Catalpol Attenuates Oxidative Stress and Inflammation via Mechanisms Involving Sirtuin-1 Activation and NF-κB Inhibition in Experimentally-Induced Chronic Kidney Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Experimental Design
- The control group was given standard feed along with saline (10 mL/kg) via oral gavage.
- The adenine group was given the same standard diet in powdered form containing 0.2% adenine (0.2 g of adenine in 100 g of powdered feed) with saline (10 mL/kg) via oral gavage.
- The CAT group was given standard feed with CAT (5 mg/kg, dissolved in saline) via oral gavage.
- The CAT + adenine group was given 0.2% adenine prepared similar to the adenine group with CAT (5 mg/kg, dissolved in saline) via oral gavage.
2.3. Homogenization of the Kidney
2.4. Biochemical Parameters
2.5. Measurement of Markers of Kidney Injury in Plasma
2.6. Assessment of Thiobarbituric Acid Reactive Substances (TBARS) and Catalase Activity in Kidney Homogenates
2.7. Measurement of Markers of Inflammation in Kidney Homogenates
2.8. DNA Damage Analysis by Comet Assay
2.9. Assessment of the Levels of Procaspase-3 and Cleaved Caspase-3 in Kidney Homogenates
2.10. Western Blot Analysis of NF-κB and Phospho-NF-κB in Kidney Homogenates
2.11. Measurement of the Concentration of Sirtuin-1 in Kidney Homogenates
2.12. Histopathological Analysis
2.13. Statistics
3. Results
3.1. The Effects of CAT on Physiological and Biochemical Parameters
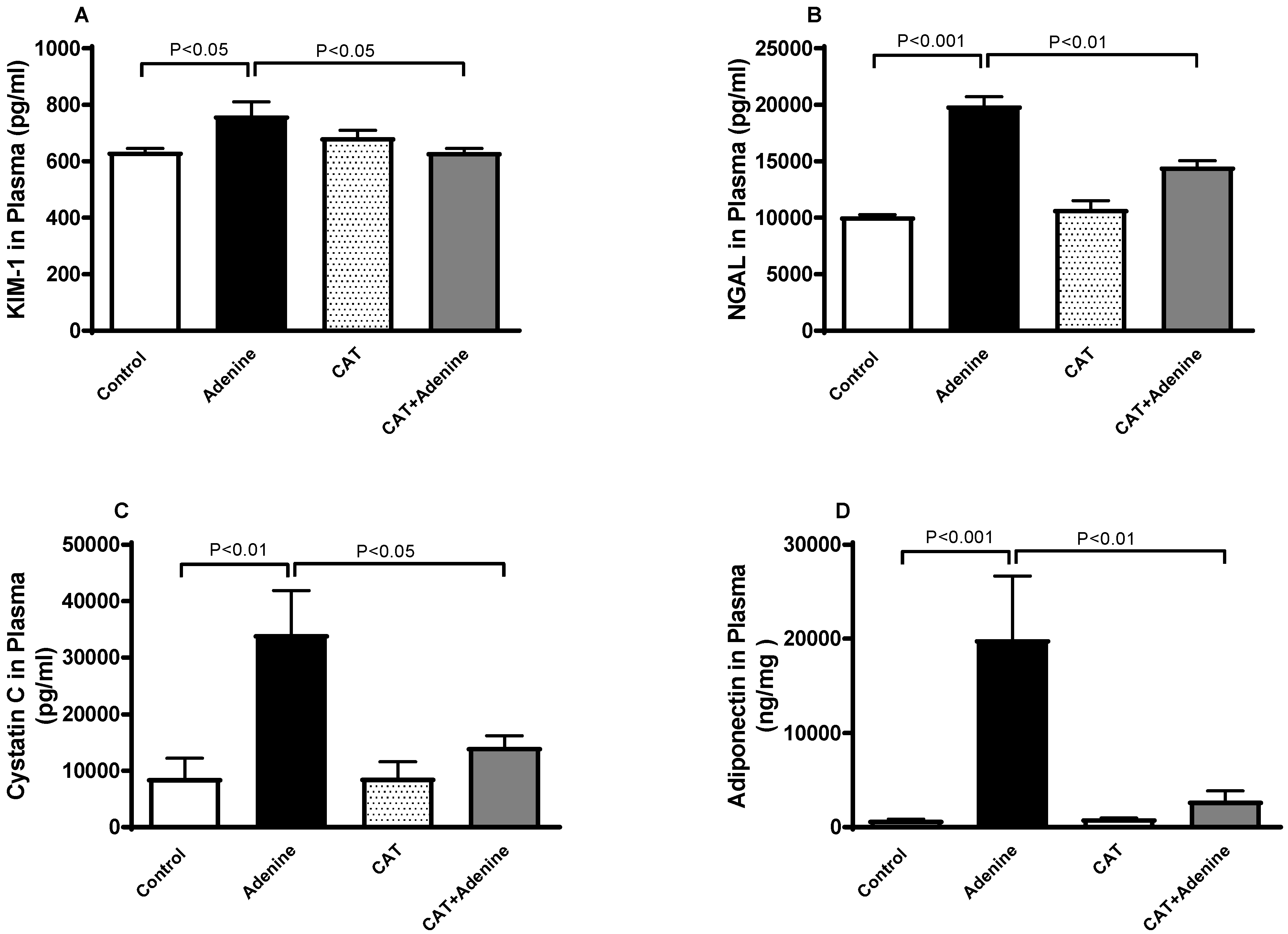
3.2. The Effects of CAT on the Concentrations of Markers of Kidney Injury in Plasma
3.3. The Effects of CAT on Lipid Peroxidation and Antioxidant Levels in Kidney Homogenates
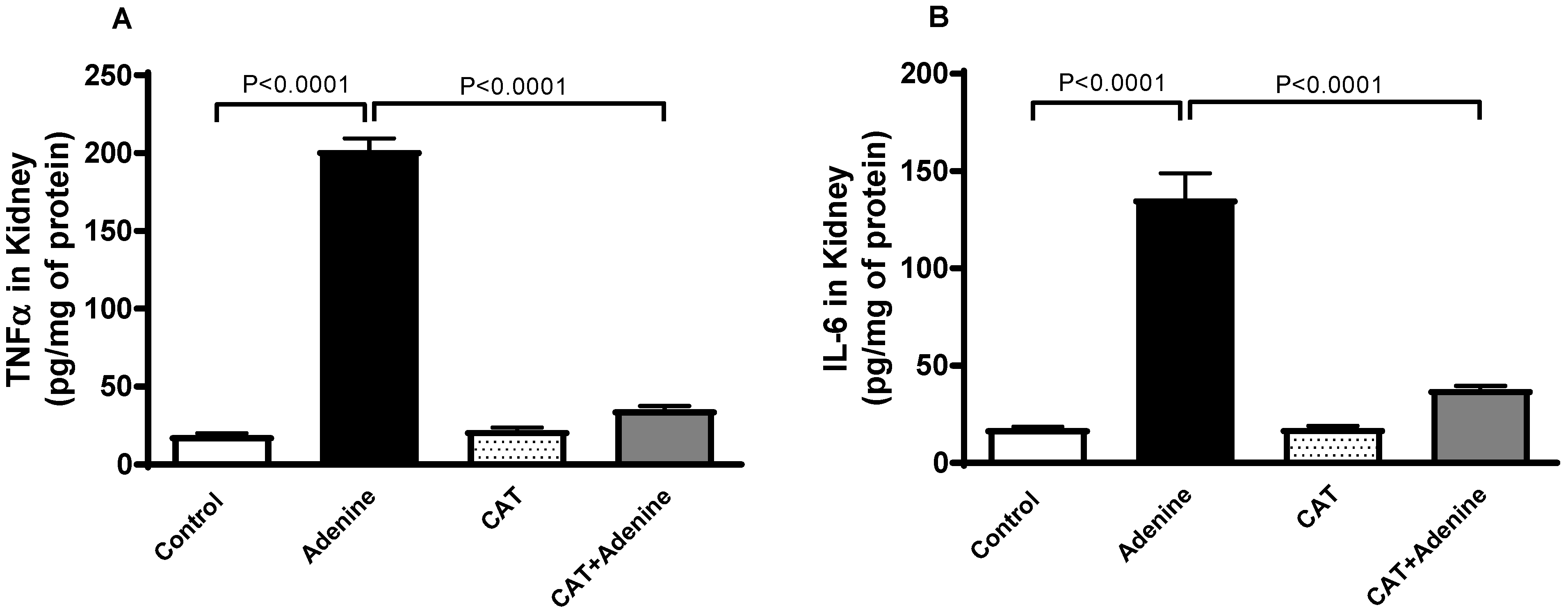
3.4. The Effects of CAT on the Levels of Proinflammatory Cytokines in Kidney Homogenates
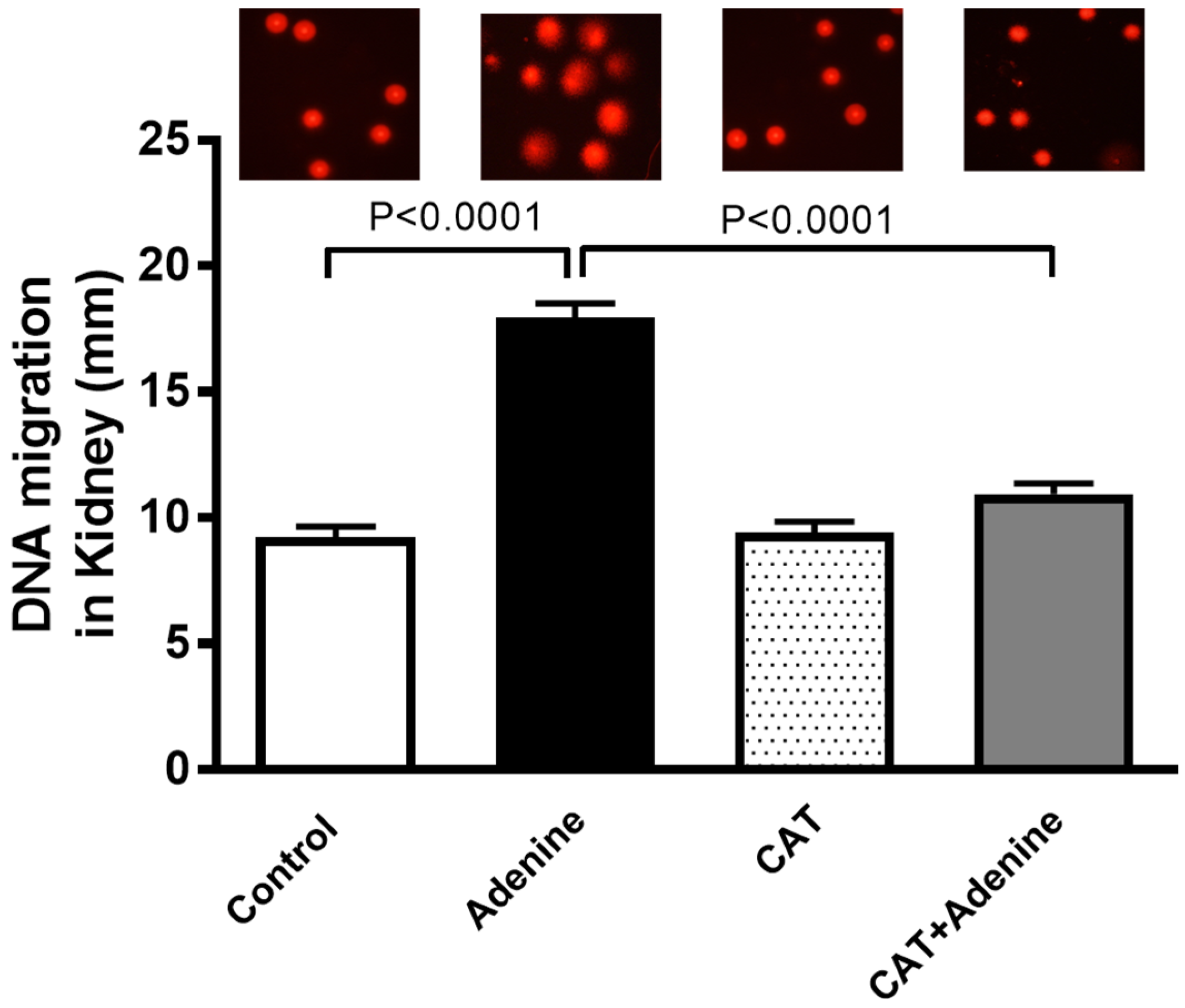
3.5. The Effects of CAT on Kidney DNA Damage
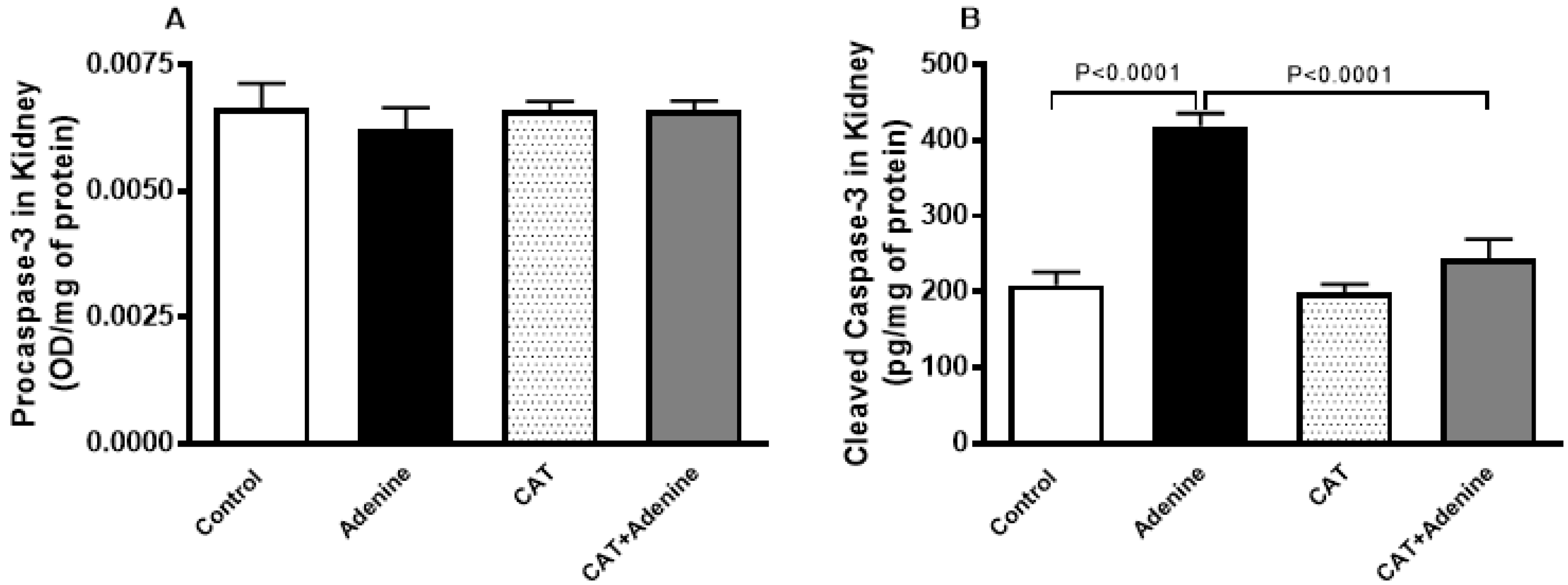
3.6. The Effects of CAT on the Levels of Procaspase-3 and Cleaved Caspase-3 in Kidney Homogenate
