Hospital Variation in Feeding Jejunostomy Policy for Minimally Invasive Esophagectomy: A Nationwide Cohort Study
Abstract
:1. Introduction
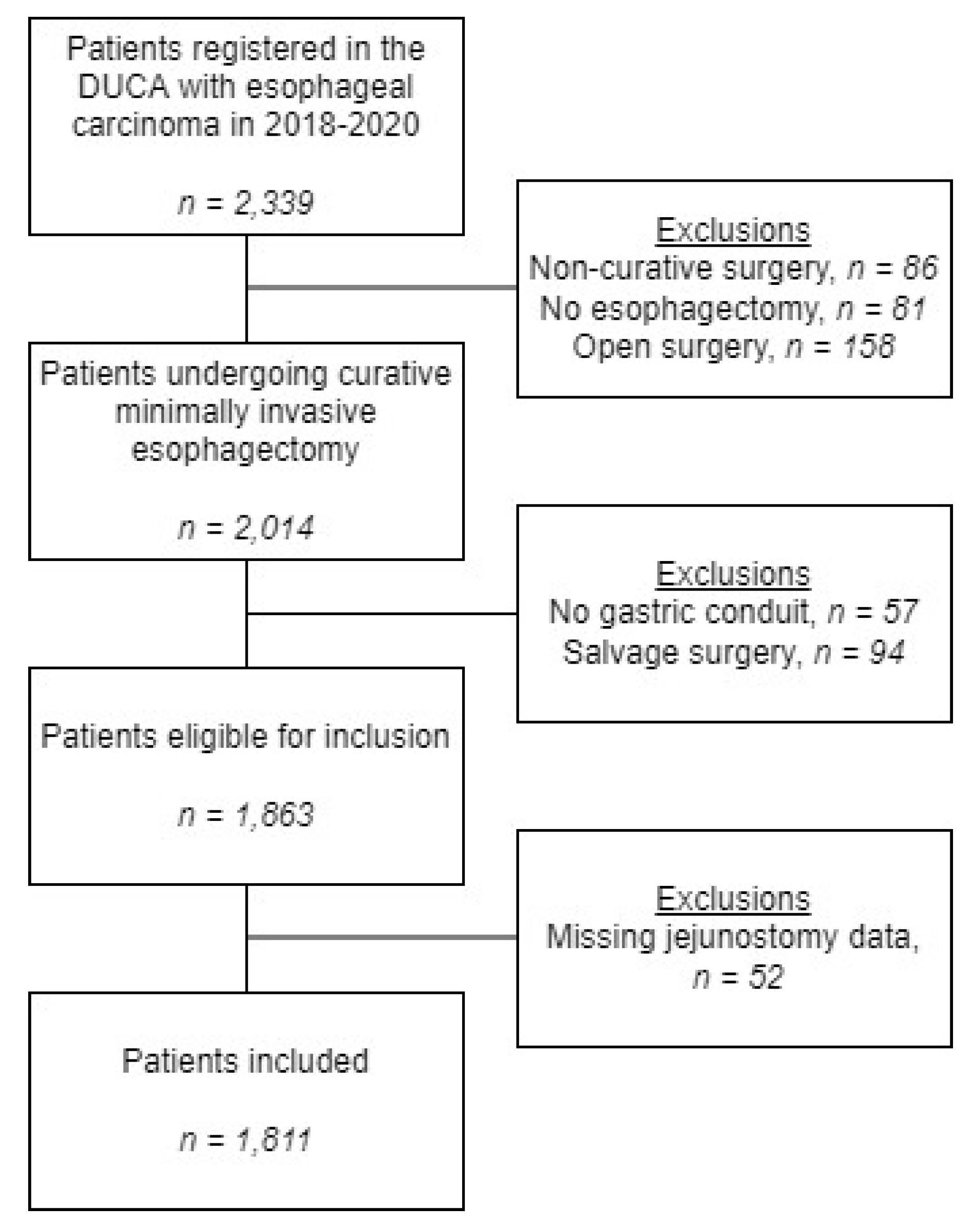
2. Materials and Methods
2.1. Study Design
2.2. Patients
2.3. Outcome Measurea
2.4. Variables for Analyses
2.5. Statistical Analysis
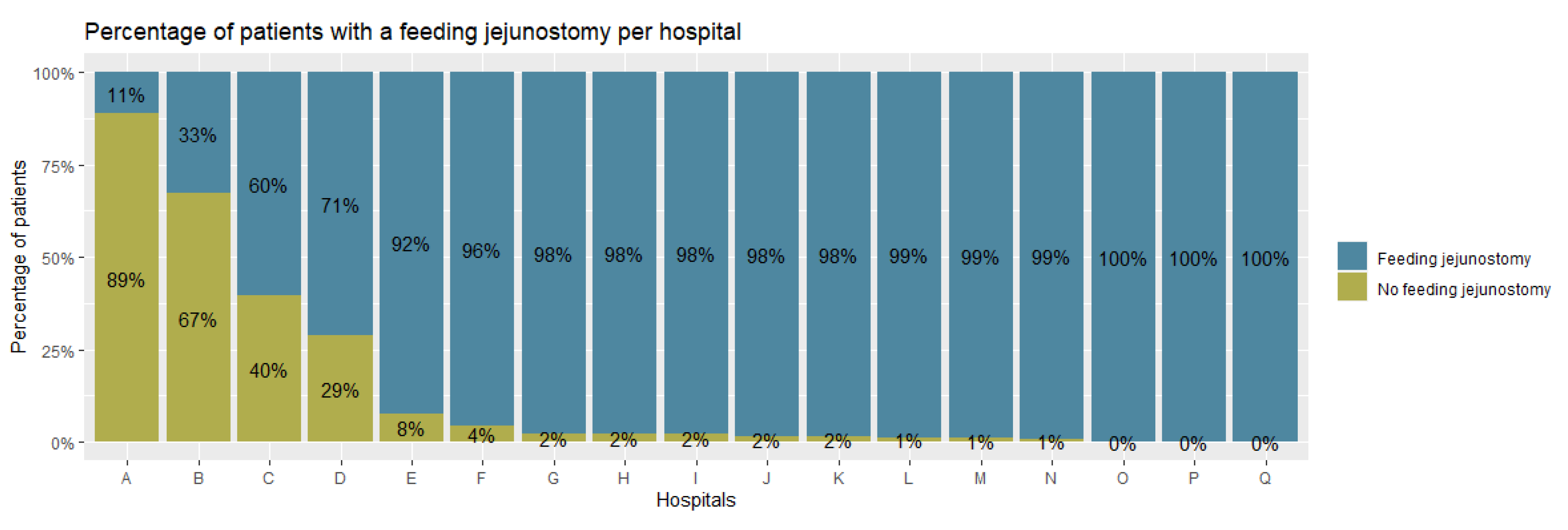
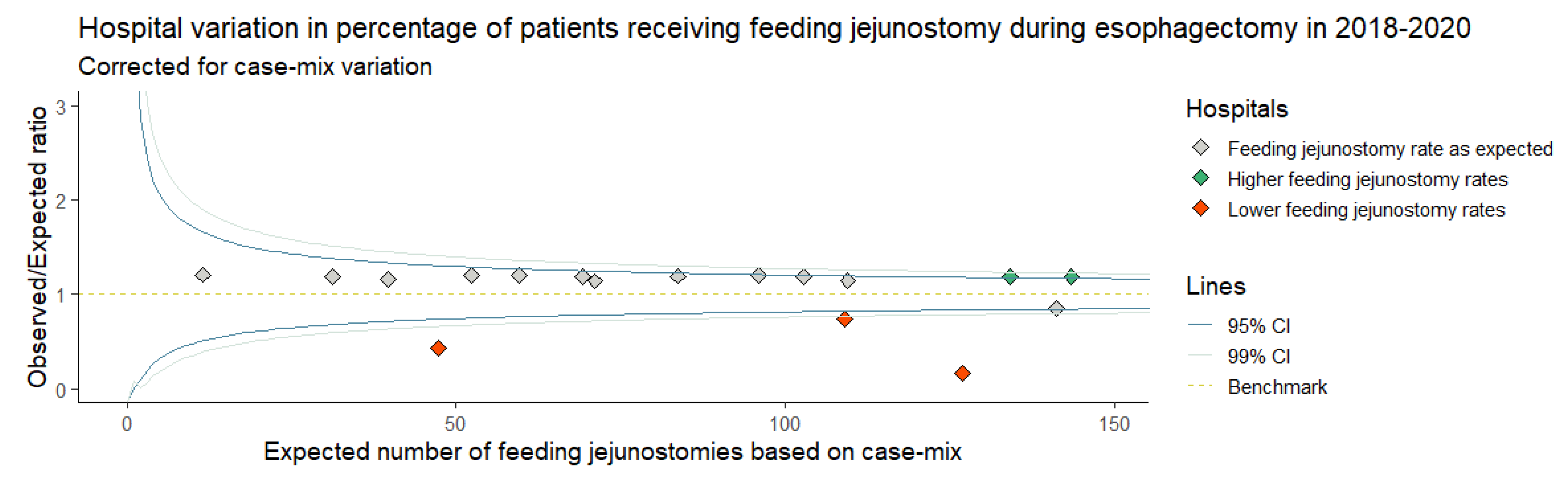
3. Results
3.1. Baseline Characteristics
3.2. Postoperative Outcomes
3.3. FJ-Related Complications
3.4. Routine vs. Non-Routine FJ Placement
3.5. Questionnaire
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2018, 68, 394. [Google Scholar] [CrossRef] [Green Version]
- Arnold, M.; Ferlay, J.; Van Berge Henegouwen, M.I.; Soerjomataram, I. Global Burden of Oesophageal and Gastric Cancer by Histology and Subsite in 2018. Gut 2020, 69, 1564. [Google Scholar] [CrossRef] [PubMed]
- Busweiler, L.A.D.; Jeremiasen, M.; Wijnhoven, B.P.L.; Lindblad, M.; Lundell, L.; van de Velde, C.J.H.; Tollenaar, R.A.E.M.; Wouters, M.W.J.M.; van Sandick, J.W.; Johansson, J.; et al. International Benchmarking in Oesophageal and Gastric Cancer Surgery. BJS Open 2019, 3, 