Vitamin D, Folic Acid and Vitamin B12 Can Reverse Vitamin D Deficiency-Induced Learning and Memory Impairment by Altering 27-Hydroxycholesterol and S-Adenosylmethionine
Abstract
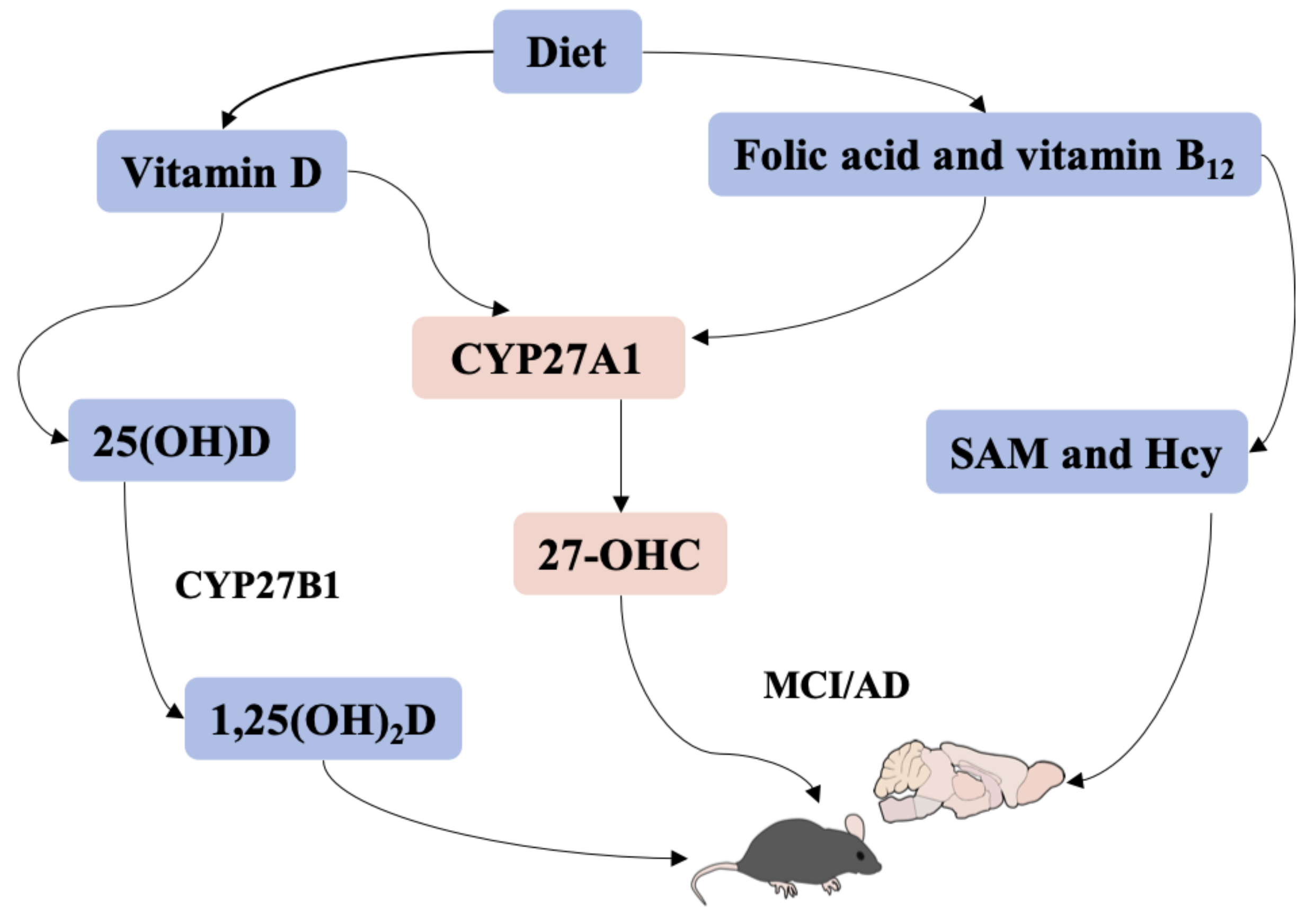
:1. Introduction
2. Materials and Methods
2.1. Animals and Models
2.2. Morris Water Maze Test
2.3. Novel Object Recognition Test
2.4. High-Performance Liquid Chromatography-Mass Spectrometry (HPLC-MS)
2.5. Enzyme-Linked Immunosorbent Assay (ELISA)
2.6. Quantitative Real-Time PCR (qRT-PCR)
2.7. Western Blot
2.8. Statistical Analysis
3. Results
3.1. Long-Term Spatial Memory Performance via Morris Water Maze
3.2. Short-Term Memory via Novel Object Recognition
3.3. Vitamin D and B Vitamins Related Factors in Serum
3.4. 27-OHC Level in Serum and Brain
3.5. Expressions of CYP27A1, CYP27B1 and VDR in Liver and Brain
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Chai, B.; Gao, F.; Wu, R.; Dong, T.; Gu, C.; Lin, Q.; Zhang, Y. Vitamin D deficiency as a risk factor for dementia and Alzheimer’s disease: An updated meta-analysis. BMC Neurol. 2019, 19, 284. [Google Scholar] [CrossRef] [PubMed]
- Keeney, J.T.; Butterfield, D.A. Vitamin D deficiency and Alzheimer disease: Common links. Neurobiol. Dis. 2015, 84, 84–98. [Google Scholar] [CrossRef] [PubMed]
- Bivona, G.; Lo Sasso, B.; Gambino, C.M.; Giglio, R.V.; Scazzone, C.; Agnello, L.; Ciaccio, M. The Role of Vitamin D as a Biomarker in Alzheimer’s Disease. Brain Sci. 2021, 11, 334. [Google Scholar] [CrossRef] [PubMed]
- Jayedi, A.; Rashidy-Pour, A.; Shab-Bidar, S. Vitamin D status and risk of dementia and Alzheimer’s disease: A meta-analysis of dose-response. Nutr. Neurosci. 2019, 22, 750–759. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.G.; Pang, Z.Q.; Wu, T.Y.; Zhang, Y.H.; Xuan, W.Q.; Wang, Z.; Yu, X.; Li, Y.C.; Guo, C.; Wang, Z.Y. Vitamin D deficiency exacerbates Alzheimer-like pathologies by reducing antioxidant capacity. Free Radic. Biol. Med. 2020, 161, 139–149. [Google Scholar] [CrossRef]
- Dai, L.; Zou, L.; Meng, L.; Qiang, G.; Yan, M.; Zhang, Z. Cholesterol Metabolism in Neurodegenerative Diseases: Molecular Mechanisms and Therapeutic Targets. Mol. Neurobiol. 2021, 58, 2183–2201. [Google Scholar] [CrossRef]
- Wang, H.L.; Wang, Y.Y.; Liu, X.G.; Kuo, S.H.; Liu, N.; Song, Q.Y.; Wang, M.W. Cholesterol, 24-Hydroxycholesterol, and 27-Hydroxycholesterol as Surrogate Biomarkers in Cerebrospinal Fluid in Mild Cognitive Impairment and Alzheimer’s Disease: A Meta-Analysis. J. Alzheimers Dis. 2016, 51, 45–55. [Google Scholar] [CrossRef]
- Loera-Valencia, R.; Goikolea, J.; Parrado-Fernandez, C.; Merino-Serrais, P.; Maioli, S. Alterations in cholesterol metabolism as a risk factor for developing Alzheimer’s disease: Potential novel targets for treatment. J. Steroid. Biochem. Mol. Biol. 2019, 190, 104–114. [Google Scholar] [CrossRef]
