Impact of Calorie-Restricted Cafeteria Diet and Treadmill Exercise on Sweet Taste in Diet-Induced Obese Female and Male Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Experiment 1
2.1.1. Animals
2.1.2. Experimental Design
2.1.3. Diets
2.1.4. Treadmill Training
2.1.5. Behavioral Testing
- Two-bottle sucrose preference test
- Taste reactivity test
- Brief-access licking test
2.1.6. Serum Analyses
2.2. Experiment 2
2.2.1. Animals, Timeline, and Experimental Design
2.2.2. Dietary and Exercise Interventions
2.2.3. Behavioral Testing
- Two-bottle sucrose preference test
- Taste reactivity test
- Brief-access licking test
2.3. Statistical Analysis
3. Results
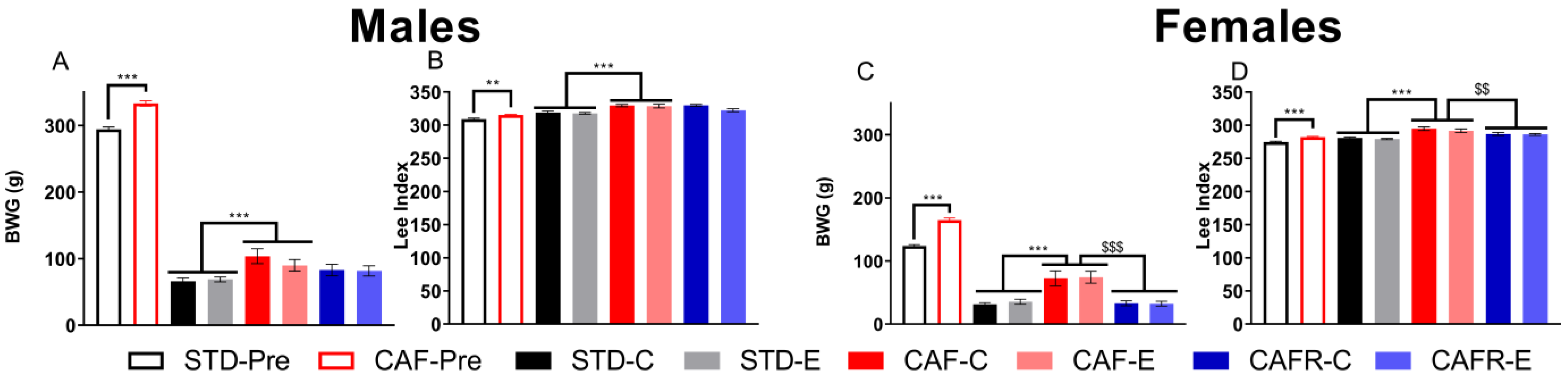
3.1. CAFR Diet Exerted Corrective Effects on CAF-Induced Alterations on Biometric Data in Females
3.2. CAFR Diet Partially Corrected the Increase in Food and Energy Intake Induced by CAF Diet in Both Sexes
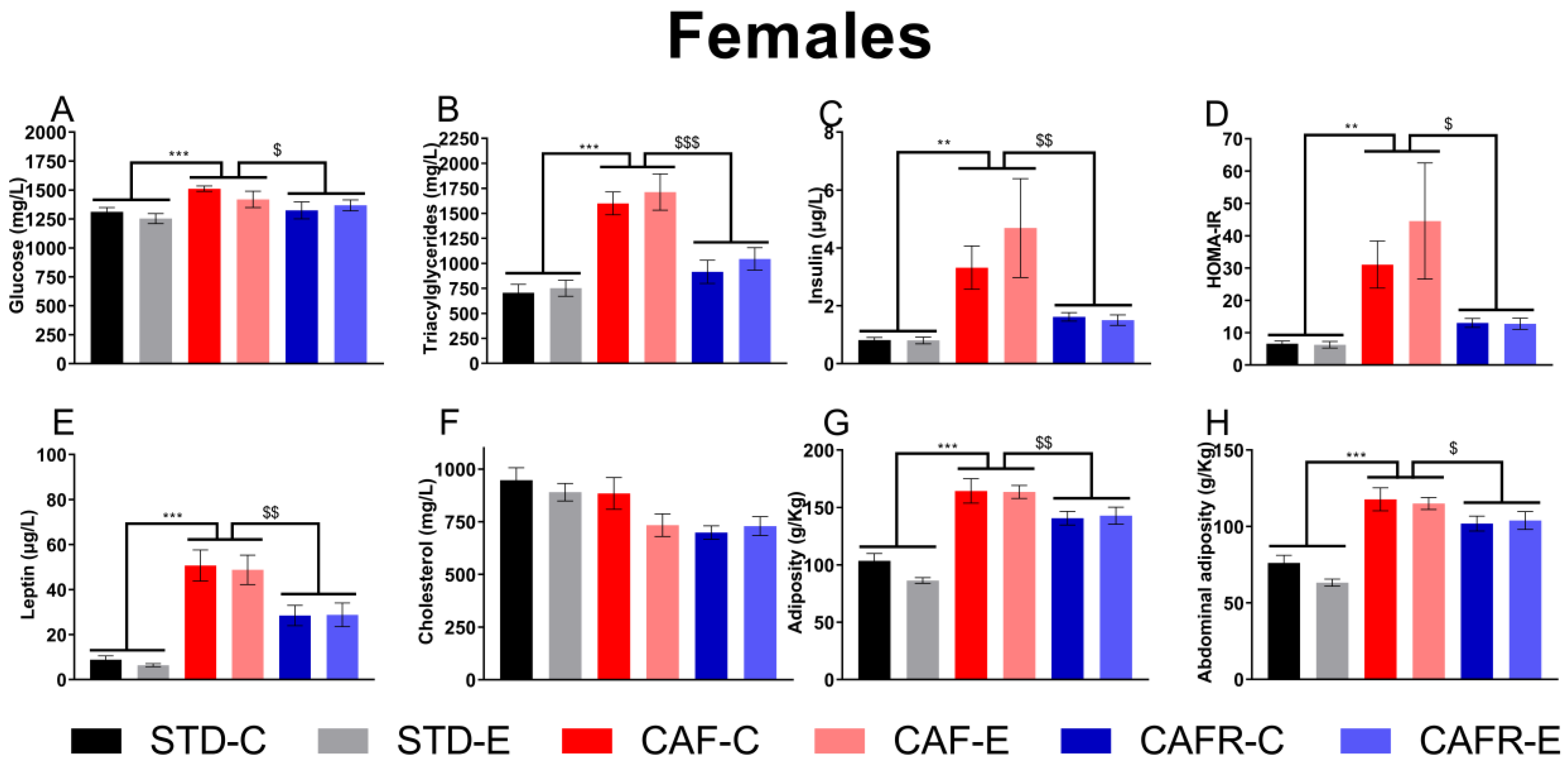
3.3. CAFR Partially Corrected the Increase in Serum MetS Biomarkers and Adiposity Induced by CAF Diet in Females
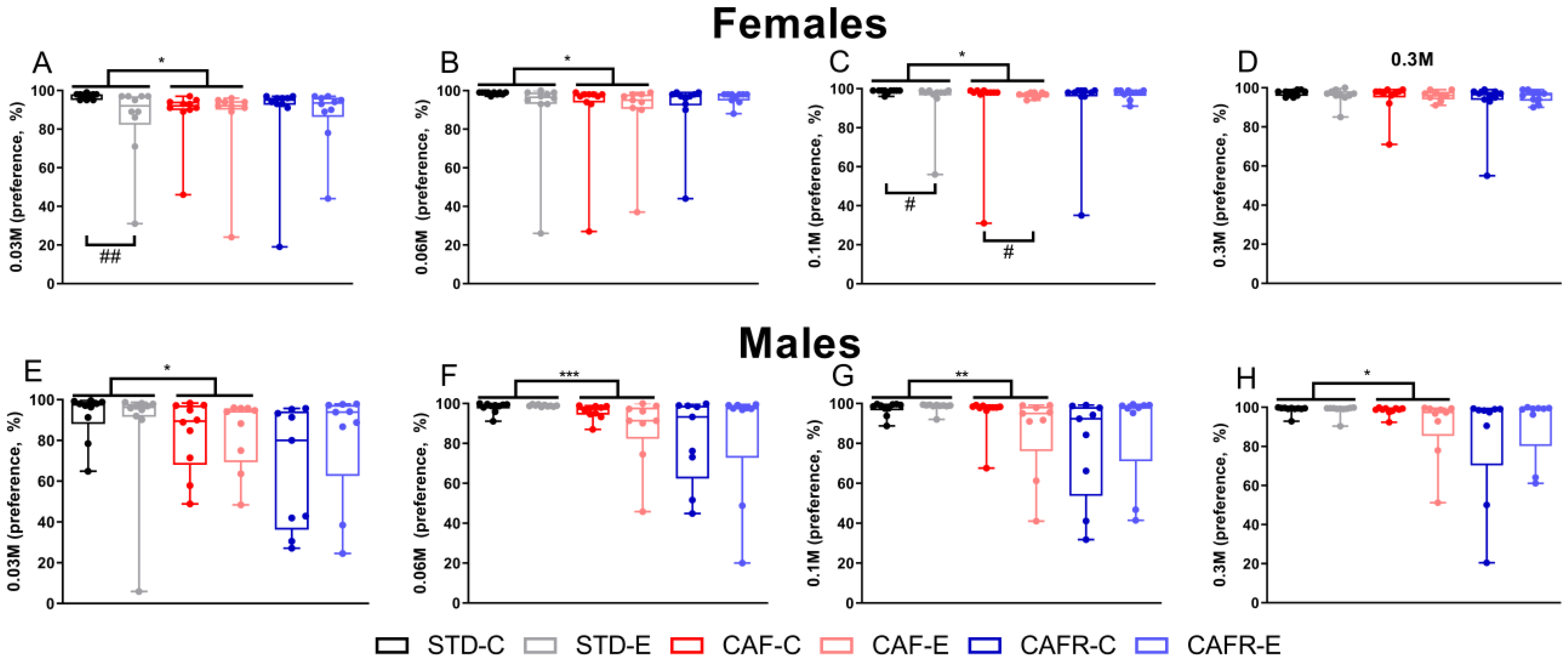
3.4. CAF Decreased Sucrose Consumption While CAFR Reverted this Effect in Females, and Exercise Decreased Sucrose Intake in the Sucrose Preference Test
3.5. CAF Decreased Hedonic Reactions to Concentrated Sucrose Solutions in Males but Not in Females
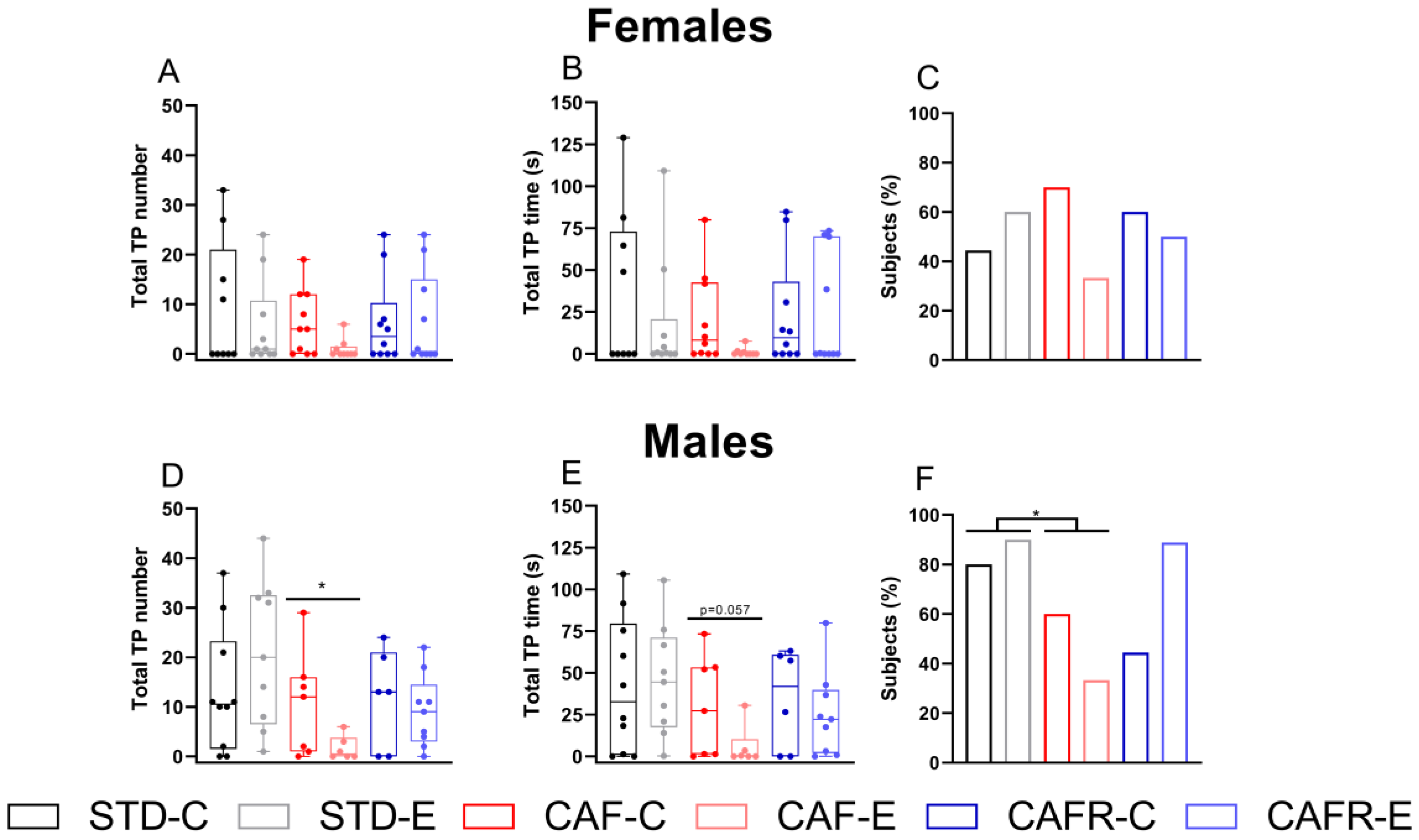
3.6. Licking Test
CAF Decreased the Lick Response in Females and Exercise Decreased It in Both Sexes
4. Discussion
5. Conclusions and Final Remarks
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chooi, Y.C.; Ding, C.; Magkos, F. The epidemiology of obesity. Metabolism 2019, 92, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.J.; Beilharz, J.E.; Maniam, J.; Reichelt, A.C.; Westbrook, R.F. Why is obesity such a problem in the 21st century? The intersection of palatable food, cues and reward pathways, stress, and cognition. Neurosci. Biobehav. Rev. 2015, 58, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Maersk, M.; Belza, A.; Stødkilde-Jørgensen, H.; Ringgaard, S.; Chabanova, E.; Thomsen, H.; Pedersen, S.B.; Astrup, A.; Richelsen, B. Sucrose-sweetened beverages increase fat storage in the liver, muscle, and visceral fat depot: A 6-mo randomized intervention study. Am. J. Clin. Nutr. 2012, 95, 283–289. [Google Scholar] [CrossRef]
- Bartoshuk, L.M.; Duffy, V.B.; Hayes, J.E.; Moskowitz, H.R.; Snyder, D.J. Psychophysics of sweet and fat perception in obesity: Problems, solutions and new perspectives. Philos. Trans. R. Soc. B Biol. Sci. 2006, 361, 1137–1148. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, R.; Fonseca, E.; Simon, S.A. The neuroscience of sugars in taste, gut-reward, feeding circuits, and obesity. Cell. Mol. Life Sci. 2020, 77, 3469–3502. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, R.; Niki, M.; Jyotaki, M.; Sanematsu, K.; Shigemura, N.; Ninomiya, Y. Modulation of sweet responses of taste receptor cells. Semin. Cell Dev. Biol. 2013, 24, 226–231. [Google Scholar] [CrossRef]
- Horio, N.; Jyotaki, M.; Yoshida, R.; Sanematsu, K.; Shigemura, N.; Ninomiya, Y. New Frontiers in Gut Nutrient Sensor Research: Nutrient Sensors in the Gastrointestinal Tract: Modulation of Sweet Taste Sensitivity by Leptin. J. Pharmacol. Sci. 2010, 112, 8–12. [Google Scholar] [CrossRef]