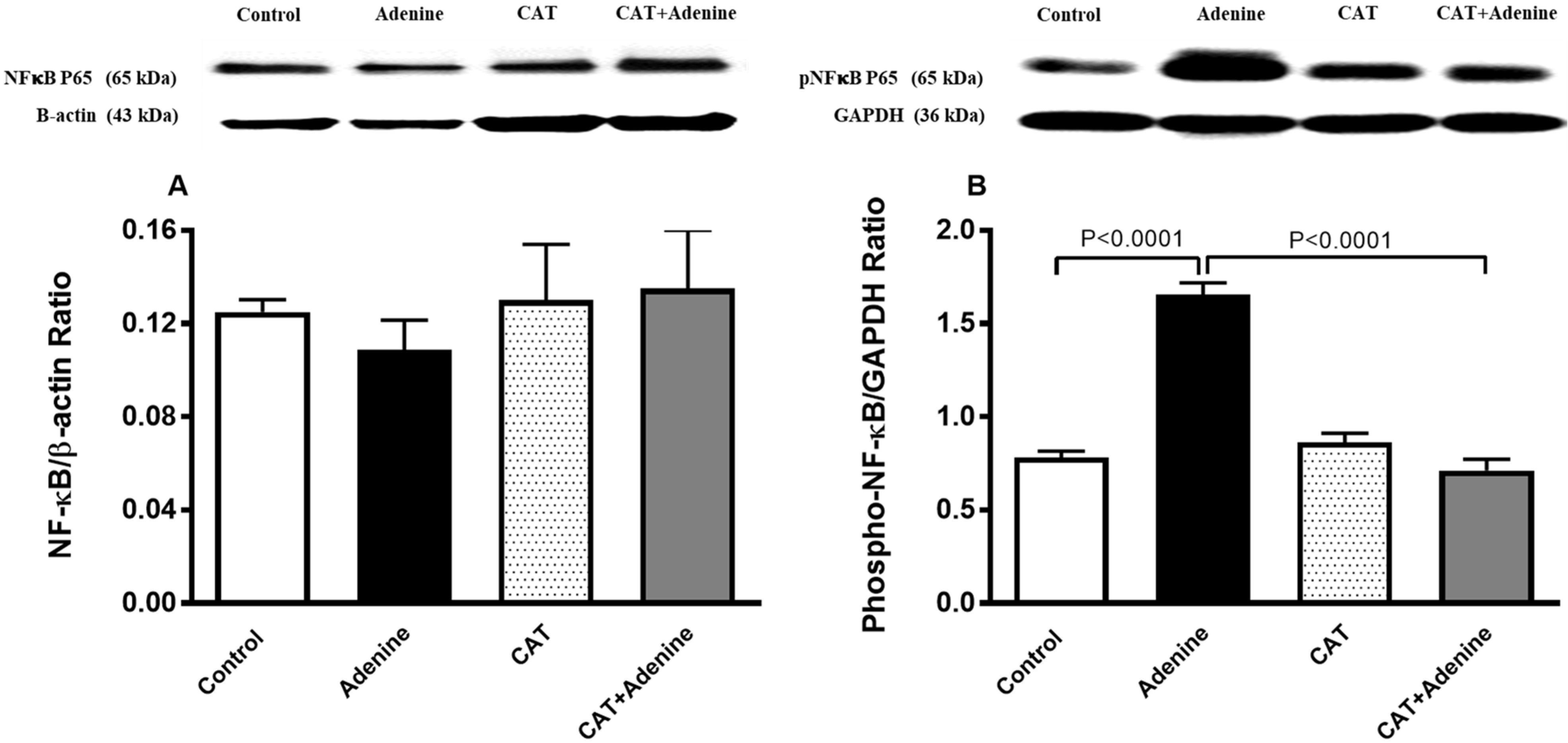
3.7. The Effects of CAT on the Expression of NF-κB and Phospho-NF-κB in Kidney Homogenate
3.8. The Effects of CAT on the Concentrations of Sirtuin-1 in Kidney Homogenate
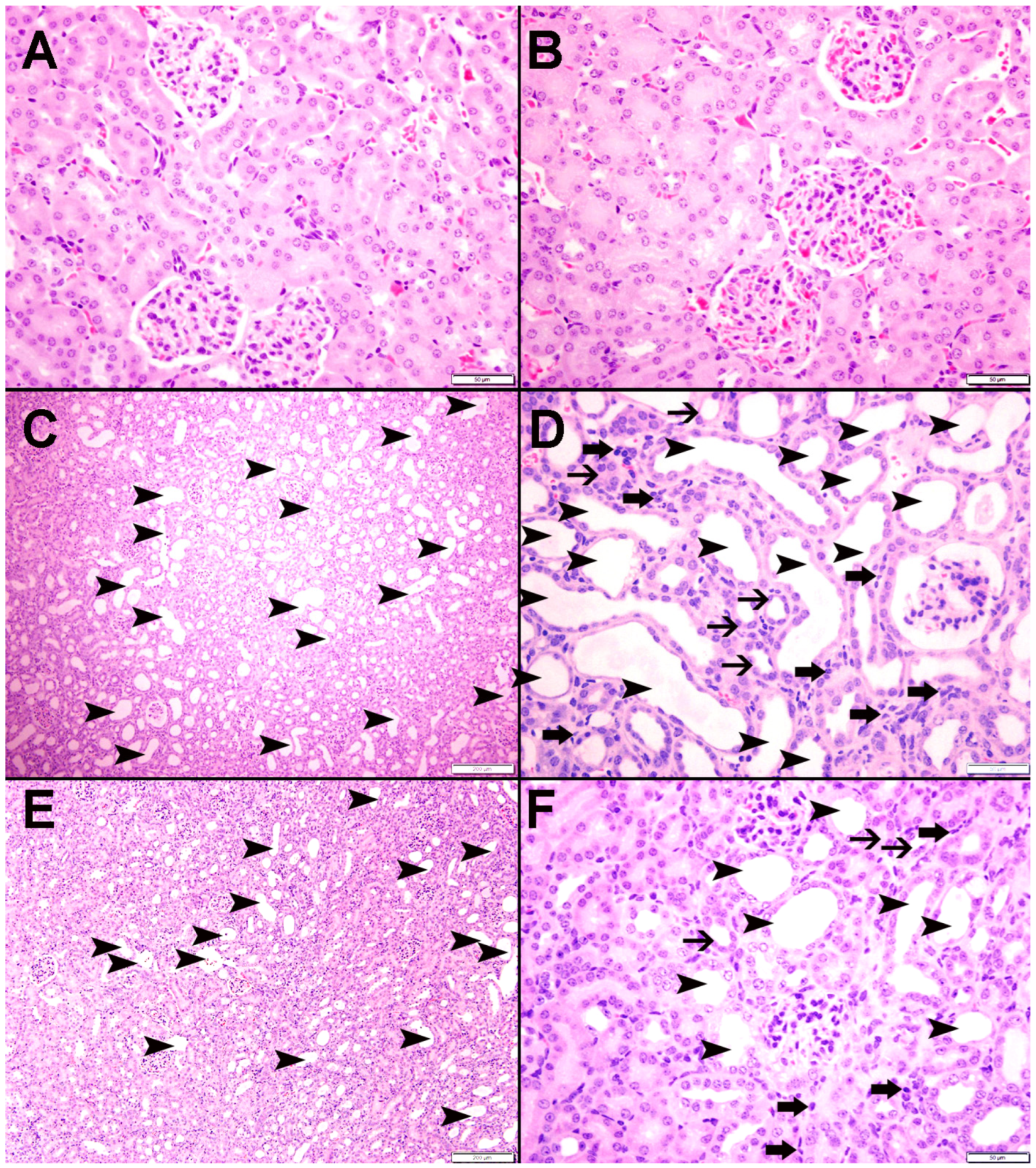
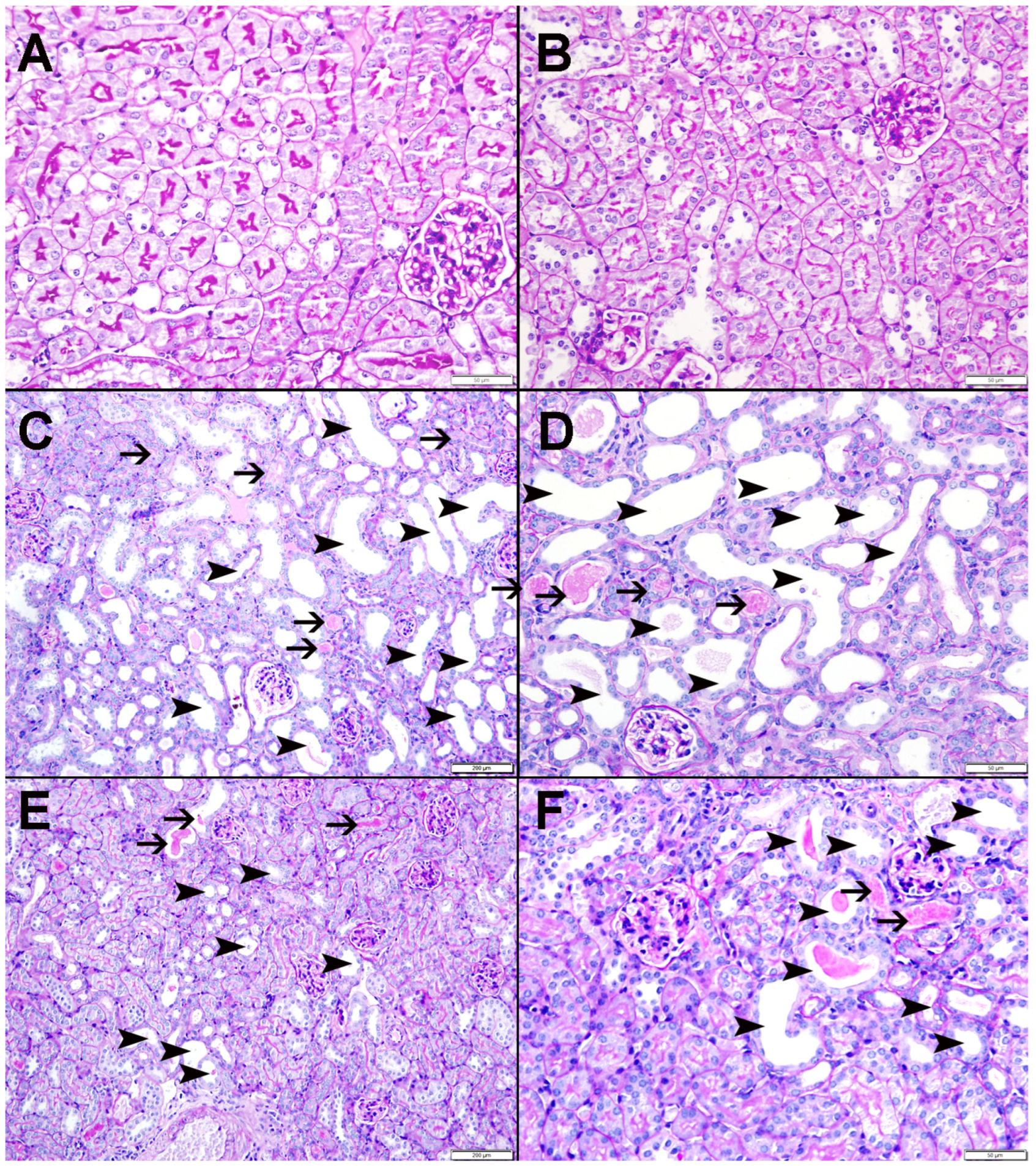
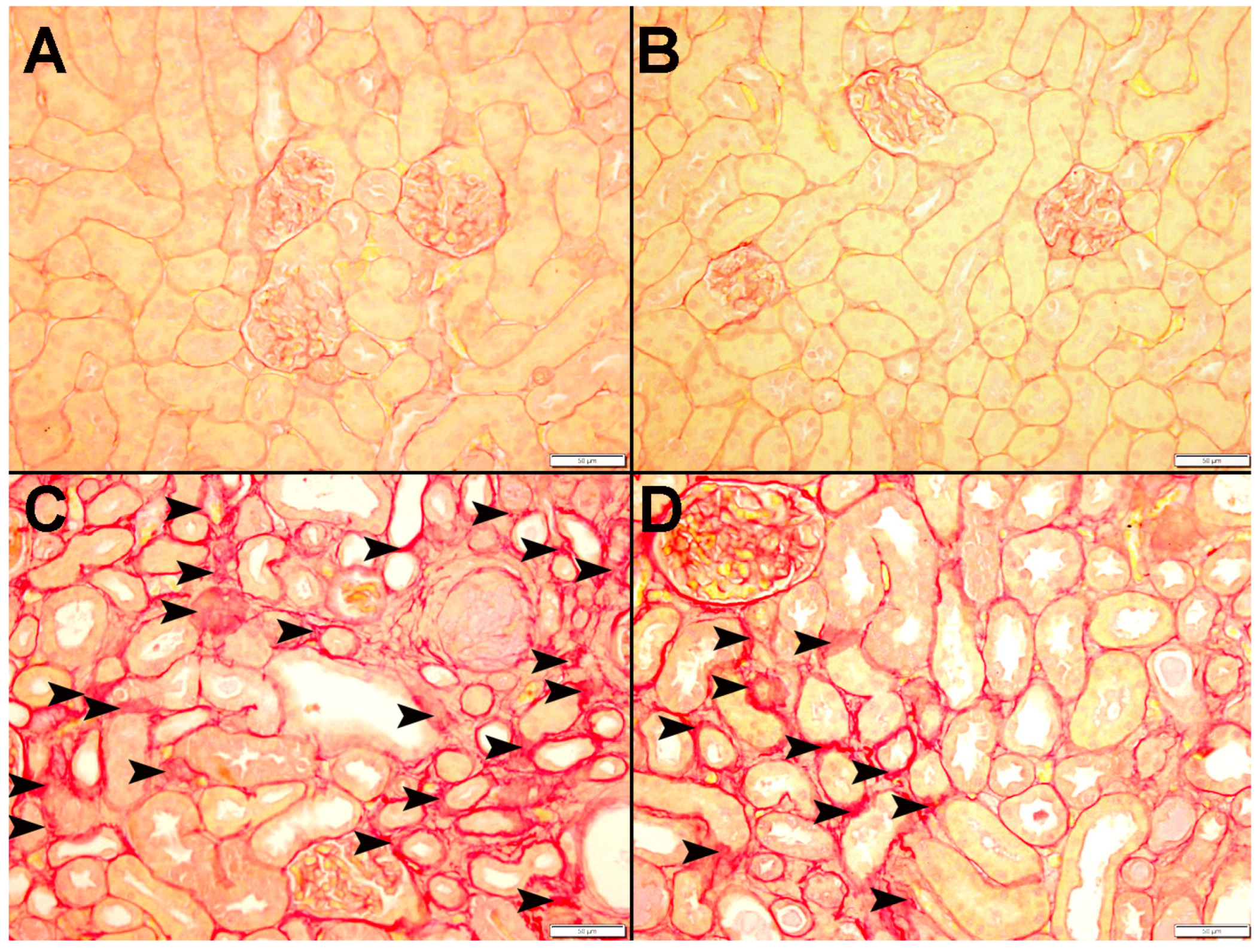
3.9. Histopathological Findings
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gusev, E.; Solomatina, L.; Zhuravleva, Y.; Sarapultsev, A. The Pathogenesis of End-Stage Renal Disease from the Standpoint of the Theory of General Pathological Processes of Inflammation. Int. J. Mol. Sci. 2021, 22, 11453. [Google Scholar] [CrossRef] [PubMed]
- Smyth, A. End-stage renal disease and renal replacement therapy in older patients. Nephro-Urol. Mon. 2012, 4, 425–430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garla, V.; Kanduri, S.; Yanes-Cardozo, L.; Lién, L.F. Management of diabetes mellitus in chronic kidney disease. Minerva Endocrinol. 2019, 44, 273–287. [Google Scholar] [CrossRef] [PubMed]
- Wen, C.P.; Cheng, T.Y.D.; Tsai, M.K.; Chang, Y.C.; Chan, H.T.; Tsai, S.P.; Chiang, P.H.; Hsu, C.C.; Sung, P.K.; Hsu, Y.H.; et al. All-cause mortality attributable to chronic kidney disease: A prospective cohort study based on 462,293 adults in Taiwan. Lancet 2008, 371, 2173–2182. [Google Scholar] [CrossRef]
- Yi, H.; Huang, C.; Shi, Y.; Cao, Q.; Chen, J.; Chen, X.-M.; Pollock, C.A. Metformin Attenuates Renal Fibrosis in a Mouse Model of Adenine-Induced Renal Injury Through Inhibiting TGF-β1 Signaling Pathways. Front. Cell Dev. Biol. 2021, 9, 603802. [Google Scholar] [CrossRef]
- Turner, J.M.; Bauer, C.; Abramowitz, M.K.; Melamed, M.L.; Hostetter, T.H. Treatment of chronic kidney disease. Kidney Int. 2012, 81, 351–362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruiz-Ortega, M.; Rayego-Mateos, S.; Lamas, S.; Ortiz, A.; Rodrigues-Diez, R.R. Targeting the progression of chronic kidney disease. Nat. Rev. Nephrol. 2020, 16, 269–288. [Google Scholar] [CrossRef]