- Liu, Q.; An, Y.; Yu, H.; Lu, Y.; Feng, L.; Wang, C.; Xiao, R. Relationship between oxysterols and mild cognitive impairment in the elderly: A case-control study. Lipids Health Dis. 2016, 15, 177. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Lv, C.; An, Y.; Liu, Q.; Rong, H.; Tao, L.; Wang, Y.; Wang, Y.; Xiao, R. Increased Levels of 27-Hydroxycholesterol Induced by Dietary Cholesterol in Brain Contribute to Learning and Memory Impairment in Rats. Mol. Nutr. Food Res. 2018, 62, 531. [Google Scholar] [CrossRef]
- Tuckey, R.C.; Cheng, C.Y.S.; Slominski, A.T. The serum vitamin D metabolome: What we know and what is still to discover. J. Steroid. Biochem. Mol. Biol. 2019, 186, 4–21. [Google Scholar] [CrossRef]
- Bouillon, R.; Manousaki, D.; Rosen, C.; Trajanoska, K.; Rivadeneira, F.; Richards, J.B. The health effects of vitamin D supplementation: Evidence from human studies. Nat. Rev. Endocrinol. 2022, 18, 96–110. [Google Scholar] [CrossRef]
- Sandebring-Matton, A.; Goikolea, J.; Björkhem, I.; Paternain, L.; Kemppainen, N.; Laatikainen, T.; Ngandu, T.; Rinne, J.; Soininen, H.; Cedazo-Minguez, A.; et al. 27-Hydroxycholesterol, cognition, and brain imaging markers in the FINGER randomized controlled trial. Alzheimers Res. Ther. 2021, 13, 56. [Google Scholar] [CrossRef]
- DuSell, C.D.; Nelson, E.R.; Wang, X.; Abdo, J.; Mödder, U.I.; Umetani, M.; Gesty-Palmer, D.; Javitt, N.B.; Khosla, S.; McDonnell, D.P. The endogenous selective estrogen receptor modulator 27-hydroxycholesterol is a negative regulator of bone homeostasis. Endocrinology 2010, 151, 3675–3685. [Google Scholar] [CrossRef] [Green Version]
- Alshahrani, F.; Aljohani, N. Vitamin D: Deficiency, sufficiency and toxicity. Nutrients 2013, 5, 3605–3616. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.X.; Wu, P.F.; Liu, Y.Z.; Jiang, Y.L.; Wan, M.D.; Xiao, X.W.; Yang, Q.J.; Jiao, B.; Liao, X.X.; Wang, J.L.; et al. Association Between Serum Vitamins and the Risk of Alzheimer’s Disease in Chinese Population. J. Alzheimers Dis. 2022, 85, 829–836. [Google Scholar] [CrossRef]
- Wang, Z.; Zhu, W.; Xing, Y.; Jia, J.; Tang, Y. B vitamins and prevention of cognitive decline and incident dementia: A systematic review and meta-analysis. Nutr. Rev. 2022, 80, 931–949. [Google Scholar] [CrossRef]
- Smith, A.D.; Refsum, H. Homocysteine, B Vitamins, and Cognitive Impairment. Annu. Rev. Nutr. 2016, 36, 211–239. [Google Scholar] [CrossRef]
- Dayon, L.; Guiraud, S.P.; Corthésy, J.; Da Silva, L.; Migliavacca, E.; Tautvydaitė, D.; Oikonomidi, A.; Moullet, B.; Henry, H.; Métairon, S.; et al. One-carbon metabolism, cognitive impairment and CSF measures of Alzheimer pathology: Homocysteine and beyond. Alzheimers Res. Ther. 2017, 9, 43. [Google Scholar] [CrossRef] [Green Version]