62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Voeten, D.M.; Busweiler, L.A.D.; van der Werf, L.R.; Wijnhoven, B.P.L.; Verhoeven, R.H.A.; van Sandick, J.W.; van Hillegersberg, R.; van Berge Henegouwen, M.I. Outcomes of Esophagogastric Cancer Surgery during Eight Years of Surgical Auditing by the Dutch Upper Gastrointestinal Cancer Audit (DUCA). Ann. Surg. 2021, 274, 866–873. [Google Scholar] [CrossRef] [PubMed]
- Findlay, J.M.; Gillies, R.S.; Millo, J.; Sgromo, B.; Marshall, R.E.K.; Maynard, N.D. Enhanced Recovery for Esophagectomy: A Systematic Review and Evidence-Based Guidelines. Ann. Surg. 2014, 259, 41. [Google Scholar] [CrossRef]
- Berkelmans, G.H.; van Workum, F.; Weijs, T.J.; Nieuwenhuijzen, G.A.; Ruurda, J.P.; Kouwenhoven, E.A.; van Det, M.J.; Rosman, C.; van Hillegersberg, R.; Luyer, M.D. The Feeding Route after Esophagectomy: A Review of Literature. J. Thorac. Dis. 2017, 9, S785. [Google Scholar] [CrossRef] [Green Version]
- Zuccari, G.; Macis, S.; Alfei, S.; Marchitto, L.; Russo, E. The Role of the Pharmacist in Selecting the Best Choice of Medication Formulation in Dysphagic Patients. J. Pers. Med. 2022, 12, 1307. [Google Scholar] [CrossRef]