- Ali, B.H.; Al-Salam, S.; Al Suleimani, Y.; Al Kalbani, J.; Al Bahlani, S.; Ashique, M.; Manoj, P.; Al Dhahli, B.; Al Abri, N.; Naser, H.T.; et al. Curcumin Ameliorates Kidney Function and Oxidative Stress in Experimental Chronic Kidney Disease. Basic Clin. Pharmacol. Toxicol. 2018, 122, 65–73. [Google Scholar] [CrossRef] [Green Version]
- Kalantar-Zadeh, K.; Jafar, T.H.; Nitsch, D.; Neuen, B.L.; Perkovic, V. Chronic kidney disease. Lancet 2021, 398, 786–802. [Google Scholar] [CrossRef]
- Diwan, V.; Brown, L.; Gobe, G.C. Adenine-induced chronic kidney disease in rats. Nephrology 2017, 23, 5–11. [Google Scholar] [CrossRef]
- Ali, B.H.; Adham, S.A.; Al Za’Abi, M.; Waly, M.; Yasin, J.; Nemmar, A.; Schupp, N. Ameliorative Effect of Chrysin on Adenine-Induced Chronic Kidney Disease in Rats. PLoS ONE 2015, 10, e0125285. [Google Scholar] [CrossRef] [PubMed]
- Nemmar, A.; Al-Salam, S.; Beegam, S.; Zaaba, N.E.; Yasin, J.; Hamadi, N.; Ali, B.H. Cardiac Inflammation, Oxidative Stress, Nrf2 Expression, and Coagulation Events in Mice with Experimental Chronic Kidney Disease. Oxidative Med. Cell. Longev. 2021, 2021, 8845607. [Google Scholar] [CrossRef] [PubMed]
- Nemmar, A.; Karaca, T.; Beegam, S.; Yuvaraju, P.; Yasin, J.; Ali, B.H. Lung Oxidative Stress, DNA Damage, Apoptosis, and Fibrosis in Adenine-Induced Chronic Kidney Disease in Mice. Front. Physiol. 2017, 8, 896. [Google Scholar] [CrossRef] [Green Version]
- Rapa, S.F.; di Iorio, B.R.; Campiglia, P.; Heidland, A.; Marzocco, S. Inflammation and oxidative stress in chronic kidney disease—Potential therapeutic role of minerals, vitamins and plant-derived metabolites. Int. J. Mol. Sci. 2020, 21, 263. [Google Scholar] [CrossRef] [Green Version]