- Heffron, S.P.; Parham, J.S.; Pendse, J.; Alemán, J.O. Treatment of obesity in mitigating metabolic risk. Circ. Res. 2021, 126, 1646–1665. [Google Scholar] [CrossRef]
- Burgess, B.; Rao, S.P.; Tepper, B.J. Changes in liking for sweet and fatty foods following weight loss in women are related to prop phenotype but not to diet. Obesity 2016, 24, 1867–1873. [Google Scholar] [CrossRef]
- Nishihara, T.; Nozaki, T.; Sawamoto, R.; Komaki, G.; Miyata, N.; Hosoi, M.; Sudo, N. Effects of Weight Loss on Sweet Taste Preference and Palatability following Cognitive Behavioral Therapy for Women with Obesity. Obes. Facts 2019, 12, 529–542. [Google Scholar] [CrossRef]
- Asao, K.; Rothberg, A.E.; Arcori, L.; Kaur, M.; Fowler, C.E.; Herman, W.H. Sweet taste preferences before and after an intensive medical weight loss intervention. Obes. Sci. Pract. 2016, 2, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Horio, T.; Kawamura, Y. Influence of Physical Exercise on Human Preferences for Various Taste Solutions. Chem. Senses 1998, 23, 417–421. [Google Scholar] [CrossRef]
- Passe, D.H.; Horn, M.; Stofan, J.; Murray, R. Palatability and Voluntary intake of Sports Beverages, Di-luted Orange Juice, and Water During exercise. Int. J. Sport. Nutr. Exerc. Metab. 2004, 14, 272–284. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, A.-C.; Guimarães, R.d.F.; Namiranian, K.; Drapeau, V.; Mathieu, M.-E. Effect of Physical Exercise on Taste Perceptions: A Systematic Review. Nutrients 2020, 12, 2741. [Google Scholar] [CrossRef]
- Feeney, E.L.; Leacy, L.; O’Kelly, M.; Leacy, N.; Phelan, A.; Crowley, L.; Stynes, E.; de Casanove, A.; Horner, K. Sweet and Umami Taste Perception Differs with Habitual Exercise in Males. Nutrients 2019, 11, 155. [Google Scholar] [CrossRef] [PubMed]
- Venditti, C.; Musa-Veloso, K.; Lee, H.Y.; Poon, T.; Mak, A.; Darch, M.; Juana, J.; Fronda, D.; Noori, D.; Pateman, E.; et al. Determinants of Sweetness Preference: A Scoping Review of Human Studies. Nutrients 2020, 12, 718. [Google Scholar] [CrossRef]
- Mozhdehi, F.J.; Abeywickrema, S.; Bremer, P.J.; Peng, M. Following Vegan, Vegetarian, or Omnivore Diets. Foods 2021, 10, 2704. [Google Scholar] [CrossRef]
- Wise, P.M.; Nattress, L.; Flammer, L.J.; Beauchamp, G.K. Reduced dietary intake of simple sugars alters perceived sweet taste intensity but not perceived pleasantness. Am. J. Clin. Nutr. 2016, 103, 50–60. [Google Scholar] [CrossRef]
- Zhou, B.; Yamanaka-Okumura, H.; Adachi, C.; Kawakami, Y.; Katayama, T.; Takeda, E. High-fat diet-related stimulation of sweetness desire is greater in women than in men despite high vegetable intake. Public Health Nutr. 2014, 18, 1272–1281. [Google Scholar] [CrossRef]
- Moskowitz, H.R.; Kumraiah, V.; Sharma, K.N.; Jacobs, H.L.; Sharma, S. Effects of hunger, satiety and glucose load upon taste intensity and taste hedonics. Physiol. Behav. 1976, 16, 471–475. [Google Scholar] [CrossRef]
- Small, L.; Brandon, A.E.; Turner, N.; Cooney, G.J. Modeling insulin resistance in rodents by alterations in diet: What have high-fat and high-calorie diets revealed? Am. J. Physiol. Metab. 2018, 314, E251–E265. [Google Scholar] [CrossRef] [PubMed]
- Sclafani, A.; Springer, D. Dietary obesity in adult rats: Similarities to hypothalamic and human obesity syndromes. Physiol. Behav. 1976, 17, 461–471. [Google Scholar] [CrossRef] [PubMed]