- Kassin, M.T.; Owen, R.M.; Perez, S.D.; Leeds, I.; Cox, J.C.; Schnier, K.; Sadiraj, V.; Sweeney, J.F. Risk Factors for 30-Day Hospital Readmission among General Surgery Patients. J. Am. Coll. Surg. 2012, 215, 322. [Google Scholar] [CrossRef] [Green Version]
- Shen, X.; Zhuo, Z.G.; Li, G.; Alai, G.H.; Song, T.N.; Xu, Z.J.; Yao, P.; Lin, Y.D. Is the Routine Placement of a Feeding Jejunostomy during Esophagectomy Worthwhile?-A Systematic Review and Meta-Analysis. Ann. Palliat. Med. 2021, 10, 4232. [Google Scholar] [CrossRef]
- Daly, J.M.; Fry, W.A.; Little, A.G.; Winchester, D.P.; McKee, R.F.; Stewart, A.K.; Fremgen, A.M. Esophageal Cancer: Results of American College of Surgeons Patient Care Evaluation Study. J. Am. Coll. Surg. 2000, 190, 562. [Google Scholar] [CrossRef]
- Martin, L.; Lagergren, J.; Lindblad, M.; Rouvelas, I.; Lagergren, P. Malnutrition after Oesophageal Cancer Surgery in Sweden. Br. J. Surg. 2007, 94, 1496. [Google Scholar] [CrossRef] [PubMed]
- Weijs, T.J.; Berkelmans, G.H.K.; Nieuwenhuijzen, G.A.P.; Ruurda, J.P.; Hillegersberg, R.V.; Soeters, P.B.; Luyer, M.D.P. Routes for Early Enteral Nutrition after Esophagectomy. A Systematic Review. Clin. Nutr. 2015, 34, 1. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, H.; Namikawa, T.; Iwabu, J.; Uemura, S.; Munekage, M.; Yokota, K.; Kobayashi, M.; Hanazaki, K. Bowel Obstruction Associated with a Feeding Jejunostomy and Its Association to Weight Loss after Thoracoscopic Esophagectomy. BMC Gastroenterol. 2019, 19, 1. [Google Scholar] [CrossRef] [Green Version]
- Kidane, B.; Kaaki, S.; Hirpara, D.H.; Shen, Y.C.; Bassili, A.; Allison, F.; Waddell, T.K.; Darling, G.E. Emergency Department Use Is High after Esophagectomy and Feeding Tube Problems Are the Biggest Culprit. J. Thorac. Cardiovasc. Surg. 2018, 156, 2340. [Google Scholar] [CrossRef]
- Spiegelhalter, D.J. Funnel Plots for Comparing Institutional Performance. Stat. Med. 2005, 24, 1185. [Google Scholar] [CrossRef] [PubMed]
- NOsborne, H.; Ko, C.Y.; Upchurch, G.R.; Dimick, J.B. The Impact of Adjusting for Reliability on Hospital Quality Rankings in Vascular Surgery. J. Vasc. Surg. 2011, 53, 1–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- RStudio Team. Rstudio; Integrated Development for R. RStudio, Inc.: Boston, MA, USA, 2019; Available online: http://www.Rstudio.com/ (accessed on 16 February 2021).