- Bai, Y.; Zhu, R.; Tian, Y.; Li, R.; Chen, B.; Zhang, H.; Xia, B.; Zhao, D.; Mo, F.; Zhang, D.; et al. Catalpol in Diabetes and its Complications: A Review of Pharmacology, Pharmacokinetics, and Safety. Molecules 2019, 24, 3302. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Wu, Z.M.; Yang, Y.P.; Shaukat, A.; Yang, J.; Guo, Y.F.; Zhang, T.; Zhu, X.Y.; Qiu, J.X.; Deng, G.Z.; et al. Catalpol ameliorates LPS-induced endometritis by inhibiting inflammation and TLR4/NF-κB signaling. J. Zhejiang Univ. Sci. B 2019, 20, 816–827. [Google Scholar] [CrossRef] [PubMed]
- Shu, A.; Du, Q.; Chen, J.; Gao, Y.; Zhu, Y.; Lv, G.; Lu, J.; Chen, Y.; Xu, H. Catalpol ameliorates endothelial dysfunction and inflammation in diabetic nephropathy via suppression of RAGE/RhoA/ROCK signaling pathway. Chem. Interactions 2021, 348, 109625. [Google Scholar] [CrossRef]
- Bi, J.; Jiang, B.; Liu, J.H.; Lei, C.; Zhang, X.L.; An, L.-J. Protective effects of catalpol against H2O2-induced oxidative stress in astrocytes primary cultures. Neurosci. Lett. 2008, 442, 224–227. [Google Scholar] [CrossRef]
- Mao, Y.-R.; Jiang, L.; Duan, Y.-L.; An, L.-J.; Jiang, B. Efficacy of catalpol as protectant against oxidative stress and mitochondrial dysfunction on rotenone-induced toxicity in mice brain. Environ. Toxicol. Pharmacol. 2007, 23, 314–318. [Google Scholar] [CrossRef]
- Ge, H.; Lin, W.; Lou, Z.; Chen, R.; Shi, H.; Zhao, Q.; Lin, Z. Catalpol alleviates myocardial ischemia reperfusion injury by activating the Nrf2/HO-1 signaling pathway. Microvasc. Res. 2022, 140, 104302. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, J.; Jiang, M.; Fu, Y.; Zhu, Y.; Jiao, N.; Liu, L.; Du, Q.; Wu, H.; Xu, H.; et al. Loganin and catalpol exert cooperative ameliorating effects on podocyte apoptosis upon diabetic nephropathy by targeting AGEs-RAGE signaling. Life Sci. 2020, 252, 117653. [Google Scholar] [CrossRef] [PubMed]
- Yap, K.H.; Yee, G.S.; Candasamy, M.; Tan, S.C.; Md, S.; Abdul Majeed, A.B.; Bhattamisra, S.K. Catalpol ameliorates insulin sensitivity and mitochondrial respiration in skeletal muscle of type-2 diabetic mice through insulin signaling pathway and ampk/sirt1/pgc-1α/ppar-γ activation. Biomolecules 2020, 10, 1360. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhu, P.; Zhang, L.; Xiong, B.; Tao, J.; Guan, W.; Li, C.; Chen, C.; Gu, J.; Duanmu, J.; et al. Autophagy inhibition attenuates the induction of anti-inflammatory effect of catalpol in liver fibrosis. Biomed. Pharmacother. 2018, 103, 1262–1271. [Google Scholar] [CrossRef]
- Jiao, N.; Chen, Y.; Zhu, Y.; Wang, W.; Liu, M.; Ding, W.; Lv, G.; Lu, J.; Yu, B.; Xu, H. Protective effects of catalpol on diabetes mellitus-induced male reproductive damage via suppression of the AGEs/RAGE/Nox4 signaling pathway. Life Sci. 2020, 256, 116736. [Google Scholar] [CrossRef]
- Nemmar, A.; Beegam, S.; Zaaba, N.E.; Alblooshi, S.; Alseiari, S.; Ali, B.H. The Salutary Effects of Catalpol on Diesel Exhaust Particles-Induced Thrombogenic Changes and Cardiac Oxidative Stress, Inflammation and Apoptosis. Biomedicines 2022, 10, 99. [Google Scholar] [CrossRef] [PubMed]
- Kauppinen, A.; Suuronen, T.; Ojala, J.; Kaarniranta, K.; Salminen, A. Antagonistic crosstalk between NF-κB and SIRT1 in the regulation of inflammation and metabolic disorders. Cell. Signal. 2013, 25, 1939–1948. [Google Scholar] [CrossRef]
- Ali, B.; Madanagopal, T.; Ramkumar, A.; Boudaka, A.; Tageldin, M.; Nemmar, A. Some physiological and histological aspects of the gastrointestinal tract in a mouse model of chronic renal failure. J. Pharmacol. Toxicol. Methods 2014, 69, 162–166. [Google Scholar] [CrossRef]
- Raza, H.; Prabu, S.K.; Robin, M.A.; Avadhani, N.G. Elevated Mitochondrial Cytochrome P450 2E1 and Glutathione S-Transferase A4-4 in Streptozotocin-Induced Diabetic Rats Tissue-Specific Variations and Roles in Oxidative Stress [Internet]. Volume 53, Diabetes. 2004. Available online: http://diabetesjournals.org/diabetes/article-pdf/53/1/185/375109/zdb00104000185.pdf (accessed on 10 October 2022).
- Nemmar, A.; Al-Salam, S.; Beegam, S.; Yuvaraju, P.; Ali, B. Aortic Oxidative Stress, Inflammation and DNA Damage Following Pulmonary Exposure to Cerium Oxide Nanoparticles in a Rat Model of Vascular Injury. Biomolecules 2019, 9, 376. [Google Scholar] [CrossRef] [Green Version]