- Lalanza, J.F.; Snoeren, E.M. The cafeteria diet: A standardized protocol and its effects on behavior. Neurosci. Biobehav. Rev. 2020, 122, 92–119. [Google Scholar] [CrossRef] [PubMed]
- Kleinert, M.; Clemmensen, C.; Hofmann, S.; Moore, M.C.; Renner, S.; Woods, S.C.; Huypens, P.; Beckers, J.; de Angelis, M.H.; Schürmann, A.; et al. Animal models of obesity and diabetes mellitus. Nat. Rev. Endocrinol. 2018, 14, 140–162. [Google Scholar] [CrossRef]
- Sampey, B.; Vanhoose, A.M.; Winfield, H.M.; Freemerman, A.J.; Muehlbauer, M.J.; Fueger, P.T.; Newgard, C.B.; Makowski, L. Cafeteria Diet Is a Robust Model of Human Metabolic Syndrome With Liver and Adipose Inflammation: Comparison to High-Fat Diet. Obesity 2011, 19, 1109–1117. [Google Scholar] [CrossRef] [PubMed]
- Gual-Grau, A.; Guirro, M.; Mayneris-Perxachs, J.; Arola, L.; Boqué, N. Impact of different hypercaloric diets on obesity features in rats: A metagenomics and metabolomics integrative approach. J. Nutr. Biochem. 2019, 71, 122–131. [Google Scholar] [CrossRef]
- Fam, J.; Clemens, K.J.; Westbrook, R.F.; Morris, M.J.; Kendig, M.D. Chronic exposure to cafeteria-style diet in rats alters sweet taste preference and reduces motivation for, but not ‘liking’ of sucrose. Appetite 2021, 168, 105742. [Google Scholar] [CrossRef]
- Shin, A.C.; Townsend, R.L.; Patterson, L.M.; Berthoud, H.-R. “Liking” and “wanting” of sweet and oily food stimuli as affected by high-fat diet-induced obesity, weight loss, leptin, and genetic predisposition. Am. J. Physiol. Integr. Comp. Physiol. 2011, 301, R1267–R1280. [Google Scholar] [CrossRef]
- Subias-Gusils, A.; Álvarez-Monell, A.; Boqué, N.; Caimari, A.; Del Bas, J.M.; Mariné-Casadó, R.; Solanas, M.; Escorihuela, R.M. Behavioral and Metabolic Effects of a Calorie-Restricted Cafeteria Diet and Oleuropein Supplementation in Obese Male Rats. Nutrients 2021, 13, 4474. [Google Scholar] [CrossRef]
- Hajnal, A.; Kovacs, P.; Ahmed, T.; Meirelles, K.; Lynch, C.J.; Cooney, R.N. Gastric bypass surgery alters behavioral and neural taste functions for sweet taste in obese rats. Am. J. Physiol. Liver Physiol. 2010, 299, G967–G979. [Google Scholar] [CrossRef]
- Tichansky, D.S.; Glatt, A.R.; Madan, A.K.; Harper, J.; Tokita, K.; Boughter, J.D. Decrease in sweet taste in rats after gastric bypass surgery. Surg. Endosc. 2010, 25, 1176–1181. [Google Scholar] [CrossRef] [PubMed]
- Cigarroa, I.; Lalanza, J.F.; Caimari, A.; del Bas, J.M.; Capdevila, L.; Arola, L.; Escorihuela, R.M. Treadmill Intervention Attenuates the Cafeteria Diet-Induced Impairment of Stress-Coping Strategies in Young Adult Female Rats. PLoS ONE 2016, 11, e0153687. [Google Scholar] [CrossRef] [PubMed]
- Thompson, Z.; Kolb, E.M.; Garland, T. High-runner mice have reduced incentive salience for a sweet-taste reward when housed with wheel access. Behav. Process. 2018, 146, 46–53. [Google Scholar] [CrossRef]
- Barragán, R.; Coltell, O.; Portolés, O.; Asensio, E.M.; Sorlí, J.V.; Ortega-Azorín, C.; González, J.I.; Sáiz, C.; Fernández-Carrión, R.; Ordovas, J.M.; et al. Bitter, Sweet, Salty, Sour and Umami Taste Perception Decreases with Age: Sex-Specific Analysis, Modulation by Genetic Variants and Taste-Preference Associations in 18 to 80 Year-Old Subjects. Nutrients 2018, 10, 1539. [Google Scholar] [CrossRef] [PubMed]
- Fischer, M.E.; Cruickshanks, K.J.; Ms, C.R.S.; Pinto, A.; Klein, B.E.K.; Klein, R.; Nieto, F.J.; Pankow, J.S.; Huang, G.-H.; Snyder, D.J. Taste intensity in the Beaver Dam Offspring Study. Laryngoscope 2013, 123, 1399–1404. [Google Scholar] [CrossRef] [PubMed]