- Mao, X.; Xing, X.; Xu, R.; Gong, Q.; He, Y.; Li, S.; Wang, H.; Liu, C.; Ding, X.; Na, R.; et al. Folic Acid and Vitamins D and B12 Correlate With Homocysteine in Chinese Patients With Type-2 Diabetes Mellitus, Hypertension, or Cardiovascular Disease. Medicine 2016, 95, e2652. [Google Scholar] [CrossRef]
- Manna, P.; Achari, A.E.; Jain, S.K. Vitamin D supplementation inhibits oxidative stress and upregulate SIRT1/AMPK/GLUT4 cascade in high glucose-treated 3T3L1 adipocytes and in adipose tissue of high fat diet-fed diabetic mice. Arch. Biochem. Biophys. 2017, 615, 22–34. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; An, Y.; Ma, W.; Yu, H.; Lu, Y.; Zhang, X.; Wang, Y.; Liu, W.; Wang, T.; Xiao, R. 27-Hydroxycholesterol contributes to cognitive deficits in APP/PS1 transgenic mice through microbiota dysbiosis and intestinal barrier dysfunction. J. Neuroinflam. 2020, 17, 199. [Google Scholar] [CrossRef] [PubMed]
- Yadang, F.S.A.; Nguezeye, Y.; Kom, C.W.; Betote, P.H.D.; Mamat, A.; Tchokouaha, L.R.Y.; Taiwé, G.S.; Agbor, G.A.; Bum, E.N. Scopolamine-Induced Memory Impairment in Mice: Neuroprotective Effects of Carissa edulis (Forssk.) Valh (Apocynaceae) Aqueous Extract. Int. J. Alzheimers Dis. 2020, 2020, 6372059. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; An, Y.; Ma, W.; Feng, L.; Wang, C.; Lu, Y.; Xiao, R. High-cholesterol diet results in elevated amyloid-β and oxysterols in rats. Mol. Med. Rep. 2018, 17, 1235–1240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.; Tang, R.; Ouyang, S.; Ma, F.; Liu, Z.; Wu, J. Folic acid prevents cardiac dysfunction and reduces myocardial fibrosis in a mouse model of high-fat diet-induced obesity. Nutr. Metab. 2017, 14, 68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tripathi, M.; Zhang, C.W.; Singh, B.K.; Sinha, R.A.; Moe, K.T.; DeSilva, D.A.; Yen, P.M. Hyperhomocysteinemia causes ER stress and impaired autophagy that is reversed by Vitamin B supplementation. Cell Death Dis. 2016, 7, e2513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, X.; Ma, X.K.; Zhang, L.; Cao, Z.B. Effects of resistance training on serum 25(OH) D concentrations in young men: A randomized controlled trial. Nutr. Metab. 2020, 17, 59. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.W.; Harvey, D.J.; Beckett, L.A.; Green, R.; Farias, S.T.; Reed, B.R.; Olichney, J.M.; Mungas, D.M.; DeCarli, C. Vitamin D Status and Rates of Cognitive Decline in a Multiethnic Cohort of Older Adults. JAMA Neurol. 2015, 72, 1295–1303. [Google Scholar] [CrossRef] [Green Version]
- Fu, L.; Chen, Y.H.; Chen, X.; Xu, S.; Yu, Z.; Xu, D.X. Vitamin D deficiency impairs neurobehavioral development in male mice. Physiol. Behav. 2017, 179, 333–339. [Google Scholar] [CrossRef]
- Al-Amin, M.M.; Sullivan, R.K.P.; Kurniawan, N.D.; Burne, T.H.J. Adult vitamin D deficiency disrupts hippocampal-dependent learning and structural brain connectivity in BALB/c mice. Brain Struct. Funct. 2019, 224, 1315–1329. [Google Scholar] [CrossRef]