- Koterazawa, Y.; Oshikiri, T.; Hasegawa, H.; Yamamoto, M.; Kanaji, S.; Yamashita, K.; Matsuda, T.; Nakamura, T.; Suzuki, S.; Kakeji, Y. Routine Placement of Feeding Jejunostomy Tube during Esophagectomy Increases Postoperative Complications and Does Not Improve Postoperative Malnutrition. Dis. Esophagus 2020, 33, doz021. [Google Scholar] [CrossRef]
- Janssen, H.J.B.; Gantxegi, A.; Fransen, L.F.C.; Nieuwenhuijzen, G.A.P.; Luyer, M.D.P. Risk Factors for Failure of Direct Oral Feeding Following a Totally Minimally Invasive Esophagectomy. Nutrients 2021, 13, 3616. [Google Scholar] [CrossRef] [PubMed]
- Fransen, L.F.C.; Janssen, T.H.J.B.; Aarnoudse, M.; Nieuwenhuijzen, G.A.P.; Luyer, M.D.P. Direct Oral Feeding After a Minimally Invasive Esophagectomy. Ann. Surg. 2020, 275, 919–923. [Google Scholar] [CrossRef]
- Holmén, A.; Hayami, M.; Szabo, E.; Rouvelas, I.; Agustsson, T.; Klevebro, F. Nutritional Jejunostomy in Esophagectomy for Cancer, a National Register-Based Cohort Study of Associations with Postoperative Outcomes and Survival. Langenbeck’s Arch. Surg. 2021, 406, 1415. [Google Scholar] [CrossRef]
- TKroese, E.; Tapias, L.; Olive, J.K.; Trager, L.E.; Morse, C.R. Routine Intraoperative Jejunostomy Placement and Minimally Invasive Oesophagectomy: An Unnecessary Step? Eur. J. Cardio-Thorac. Surg. 2019, 56, 746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, R.; Rios-Diaz, A.J.; Liem, S.; Devin, C.L.; Evans, N.R.; Rosato, E.L.; Palazzo, F.; Berger, A.C. Is the Placement of Jejunostomy Tubes in Patients with Esophageal Cancer Undergoing Esophagectomy Associated with Increased Inpatient Healthcare Utilization? An Analysis of the National Readmissions Database. Am. J. Surg. 2021, 221, 141. [Google Scholar] [CrossRef] [PubMed]
- Zheng, R.; Devin, C.L.; Pucci, M.J.; Berger, A.C.; Rosato, E.L.; Palazzo, F. Optimal Timing and Route of Nutritional Support after Esophagectomy: A Review of the Literature. World J. Gastroenterol. 2019, 25, 4427. [Google Scholar] [CrossRef]
- Weijs, T.J.; Berkelmans, G.H.K.; Nieuwenhuijzen, G.A.P.; Dolmans, A.C.P.; Kouwenhoven, E.A.; Rosman, C.; Ruurda, J.P.; van Workum, F.; van Det, M.J.; Corten, L.C.S.; et al. Immediate Postoperative Oral Nutrition Following Esophagectomy: A Multicenter Clinical Trial. Ann. Thorac. Surg. 2016, 102, 1141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berkelmans, G.H.K.; Fransen, L.F.C.; Dolmans-Zwartjes, A.C.P.; Kouwenhoven, E.A.; van Det, M.J.; Nilsson, M.; Nieuwenhuijzen, G.A.P.; Luyer, M.D.P. Direct Oral Feeding Following Minimally Invasive Esophagectomy (NUTRIENT II Trial): An International, Multicenter, Open-Label Randomized Controlled Trial. Ann. Surg. 2020, 271, 41. [Google Scholar] [CrossRef]