- Yoshinaka, M.; Ikebe, K.; Uota, M.; Ogawa, T.; Okada, T.; Inomata, C.; Takeshita, H.; Mihara, Y.; Gondo, Y.; Masui, Y.; et al. Age and sex differences in the taste sensitivity of young adult, young-old and old-old Japanese. Geriatr. Gerontol. Int. 2016, 16, 1281–1288. [Google Scholar] [CrossRef]
- Valenstein, E.S.; Kakolewski, J.W.; Cox, V.C. Sex Differences in Taste Preference for Glucose and Saccharin Solutions. Science 1979, 156, 942–943. [Google Scholar] [CrossRef]
- Martin, L.J.; Sollars, S.I. Contributory role of sex differences in the variations of gustatory function. J. Neurosci. Res. 2017, 95, 594–603. [Google Scholar] [CrossRef]
- Berridge, K.C. Wanting and Liking: Observations from the Neuroscience and Psychology Laboratory. Inquiry 2009, 52, 378–398. [Google Scholar] [CrossRef]
- Meyerolbersleben, L.; Winter, C.; Bernhardt, N. Dissociation of wanting and liking in the sucrose preference test in dopamine transporter overexpressing rats. Behav. Brain Res. 2020, 378, 112244. [Google Scholar] [CrossRef]
- Robinson, S.; Sandstrom, S.M.; Denenberg, V.H.; Palmiter, R.D. Distinguishing Whether Dopamine Regulates Liking, Wanting, and/or Learning About Rewards. Behav. Neurosci. 2005, 119, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.S.; Berridge, K.C.; Aldridge, J.W. Disentangling pleasure from incentive salience and learning signals in brain reward circuitry. Proc. Natl. Acad. Sci. USA 2011, 108, E255–E264. [Google Scholar] [CrossRef] [PubMed]
- Lalanza, J.F.; Caimari, A.; Del Bas, J.M.; Torregrosa, D.; Cigarroa, I.; Pallàs, M.; Capdevila, L.; Arola, L.; Escorihuela, R.M. Effects Of A Post-Weaning Cafeteria Diet In Young Rats: Metabolic Syndrome, Reduced Activity And Low Anxiety-Like Behaviour. PLoS ONE 2014, 9, e85049. [Google Scholar] [CrossRef]
- Alvarez-Monell, A.; Subias-Gusils, A.; Mariné-Casadó, R.; Belda, X.; Gagliano, H.; Pozo, O.J.; Boqué, N.; Caimari, A.; Armario, A.; Solanas, M.; et al. Restricted cafeteria feeding and treadmill exercise improved body composition, metabolic profile and exploratory behavior in obese male rats. Sci. Rep. 2022, 12, 19545. [Google Scholar] [CrossRef]
- Berridge, K. Measuring hedonic impact in animals and infants: Microstructure of affective taste reactivity patterns. Neurosci. Biobehav. Rev. 2000, 24, 173–198. [Google Scholar] [CrossRef] [PubMed]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Foright, R.; Johnson, G.C.; Kahn, D.; Charleston, C.A.; Presby, D.M.; Bouchet, C.; Wellberg, E.A.; Sherk, V.D.; Jackman, M.R.; Greenwood, B.N.; et al. Compensatory eating behaviors in male and female rats in response to exercise training. Am. J. Physiol. Integr. Comp. Physiol. 2020, 319, R171–R183. [Google Scholar] [CrossRef]
- Lalanza, J.F.; Sanchez-Roige, S.; Gagliano, H.; Fuentes, S.; Bayod, S.; Camins, A.; Pallàs, M.; Armario, A.; Escorihuela, R.M. Physiological and behavioural consequences of long-term moderate treadmill exercise. Psychoneuroendocrinology 2012, 37, 1745–1754. [Google Scholar] [CrossRef]
- Treesukosol, Y.; Boersma, G.J.; Oros, H.; Choi, P.; Tamashiro, K.L.; Moran, T.H. Similarities and differences between “proactive” and “passive” stress-coping rats in responses to sucrose, NaCl, citric acid, and quinine. Chem. Senses 2014, 39, 333–342. [Google Scholar] [CrossRef]
