Yin, H.Y.; Liu, X. Capsaicin has an anti-obesity effect through alterations in gut microbiota populations and short-chain fatty acid concentrations. Food Nutr. Res. 2020, 64, 3615–3623. [Google Scholar] [CrossRef] [PubMed]
- Depommier, C.; Van Hul, M.; Everard, A.; Delzenne, N.M.; De Vos, W.M.; Cani, P.D. Pasteurized Akkermansia muciniphila increases whole-body energy expenditure and fecal energy excretion in diet-induced obese mice. Gut Microbes 2020, 11, 1231–1245. [Google Scholar] [CrossRef]
- Rao, Y.; Kuang, Z.Q.; Li, C.; Guo, S.Y.; Xu, Y.H.; Zhao, D.N.; Hu, Y.T.; Song, B.B.; Jiang, Z.; Ge, Z.H.; et al. Gut Akkermansia muciniphila ameliorates metabolic dysfunction-associated fatty liver disease by regulating the metabolism of L-aspartate via gut-liver axis. Gut Microbes 2021, 13, 1–19. [Google Scholar] [CrossRef]
- Li, J.; Lin, S.Q.; Vanhoutte, P.M.; Woo, C.W.; Xu, A.M. Akkermansia Muciniphila Protects Against Atherosclerosis by Preventing Metabolic Endotoxemia-Induced Inflammation in Apoe(-/-) Mice. Circulation 2016, 133, 2434–2446. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Liao, X.; Yin, X.; Deng, Z.; Hu, G.; Zhang, W.; Jiang, F.; Zhao, L. Gut Microbiome and Serum Metabolome Profiles of Capsaicin with Cognitive Benefits in APP/PS1 Mice. Nutrients 2023, 15, 118. https://doi.org/10.3390/nu15010118
Li J, Liao X, Yin X, Deng Z, Hu G, Zhang W, Jiang F, Zhao L. Gut Microbiome and Serum Metabolome Profiles of Capsaicin with Cognitive Benefits in APP/PS1 Mice. Nutrients. 2023; 15(1):118. https://doi.org/10.3390/nu15010118
Chicago/Turabian StyleLi, Jun, Xiaojun Liao, Xuedong Yin, Zimeng Deng, Guangfen Hu, Weiwei Zhang, Feng Jiang, and Liang Zhao. 2023. "Gut Microbiome and Serum Metabolome Profiles of Capsaicin with Cognitive Benefits in APP/PS1 Mice" Nutrients 15, no. 1: 118. https://doi.org/10.3390/nu15010118
APA StyleLi, J., Liao, X., Yin, X., Deng, Z., Hu, G., Zhang, W., Jiang, F., & Zhao, L. (2023). Gut Microbiome and Serum Metabolome Profiles of Capsaicin with Cognitive Benefits in APP/PS1 Mice. Nutrients, 15(1), 118. https://doi.org/10.3390/nu15010118