- Nakajima, M.; Ueda, M.; Yamakage, K.; Nakagawa, Y.; Nakagawa, M.; Ohyama, W.; Omori, T.; Asano, N.; Hayashi, M.; Uno, Y. Tissue Sample Preparation for In Vivo Rodent Alkaline Comet Assay. Genes Environ. 2012, 34, 50–54. [Google Scholar] [CrossRef] [Green Version]
- Nemmar, A.; Melghit, K.; Al-Salam, S.; Zia, S.; Dhanasekaran, S.; Attoub, S.; Al-Amri, I.; Ali, B.H. Acute respiratory and systemic toxicity of pulmonary exposure to rutile Fe-doped TiO2 nanorods. Toxicology 2011, 279, 167–175. [Google Scholar] [CrossRef]
- Al Za’abi, M.; Ali, B.H.; Al Suleimani, Y.; Adham, S.A.; Ali, H.; Manoj, P.; Ashique, M.; Nemmar, A. The effect of metformin in diabetic and non-diabetic rats with experimentally-induced chronic kidney disease. Biomolecules 2021, 11, 814. [Google Scholar] [CrossRef] [PubMed]
- Ali, B.H.; Al-Husseni, I.; Beegam, S.; Al-Shukaili, A.; Nemmar, A.; Schierling, S.; Queisser, N.; Schupp, N. Effect of Gum Arabic on Oxidative Stress and Inflammation in Adenine-Induced Chronic Renal Failure in Rats. PLoS ONE 2013, 8, e55242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Courtoy, G.E.; Leclercq, I.; Froidure, A.; Schiano, G.; Morelle, J.; Devuyst, O.; Huaux, F.; Bouzin, C. Digital Image Analysis of Picrosirius Red Staining: A Robust Method for Multi-Organ Fibrosis Quantification and Characterization. Biomolecules 2020, 10, 1585. [Google Scholar] [CrossRef] [PubMed]
- Webster, A.C.; Nagler, E.V.; Morton, R.L.; Masson, P. Chronic Kidney Disease. Lancet 2017, 389, 1238–1252. [Google Scholar] [CrossRef]
- Irazabal, M.V.; Torres, V.E. Reactive Oxygen Species and Redox Signaling in Chronic Kidney Disease. Cells 2020, 9, 1342. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.A.; Kim, J.E.; Jo, M.; Ko, G.-J. The Role of Sirtuins in Kidney Diseases. Int. J. Mol. Sci. 2020, 21, 6686. [Google Scholar] [CrossRef] [PubMed]