- Morello, M.; Landel, V.; Lacassagne, E.; Baranger, K.; Annweiler, C.; Féron, F.; Millet, P. Vitamin D Improves Neurogenesis and Cognition in a Mouse Model of Alzheimer’s Disease. Mol. Neurobiol. 2018, 55, 6463–6479. [Google Scholar] [CrossRef] [Green Version]
- van Wijngaarden, J.P.; Dhonukshe-Rutten, R.A.; van Schoor, N.M.; van der Velde, N.; Swart, K.M.; Enneman, A.W.; van Dijk, S.C.; Brouwer-Brolsma, E.M.; Zillikens, M.C.; van Meurs, J.B.; et al. Rationale and design of the B-PROOF study, a randomized controlled trial on the effect of supplemental intake of vitamin B12 and folic acid on fracture incidence. BMC Geriatr. 2011, 11, 80. [Google Scholar] [CrossRef] [Green Version]
- Saponaro, F.; Saba, A.; Zucchi, R. An Update on Vitamin D Metabolism. Int. J. Mol. Sci. 2020, 21, 6573. [Google Scholar] [CrossRef]
- Kong, M.; Zhu, L.; Bai, L.; Zhang, X.; Chen, Y.; Liu, S.; Zheng, S.; Pandol, S.J.; Han, Y.P.; Duan, Z. Vitamin D deficiency promotes nonalcoholic steatohepatitis through impaired enterohepatic circulation in animal model. Am. J. Physiol. Gastrointest. Liver Physiol. 2014, 307, G883–G893. [Google Scholar] [CrossRef] [Green Version]
- Maia-Ceciliano, T.C.; Dutra, R.R.; Aguila, M.B.; Mandarim-De-Lacerda, C.A. The deficiency and the supplementation of vitamin D and liver: Lessons of chronic fructose-rich diet in mice. J. Steroid. Biochem. Mol. Biol. 2019, 192, 105399. [Google Scholar] [CrossRef]
- Cielen, N.; Heulens, N.; Maes, K.; Carmeliet, G.; Mathieu, C.; Janssens, W.; Gayan-Ramirez, G. Vitamin D deficiency impairs skeletal muscle function in a smoking mouse model. J. Endocrinol. 2016, 229, 97–108. [Google Scholar] [CrossRef] [Green Version]
- Going, C.C.; Alexandrova, L.; Lau, K.; Yeh, C.Y.; Feldman, D.; Pitteri, S.J. Vitamin D supplementation decreases serum 27-hydroxycholesterol in a pilot breast cancer trial. Breast Cancer Res. Treat. 2018, 167, 797–802. [Google Scholar] [CrossRef]
- Sundarakumar, J.S.; Shahul Hameed, S.K.; Ravindranath, V. Burden of Vitamin D, Vitamin B12 and Folic Acid Deficiencies in an Aging, Rural Indian Community. Front. Public Health 2021, 9, 707036. [Google Scholar] [CrossRef]
- Linnebank, M.; Popp, J.; Smulders, Y.; Smith, D.; Semmler, A.; Farkas, M.; Kulic, L.; Cvetanovska, G.; Blom, H.; Stoffel-Wagner, B.; et al. S-adenosylmethionine is decreased in the cerebrospinal fluid of patients with Alzheimer’s disease. Neurodegener. Dis. 2010, 7, 373–378. [Google Scholar] [CrossRef] [Green Version]
- Fuso, A.; Nicolia, V.; Ricceri, L.; Cavallaro, R.A.; Isopi, E.; Mangia, F.; Fiorenza, M.T.; Scarpa, S. S-adenosylmethionine reduces the progress of the Alzheimer-like features induced by B-vitamin deficiency in mice. Neurobiol. Aging 2012, 33, 1482.e1–1482.e16. [Google Scholar] [CrossRef]