- Boersma, G.J.; Treesukosol, Y.; Cordner, Z.A.; Kastelein, A.; Choi, P.; Moran, T.H.; Tamashiro, K.L. Exposure to activity-based anorexia impairs contextual learning in weight-restored rats without affecting spatial learning, taste, anxiety, or dietary-fat preference. Int. J. Eat. Disord. 2016, 49, 167–179. [Google Scholar] [CrossRef]
- Bin Lin, X.; Pierce, D.R.; Light, K.E.; Hayar, A. The fine temporal structure of the rat licking pattern: What causes the variabiliy in the interlick intervals and how is it affected by the drinking solution? Chem. Senses 2013, 38, 685–704. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Johnson, A.W. Characterizing ingestive behavior through licking microstructure: Underlying neurobiology and its use in the study of obesity in animal models. Int. J. Dev. Neurosci. 2018, 64, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Martire, S.I.; Holmes, N.; Westbrook, R.F.; Morris, M.J. Altered Feeding Patterns in Rats Exposed to a Palatable Cafeteria Diet: Increased Snacking and Its Implications for Development of Obesity. PLoS ONE 2013, 8, e60407. [Google Scholar] [CrossRef] [PubMed]
- Maric, I.; Krieger, J.-P.; van der Velden, P.; Börchers, S.; Asker, M.; Vujicic, M.; Asterholm, I.W.; Skibicka, K.P. Sex and Species Differences in the Development of Diet-Induced Obesity and Metabolic Disturbances in Rodents. Front. Nutr. 2022, 9, 141. [Google Scholar] [CrossRef] [PubMed]
- Guerra-Cantera, S.; Frago, L.M.; Collado-Pérez, R.; Canelles, S.; Ros, P.; Freire-Regatillo, A.; Jiménez-Hernaiz, M.; Barrios, V.; Argente, J.; Chowen, J.A. Sex Differences in Metabolic Recuperation After Weight Loss in High Fat Diet-Induced Obese Mice. Front. Endocrinol. 2021, 12, 796661. [Google Scholar] [CrossRef]
- Subias-Gusils, A.; Boqué, N.; Caimari, A.; Del Bas, J.M.; Mariné-Casadó, R.; Solanas, M.; Escorihuela, R.M. A restricted cafeteria diet ameliorates biometric and metabolic profile in a rat diet-induced obesity model. Int. J. Food Sci. Nutr. 2021, 72, 767–780. [Google Scholar] [CrossRef]
- Leigh, S.-J.; Kaakoush, N.O.; Escorihuela, R.M.; Westbrook, R.F.; Morris, M.J. Treadmill exercise has minimal impact on obesogenic diet-related gut microbiome changes but alters adipose and hypothalamic gene expression in rats. Nutr. Metab. 2020, 17, 1–13. [Google Scholar] [CrossRef]
- Mayengbam, S.; Mickiewicz, B.; Trottier, S.K.; Mu, C.; Wright, D.C.; Reimer, R.A.; Vogel, H.J.; Shearer, J. Distinct Gut Microbiota and Serum Metabolites in Response to Weight Loss Induced by Either Dairy or Exercise in a Rodent Model of Obesity. J. Proteome Res. 2019, 18, 3867–3875. [Google Scholar] [CrossRef]
- Mifune, H.; Tajiri, Y.; Nishi, Y.; Hara, K.; Iwata, S.; Tokubuchi, I.; Mitsuzono, R.; Yamada, K.; Kojima, M. Voluntary exercise contributed to an amelioration of abnormal feeding behavior, locomotor activity and ghrelin production concomitantly with a weight reduction in high fat diet-induced obese rats. Peptides 2015, 71, 49–55. [Google Scholar] [CrossRef]
- Sanchís-Ollé, M.; Sánchez-Benito, L.; Fuentes, S.; Gagliano, H.; Belda, X.; Molina, P.; Carrasco, J.; Nadal, R.; Armario, A. Male long-Evans rats: An outbred model of marked hypothalamic-pituitary-adrenal hyperactivity. Neurobiol. Stress 2021, 15, 100355. [Google Scholar] [CrossRef]