- Yang, Y.H.; Park, S.Y.; Kim, D.J. Chyle Leakage after Esophageal Cancer Surgery. Korean J. Thorac. Cardiovasc. Surg. 2020, 53, 191. [Google Scholar] [CrossRef]
- Wouters, M.W.J.M.; Gooiker, G.A.; van Sandick, J.W.; Tollenaar, R.A.E.M. The Volume-Outcome Relation in the Surgical Treatment of Esophageal Cancer: A Systematic Review and Meta-Analysis. Cancer 2012, 118, 1754. [Google Scholar] [CrossRef]
- Markar, S.R.; Karthikesalingam, A.; Thrumurthy, S.; Low, D.E. Volume-Outcome Relationship in Surgery for Esophageal Malignancy: Systematic Review and Meta-Analysis 2000–2011. J. Gastrointest. Surg. Off. J. Soc. Surg. Aliment. Tract 2012, 16, 1055. [Google Scholar] [CrossRef] [PubMed]
- Voeten, D.M.; Gisbertz, S.S.; Ruurda, J.P.; Wilschut, J.A.; Ferri, L.E.; van Hillegersberg, R.; van Berge Henegouwen, M.I. Overall Volume Trends in Esophageal Cancer Surgery Results From the Dutch Upper Gastrointestinal Cancer Audit. Ann. Surg. 2021, 274, 449. [Google Scholar] [CrossRef]
| Patients without FJ N (%) | Patient with FJ N (%) | Total N (%) | p-Value (χ2/Fisher) | |
|---|---|---|---|---|
| Total | 330 (100%) | 1481 (100%) | 1811 (100%) | |
| Sex | 0.054 | |||
| Male | 243 (73.6%) | 1163 (78.5%) | 1406 (77.6%) | |
| Female | 87 (26.4%) | 318 (21.5%) | 405 (22.4%) | |
| Age in years | 0.132 | |||
| <65 | 135 (40.9%) | 539 (36.4%) | 674 (37.2%) | |
| 65–75 | 163 (49.4%) | 748 (50.5%) | 911 (50.3%) | |
| >75 | 32 (9.7%) | 194 (13.1%) | 226 (12.5%) | |
| Preoperative weight loss (kg) | 0.150 | |||
| No weight loss | 104 (31.5%) | 460 (31.1%) | 564 (31.1%) | |
| 1–5 | 81 (24.5%) | 462 (31.2%) | 543 (30.0%) | |
| 6–10 | 84 (25.5%) | 324 (21.9%) | 408 (22.5%) | |
| >10 | 48 (14.5%) | 181 (12.2%) | 229 (12.6%) | |
| Missing | 13 (3.9%) | 54 (3.6%) | 67 (3.7%) | |
| Body Mass Index (BMI) (kg/m2) | 0.059 | |||
| <20 | 14 (4.2%) | 102 (6.9%) | 116 (6.4%) | |
| 20–25 | 155 (47.0%) | 690 (46.6%) | 845 (46.7%) | |
| 26–20 | 108 (32.7%) | 519 (35.0%) | 627 (34.6%) | |
| >30 | 51 (15.5%) | 166 (11.2%) | 217 (12.0%) | |
| Missing | 2 (0.6%) | 4 (0.3%) | 6 (0.3%) | |
| Charlson Comorbidity Index | 0.010 | |||
| 0 | 126 (38.2%) | 673 (45.4%) | 799 (44.1%) | |
| 1 | 72 (21.8%) | 371 (25.1%) | 443 (24.5%) | |
| 2+ | 122 (37.0%) | 437 (29.5%) | 559 (30.9%) | |
| Missing | 10 (3.0%) | 0 (0%) | 10 (0.6%) | |
| ASA | 0.894 | |||
| 1–2 | 227 (68.8%) | 1007 (68.0%) | 1234 (68.1%) | |
| 3+ | 103 (31.2%) | 465 (31.4%) | 568 (31.4%) | |
| Missing | 0 (0%) | 9 (0.6%) | 9 (0.5%) | |
| Diabetes | 0.393 | |||
| No | 269 (81.5%) | 1220 (82.4%) | 1489 (82.2%) | |
| Yes | 48 (14.5%) | 252 (17.0%) | 300 (16.6%) | |
| Missing | 13 (3.9%) | 9 (0.6%) | 22 (1.2%) | |
| Previous esophagogastric surgery | 0.133 | |||