- Chen, H.; Liu, S.; Ji, L.; Wu, T.; Ji, Y.; Zhou, Y.; Zheng, M.; Zhang, M.; Xu, W.; Huang, G. Folic Acid Supplementation Mitigates Alzheimer’s Disease by Reducing Inflammation: A Randomized Controlled Trial. Mediators Inflamm. 2016, 2016, 5912146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lam, A.B.; Kervin, K.; Tanis, J.E. Vitamin B(12) impacts amyloid beta-induced proteotoxicity by regulating the methionine/S-adenosylmethionine cycle. Cell Rep. 2021, 36, 109753. [Google Scholar] [CrossRef] [PubMed]
- Rai, S.N.; Singh, P.; Steinbusch, H.W.M.; Vamanu, E.; Ashraf, G.; Singh, M.P. The Role of Vitamins in Neurodegenerative Disease: An Update. Biomedicines 2021, 9, 1284. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Hu, J.; Huo, X.; Miao, R.; Zhang, Y.; Ma, F. Effects of vitamin D supplementation on cognitive function and blood Aβ-related biomarkers in older adults with Alzheimer’s disease: A randomised, double-blind, placebo-controlled trial. J. Neurol. Neurosurg. Psychiatry 2019, 90, 1347–1352. [Google Scholar] [CrossRef]
- Chen, H.; Liu, S.; Ge, B.; Zhou, D.; Li, M.; Li, W.; Ma, F.; Liu, Z.; Ji, Y.; Huang, G. Effects of Folic Acid and Vitamin B12 Supplementation on Cognitive Impairment and Inflammation in Patients with Alzheimer’s Disease: A Randomized, Single-Blinded, Placebo-Controlled Trial. J. Prev. Alzheimers Dis. 2021, 8, 249–256. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Zhou, C.; Yu, H.; Hao, L.; Ju, M.; Feng, W.; Guo, Z.; Sun, X.; Fan, Q.; Xiao, R. Vitamin D, Folic Acid and Vitamin B12 Can Reverse Vitamin D Deficiency-Induced Learning and Memory Impairment by Altering 27-Hydroxycholesterol and S-Adenosylmethionine. Nutrients 2023, 15, 132. https://doi.org/10.3390/nu15010132
Wang L, Zhou C, Yu H, Hao L, Ju M, Feng W, Guo Z, Sun X, Fan Q, Xiao R. Vitamin D, Folic Acid and Vitamin B12 Can Reverse Vitamin D Deficiency-Induced Learning and Memory Impairment by Altering 27-Hydroxycholesterol and S-Adenosylmethionine. Nutrients. 2023; 15(1):132. https://doi.org/10.3390/nu15010132
Chicago/Turabian StyleWang, Lijing, Cui Zhou, Huiyan Yu, Ling Hao, Mengwei Ju, Wenjing Feng, Zhiting Guo, Xuejing Sun, Qiushi Fan, and Rong Xiao. 2023. "Vitamin D, Folic Acid and Vitamin B12 Can Reverse Vitamin D Deficiency-Induced Learning and Memory Impairment by Altering 27-Hydroxycholesterol and S-Adenosylmethionine" Nutrients 15, no. 1: 132. https://doi.org/10.3390/nu15010132
APA StyleWang, L., Zhou, C., Yu, H., Hao, L., Ju, M., Feng, W., Guo, Z., Sun, X., Fan, Q., & Xiao, R. (2023). Vitamin D, Folic Acid and Vitamin B12 Can Reverse Vitamin D Deficiency-Induced Learning and Memory Impairment by Altering 27-Hydroxycholesterol and S-Adenosylmethionine. Nutrients, 15(1), 132. https://doi.org/10.3390/nu15010132