- Liu, M.-Y.; Yin, C.-Y.; Zhu, L.-J.; Zhu, X.-H.; Xu, C.; Luo, C.-X.; Chen, H.; Zhu, D.-Y.; Zhou, Q.-G. Sucrose preference test for measurement of stress-induced anhedonia in mice. Nat. Protoc. 2018, 13, 1686–1698. [Google Scholar] [CrossRef]
- Smith, G. John Davis and the meanings of licking. Appetite 2001, 36, 84–92. [Google Scholar] [CrossRef]
- Davis, J.F.; Tracy, A.L.; Schurdak, J.D.; Tschöp, M.H.; Lipton, J.W.; Clegg, D.J.; Benoit, S.C. Exposure to elevated levels of dietary fat attenuates psychostimulant reward and mesolimbic dopamine turnover in the rat. Behav. Neurosci. 2008, 122, 1257–1263. [Google Scholar] [CrossRef] [PubMed]
- Vucetic, Z.; Kimmel, J.; Reyes, T.M. Chronic High-Fat Diet Drives Postnatal Epigenetic Regulation of μ-Opioid Receptor in the Brain. Neuropsychopharmacology 2011, 36, 1199–1206. [Google Scholar] [CrossRef] [PubMed]
- Johnson, P.M.; Kenny, P.J. Dopamine D2 receptors in addiction-like reward dysfunction and compulsive eating in obese rats. Nat. Neurosci. 2010, 13, 635–641. [Google Scholar] [CrossRef]
- Ong, Z.Y.; Wanasuria, A.F.; Lin, M.Z.; Hiscock, J.; Muhlhausler, B.S. Chronic intake of a cafeteria diet and subsequent abstinence. Sex-specific effects on gene expression in the mesolimbic reward system. Appetite 2013, 65, 189–199. [Google Scholar] [CrossRef]
- Hajnal, A.; Norgren, R. Accumbens dopamine mechanisms in sucrose intake. Brain Res. 2001, 904, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.; Lee, M.J.; Han, J.; Kim, S.J.; Ryu, I.; Ju, X.; Ryu, M.J.; Chung, W.; Oh, E.; Kweon, G.R.; et al. A High-fat Diet Induces a Loss of Midbrain Dopaminergic Neuronal Function That Underlies Motor Abnormalities. Exp. Neurobiol. 2017, 26, 104–112. [Google Scholar] [CrossRef]
- Chen, W.; Li, J.; Liu, J.; Wang, D.; Hou, L. Aerobic Exercise Improves Food Reward Systems in Obese Rats via Insulin Signaling Regulation of Dopamine Levels in the Nucleus Accumbens. ACS Chem. Neurosci. 2019, 10, 2801–2808. [Google Scholar] [CrossRef]
- Volkow, N.D.; Wang, G.-J.; Tomasi, D.; Baler, R.D. Obesity and addiction: Neurobiological overlaps. Obes. Rev. 2012, 14, 2–18. [Google Scholar] [CrossRef]
- Abildgaard, A.; Solskov, L.; Volke, V.; Harvey, B.H.; Lund, S.; Wegener, G. A high-fat diet exacerbates depressive-like behavior in the Flinders Sensitive Line (FSL) rat, a genetic model of depression. Psychoneuroendocrinology 2011, 36, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Macht, V.; Vazquez, M.; Petyak, C.; Grillo, C.; Kaigler, K.; Enos, R.; McClellan, J.; Cranford, T.; Murphy, E.; Nyland, J.; et al. Leptin resistance elicits depressive-like behaviors in rats. Brain Behav. Immun. 2017, 60, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Grillo, C.A.; Piroli, G.G.; Kaigler, K.F.; Wilson, S.P.; Wilson, M.A.; Reagan, L.P. Downregulation of hypothalamic insulin receptor expression elicits depressive-like behaviors in rats. Behav. Brain Res. 2011, 222, 230–235. [Google Scholar] [CrossRef]
- Carey, M.; Small, H.; Yoong, S.L.; Boyes, A.; Bisquera, A.; Sanson-Fisher, R. Prevalence of comorbid depression and obesity in general practice: A cross-sectional survey. Br. J. Gen. Pract. 2014, 64, e122–e127. [Google Scholar] [CrossRef]
- Patsalos, O.; Keeler, J.; Schmidt, U.; Penninx, B.; Young, A.; Himmerich, H. Diet, Obesity, and Depression: A Systematic Review. J. Pers. Med. 2021, 11, 176. [Google Scholar] [CrossRef] [PubMed]