- Jia, T.; Olauson, H.; Lindberg, K.; Amin, R.; Edvardsson, K.; Lindholm, B.; Andersson, G.; Wernerson, A.; Sabbagh, Y.; Schiavi, S.; et al. A novel model of adenine-induced tubulointerstitial nephropathy in mice. BMC Nephrol. 2013, 14, 116. [Google Scholar] [CrossRef] [Green Version]
- Awad, A.M.; Saleh, M.A.; Abu-Elsaad, N.M.; Ibrahim, T.M. Erlotinib can halt adenine induced nephrotoxicity in mice through modulating ERK1/2, STAT3, p53 and apoptotic pathways. Sci. Rep. 2020, 10, 11524. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, Q. Catalpol ameliorates doxorubicin induced inflammation and oxidative stress in H9C2 cells through PPAR γ activation. Exp. Ther. Med. 2020, 20, 1003–1011. [Google Scholar] [CrossRef]
- Tani, T.; Orimo, H.; Shimizu, A.; Tsuruoka, S. Development of a novel chronic kidney disease mouse model to evaluate the progression of hyperphosphatemia and associated mineral bone disease. Sci. Rep. 2017, 7, 2233. [Google Scholar] [CrossRef]
- Ali, B.H.; Al-Salam, S.; Al Za’Abi, M.; Waly, M.I.; Ramkumar, A.; Beegam, S.; Al-Lawati, I.; Adham, S.A.; Nemmar, A. New model for adenine-induced chronic renal failure in mice, and the effect of gum acacia treatment thereon: Comparison with rats. J. Pharmacol. Toxicol. Methods 2013, 68, 384–393. [Google Scholar] [CrossRef] [PubMed]
- Santana, A.C.; Degaspari, S.; Catanozi, S.; Dellê, H.; de Sa Lima, L.; Silva, C.; Blanco, P.; Solez, K.; Scavone, C.; Noronha, I.L. Thalidomide suppresses inflammation in adenine-induced CKD with uraemia in mice. Nephrol. Dial. Transplant. 2013, 28, 1140–1149. [Google Scholar] [CrossRef] [PubMed]
- Mak, R.H.; Ikizler, A.T.; Kovesdy, C.P.; Raj, D.S.; Stenvinkel, P.; Kalantar-Zadeh, K. Wasting in chronic kidney disease. J. Cachexia Sarcopenia Muscle 2011, 2, 9–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takezawa, K.; Kuribayashi, S.; Okada, K.; Sekii, Y.; Inagaki, Y.; Fukuhara, S.; Kiuchi, H.; Abe, T.; Fujita, K.; Uemura, M.; et al. Decreased renal function increases the nighttime urine volume rate by carryover of salt excretion to the nighttime. Sci. Rep. 2021, 11, 10587. [Google Scholar] [CrossRef] [PubMed]