| Yes | 6 (1.8%) | 13 (0.9%) | 19 (1.0%) | |
| No | 324 (98.2%) | 1458 (98.4%) | 1782 (98.4%) | |
| Unknown/missing | 0 (0%) | 10 (0.7%) | 10 (0.6%) | |
| Tumor location | 0.708 | |||
| Intrathoracic esophagus | 256 (77.6%) | 1170 (79.0%) | 1426 (78.7%) | |
| Gastro-esophageal junction | 72 (21.8%) | 298 (20.1%) | 370 (20.4%) | |
| Unknown/missing | 2 (0.6%) | 13 (0.9%) | 15 (0.8%) | |
| Histology | 0.091 | |||
| Adenocarcinoma | 278 (84.2%) | 1191 (80.4%) | 1469 (81.1%) | |
| Squamous cell carcinoma | 40 (12.1%) | 252 (17.0%) | 292 (16.1%) | |
| Other/unknown | 8 (2.4%) | 29 (2.0%) | 37 (2.0%) | |
| Missing | 4 (1.2%) | 9 (0.6%) | 13 (0.7%) | |
| Clinical tumor stage | 0.015 | |||
| T0–2 | 59 (17.9%) | 308 (20.8%) | 367 (20.3%) | |
| T3–4 | 250 (75.8%) | 1119 (75.6%) | 1369 (75.6%) | |
| Tx | 21 (6.4%) | 47 (3.2%) | 68 (3.8%) | |
| Missing | 0 (0%) | 7 (0.5%) | 7 (0.4%) | |
| Clinical node stage | 0.017 | |||
| N0 | 125 (37.9%) | 552 (37.3%) | 677 (37.4%) | |
| N+ | 186 (56.4%) | 882 (59.6%) | 1068 (59.0%) | |
| Nx | 19 (5.8%) | 40 (2.7%) | 59 (3.3%) | |
| Missing | 0 (0%) | 7 (0.5%) | 7 (0.4%) | |
| Neoadjuvant therapy | 0.930 | |||
| None | 17 (5.2%) | 79 (5.3%) | 96 (5.3%) | |
| Chemoradiotherapy | 281 (85.2%) | 1267 (85.6%) | 1548 (85.5%) | |
| Chemotherapy | 32 (9.7%) | 134 (9.0%) | 32 (9.7%) | |
| Other/missing | 0 (0%) | 1 (0.1%) | 0 (0%) | |
| Surgical procedure | <0.001 | |||
| Transhiatal | 16 (4.8%) | 166 (11.2%) | 182 (10.0%) | |
| Transthoracic | 314 (95.2%) | 1315 (88.8%) | 1629 (90.0%) | |
| Anastomotic location | <0.001 | |||
| Intrathoracic | 246 (74.5%) | 856 (57.8%) | 1102 (60.9%) | |
| Cervical | 84 (25.5%) | 621 (41.9%) | 705 (38.9%) | |
| None/other/missing | 0 (0%) | 4 (0.2%) | 4 (0.2%) | |
| Hospital volume (esophageal resections per year) | <0.001 | |||
| ≤40 | 51 (15.5%) | 418 (28.2%) | 469 (25.9%) | |
| >40 | 279 (84.5%) | 1056 (71.3%) | 1335 (73.7%) | |
| Missing | 0 (0%) | 7 (0.5%) | 7 (0.4%) |
| FJ Placement | Outcome Incidence (%) | Corrected for a | aOR a | 95% CI b | p-Value | |
|---|---|---|---|---|---|---|
| Overall intraoperative complications (yes) | No Yes | 13 (4.3%) 49 (3.5%) | No relevant confounders identified | 1–0.81 | 0.45–1.58 | 0.510 |
| Overall postoperative complications (yes) | No Yes | 180 (59.2%) 901 (64.3%) | All | 1–1.27 | 0.88–1.83 | 0.199 |
| Severe complications c (yes) | No Yes | 96 (31.6%) 427 (30.5%) | All | 1–1.04 | 0.73–1.48 | 0.822 |
| 30-day/in-hospital mortality (yes) | No Yes | 5 (1.6%) 42 (3.0%) | No relevant confounders identified | 1–1.85 | 0.80–5.39 | 0.198 |
| Chyle leakage (yes) | No Yes | 14 (4.6%) 133 (9.5%) | No relevant confounders identified | 1–2.16 | 1.27–3.98 | 0.007 |