- Milaneschi, Y.; Simmons, W.K.; Van Rossum, E.F.C.; Penninx, B.W. Depression and obesity: Evidence of shared biological mechanisms. Mol. Psychiatry 2019, 24, 18–33. [Google Scholar] [CrossRef] [PubMed]
- Ambrósio, G.; Kaufmann, F.N.; Manosso, L.; Platt, N.; Ghisleni, G.; Rodrigues, A.L.S.; Rieger, D.K.; Kaster, M.P. Depression and peripheral inflammatory profile of patients with obesity. Psychoneuroendocrinology 2018, 91, 132–141. [Google Scholar] [CrossRef]
- Fuller, N.R.; Burns, J.; Sainsbury, A.; Horsfield, S.; da Luz, F.; Zhang, S.; Denyer, G.; Markovic, T.P.; Caterson, I.D. Examining the association between depression and obesity during a weight management programme. Clin. Obes. 2017, 7, 354–359. [Google Scholar] [CrossRef]
- Smith, M.A.; Schmidt, K.T.; Iordanou, J.C.; Mustroph, M.L. Aerobic exercise decreases the positive-reinforcing effects of cocaine. Drug Alcohol Depend. 2008, 98, 129–135. [Google Scholar] [CrossRef]
- Smith, M.A.; Lynch, W.J. Exercise as a Potential Treatment for Drug Abuse: Evidence from Preclinical Studies. Front. Psychiatry 2012, 2, 82. [Google Scholar] [CrossRef]
- Chen, H.I.; Kuo, Y.M.; Liao, C.-H.; Jen, C.J.; Huang, A.M.; Cherng, C.G.; Su, S.-W.; Yu, L. Long-term compulsive exercise reduces the rewarding efficacy of 3,4-methylenedioxymethamphetamine. Behav. Brain Res. 2008, 187, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Avena, N.M.; Rada, P.; Hoebel, B.G. Evidence for sugar addiction: Behavioral and neurochemical effects of intermittent, excessive sugar intake. Neurosci. Biobehav. Rev. 2008, 32, 20–39. [Google Scholar] [CrossRef] [PubMed]
- Sadat-Shirazi, M.; Zadeh-Tehrani, S.N.; Akbarabadi, A.; Mokri, A.; Kasgari, B.T.Z.; Zarrindast, M. Exercise can restore behavioural and molecular changes of intergenerational morphine effects. Addict. Biol. 2021, 27, e13122. [Google Scholar] [CrossRef]
- Rosa, H.; Segat, H.; Barcelos, R.; Roversi, K.; Rossato, D.; de Brum, G.; Burger, M. Involvement of the endogenous opioid system in the beneficial influence of physical exercise on amphetamine-induced addiction parameters. Pharmacol. Biochem. Behav. 2020, 197, 173000. [Google Scholar] [CrossRef]
- Robison, L.S.; Swenson, S.; Hamilton, J.; Thanos, P.K. Exercise Reduces Dopamine D1R and Increases D2R in Rats: Implications for Addiction. Med. Sci. Sports Exerc. 2018, 50, 1596–1602. [Google Scholar] [CrossRef]
- Jonasson, L.S.; Nyberg, L.; Axelsson, J.; Kramer, A.F.; Riklund, K.; Boraxbekk, C.-J. Higher striatal D2-receptor availability in aerobically fit older adults but non-selective intervention effects after aerobic versus resistance training. NeuroImage 2019, 202, 116044. [Google Scholar] [CrossRef] [PubMed]
- Castro, D.C.; Berridge, K. Opioid Hedonic Hotspot in Nucleus Accumbens Shell: Mu, Delta, and Kappa Maps for Enhancement of Sweetness “Liking” and “Wanting”. J. Neurosci. 2014, 34, 4239–4250. [Google Scholar] [CrossRef]
- Rahman, N.; Mihalkovic, A.; Geary, O.; Haffey, R.; Hamilton, J.; Thanos, P.K. Chronic aerobic exercise: Autoradiographic assessment of GABA(a) and mu-opioid receptor binding in adult rats. Pharmacol. Biochem. Behav. 2020, 196, 172980. [Google Scholar] [CrossRef]
- Yoshida, R.; Noguchi, K.; Shigemura, N.; Jyotaki, M.; Takahashi, I.; Margolskee, R.F.; Ninomiya, Y. Leptin Suppresses Mouse Taste Cell Responses to Sweet Compounds. Diabetes 2015, 64, 3751–3762. [Google Scholar] [CrossRef]