| Anastomotic leakage (yes) | No Yes | 40 (13.2%) 259 (18.5%) | All | 1–1.36 | 0.84–2.22 | 0.210 |
| Pulmonary complication (yes) | No Yes | 93 (30.6%) 441 (31.5%) | All | 1–1.06 | 0.82–1.41 | 0.670 |
| Pneumonia (yes) | No Yes | 49 (16.1%) 311 (22.2%) | All | 1–1.38 | 0.90–2.11 | 0.147 |
| Wound infection (yes) | No Yes | 9 (3.0%) 51 (3.6%) | Type of esophagectomy, anastomotic location, and hospital volume | 1–0.80 | 0.39–1.80 | 0.560 |
| Length of hospital stay (≤ 10 days) | No Yes | 196 (64.5%) 706 (50.4%) | All | 1–0.62 | 0.42–0.90 | 0.013 |
| Prolonged hospital stay (>30 days) | No Yes | 24 (7.9%) 139 (9.9%) | No relevant confounders identified | 1–1.06 | 0.57–1.96 | 0.856 |
| 30-day readmission (yes) | No Yes | 49 (16.1%) 217 (15.5%) | All | 1–0.91 | 0.65–1.31 | 0.630 |
| Reintervention (yes) | No Yes | 86 (28.3%) 348 (24.8%) | All | 1–0.88 | 0.61–1.27 | 0.500 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Visser, M.R.; Straatman, J.; Voeten, D.M.; Gisbertz, S.S.; Ruurda, J.P.; Luyer, M.D.P.; van der Sluis, P.C.; van der Peet, D.L.; van Berge Henegouwen, M.I.; van Hillegersberg, R., on behalf of the Dutch Upper Gastrointestinal Cancer Audit (DUCA) Group. Hospital Variation in Feeding Jejunostomy Policy for Minimally Invasive Esophagectomy: A Nationwide Cohort Study. Nutrients 2023, 15, 154. https://doi.org/10.3390/nu15010154
Visser MR, Straatman J, Voeten DM, Gisbertz SS, Ruurda JP, Luyer MDP, van der Sluis PC, van der Peet DL, van Berge Henegouwen MI, van Hillegersberg R on behalf of the Dutch Upper Gastrointestinal Cancer Audit (DUCA) Group. Hospital Variation in Feeding Jejunostomy Policy for Minimally Invasive Esophagectomy: A Nationwide Cohort Study. Nutrients. 2023; 15(1):154. https://doi.org/10.3390/nu15010154
Chicago/Turabian StyleVisser, Maurits R., Jennifer Straatman, Daan M. Voeten, Suzanne S. Gisbertz, Jelle. P. Ruurda, Misha D. P. Luyer, Pieter C. van der Sluis, Donald L. van der Peet, Mark I. van Berge Henegouwen, and Richard van Hillegersberg on behalf of the Dutch Upper Gastrointestinal Cancer Audit (DUCA) Group. 2023. "Hospital Variation in Feeding Jejunostomy Policy for Minimally Invasive Esophagectomy: A Nationwide Cohort Study" Nutrients 15, no. 1: 154. https://doi.org/10.3390/nu15010154
APA StyleVisser, M. R., Straatman, J., Voeten, D. M., Gisbertz, S. S., Ruurda, J. P., Luyer, M. D. P., van der Sluis, P. C., van der Peet, D. L., van Berge Henegouwen, M. I., & van Hillegersberg, R., on behalf of the Dutch Upper Gastrointestinal Cancer Audit (DUCA) Group. (2023). Hospital Variation in Feeding Jejunostomy Policy for Minimally Invasive Esophagectomy: A Nationwide Cohort Study. Nutrients, 15(1), 154. https://doi.org/10.3390/nu15010154