- Kawai, K.; Sugimoto, K.; Nakashima, K.; Miura, H.; Ninomiya, Y.; Meyer, A.L.; Trollmo, C.; Crawford, F.; Marrack, P.; Steere, A.C.; et al. Leptin as a modulator of sweet taste sensitivities in mice. Proc. Natl. Acad. Sci. USA 2000, 97, 11044–11049. [Google Scholar] [CrossRef]



| STD-Pre | CAF-Pre | STD-C | STD-E | CAF-C | CAF-E | CAFR-C | CAFR-E | ||
|---|---|---|---|---|---|---|---|---|---|
| Total Food intake (g/Kg) | Males | 90.7 ± 2.48 | 265.3 ± 24.33 *** | 42.95 ± 2.87 | 46.18 ± 2.63 | 111.93 ± 13.74 *** | 109.26 ± 17.15 *** | 78.74 ± 7.73 $$$ | 76.71 ± 6.61 $$$ |
| Females | 88.9 ± 3.05 | 229.8 ± 30.76 *** | 56.23 ± 4.09 | 56.23 ± 2.38 | 130.9 ± 13.31 *** | 123.3 ± 16.9 *** | 62.03 ± 6.69 $$$ | 46.5 ± 7.11 $$$ | |
| Chow energy intake (Kcal/Kg) | Males | 263.0 ± 7.2 | 86.3 ± 14.4 *** | 124.5 ± 8.33 | 133.9 ± 7.61 | 24.5 ± 8.21 *** | 22.8 ± 10.73 *** | 47.4 ± 7.77 $$$ | 47.3 ± 9.53 $$$ |
| Females | 257.9 ± 8.86 | 44.8 ± 13.54 *** | 163.08 ± 11.87 | 163.07 ± 6.92 | 15.61 ± 5.20 *** | 22.28 ± 9.31 *** | 41.29 ± 6.5 $$$ | 45.04 ± 10.24 $$$ | |
| Non-chow energy intake (Kcal/Kg) | Males | - | 514.7 ± 9.96 | - | - | 233.1 ± 6.20 | 231.1 ± 6.86 | 129.2 ± 5.07 $$$ | 126.4 ± 4.02 $$$ |
| Females | - | 518.7 ± 7.97 | - | - | 283.4 ± 11.89 | 275.9 ± 11.52 | 139.1 ± 4.17 $$$ | 140.6 ± 3.25 $$$ | |
| Chow intake (% of total energy intake) | Males | 100 | 14.38 ± 0.47 *** | 100 | 100 | 9.61 ± 1.07 *** | 8.99 ± 1.31 *** | 26.94 ± 1.36 $$$ | 27.22 ± 1.67 $$$ |
| Females | 100 | 7.96 ± 0.55 *** | 100 | 100 | 5.32 ± 0.97 *** | 7.30 ± 1.26 *** | 22.92 ± 1.71 $$$ | 24.16 ± 2.10 $$$ | |
| Non-chow intake (% of total energy intake) | Males | - | 85.62 ± 0.47 | - | - | 90.39 ± 1.07 | 91.01 ± 1.31 | 73.06 ± 1.36 $$$ | 72.78 ± 1.67 $$$ |
| Females | - | 92.04 ± 0.55 | - | - | 94.68 ± 0.97 | 92.70 ± 1.26 | 77.08 ± 1.71 $$$ | 75.84 ± 2.10 $$$ | |
| Simple sugars (Kcal/Kg) | Males | - | 167.6 ± 4.25 | - | - | 72.8 ± 5.18 | 69.87 ± 6.03 | 36.91 ± 2.04 $$$ | 34.75 ± 1.83 $$$ |
| Females | - | 157.3 ± 6.03 | - | - | 84.95 ± 4.67 | 81.43 ± 5.57 | 31.76 ± 2.28 $$$ | 33.69 ± 2.30 $$$ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alvarez-Monell, A.; Subias-Gusils, A.; Mariné-Casadó, R.; Boqué, N.; Caimari, A.; Solanas, M.; Escorihuela, R.M. Impact of Calorie-Restricted Cafeteria Diet and Treadmill Exercise on Sweet Taste in Diet-Induced Obese Female and Male Rats. Nutrients 2023, 15, 144. https://doi.org/10.3390/nu15010144
Alvarez-Monell A, Subias-Gusils A, Mariné-Casadó R, Boqué N, Caimari A, Solanas M, Escorihuela RM. Impact of Calorie-Restricted Cafeteria Diet and Treadmill Exercise on Sweet Taste in Diet-Induced Obese Female and Male Rats. Nutrients. 2023; 15(1):144. https://doi.org/10.3390/nu15010144
Chicago/Turabian StyleAlvarez-Monell, Adam, Alex Subias-Gusils, Roger Mariné-Casadó, Noemi Boqué, Antoni Caimari, Montserrat Solanas, and Rosa M. Escorihuela. 2023. "Impact of Calorie-Restricted Cafeteria Diet and Treadmill Exercise on Sweet Taste in Diet-Induced Obese Female and Male Rats" Nutrients 15, no. 1: 144. https://doi.org/10.3390/nu15010144
APA StyleAlvarez-Monell, A., Subias-Gusils, A., Mariné-Casadó, R., Boqué, N., Caimari, A., Solanas, M., & Escorihuela, R. M. (2023). Impact of Calorie-Restricted Cafeteria Diet and Treadmill Exercise on Sweet Taste in Diet-Induced Obese Female and Male Rats. Nutrients, 15(1), 144. https://doi.org/10.3390/nu15010144