- Panizo, S.; Martínez-Arias, L.; Alonso-Montes, C.; Cannata, P.; Martín-Carro, B.; Fernández-Martín, J.; Naves-Díaz, M.; Carrillo-López, N.; Cannata-Andía, J. Fibrosis in Chronic Kidney Disease: Pathogenesis and Consequences. Int. J. Mol. Sci. 2021, 22, 408. [Google Scholar] [CrossRef]
- Chen, T.K.; Knicely, D.H.; Grams, M.E. Chronic Kidney Disease Diagnosis and Management: A Review. JAMA J. Am. Med. Association. Am. Med. Assoc. 2019, 322, 1294–1304. [Google Scholar] [CrossRef] [PubMed]
- Pandya, D.; Nagrajappa, A.K.; Ravi, K.S. Assessment and correlation of urea and creatinine levels in saliva and serum of patients with chronic kidney disease, diabetes and hypertension—A research study. J. Clin. Diagn. Res. 2016, 10, ZC58–ZC62. [Google Scholar] [CrossRef]
- Benoit, S.W.; Ciccia, E.A.; Devarajan, P. Cystatin C as a biomarker of chronic kidney disease: Latest developments. Expert Rev. Mol. Diagn. 2020, 20, 1019–1026. [Google Scholar] [CrossRef]
- Al Za’abi, M.; Al Busaidi, M.; Yasin, J.; Schupp, N.; Nemmar, A.; Ali, B.H. Development of a new model for the induction of chronic kidney disease via intraperitoneal adenine administration, and the effect of treatment with gum acacia thereon. Am. J. Transl. Res. 2015, 7, 28–38. [Google Scholar] [CrossRef]
- Song, S.; Meyer, M.; Turk, T.R.; Wilde, B.; Feldkamp, T.; Assert, R.; Wu, K.; Kribben, A.; Witzke, O. Serum cystatin C in mouse models: A reliable and precise marker for renal function and superior to serum creatinine. Nephrol. Dial. Transplant. 2008, 24, 1157–1161. [Google Scholar] [CrossRef]
- Zaaba, N.E.; Beegam, S.; Elzaki, O.; Yasin, J.; Nemmar, B.M.; Ali, B.H.; Adeghate, E.; Nemmar, A. The Nephroprotective Effects of α-Bisabolol in Cisplatin-Induced Acute Kidney Injury in Mice. Biomedicines 2022, 10, 842. [Google Scholar] [CrossRef] [PubMed]
- Gil, A.; Brod, V.; Awad, H.; Heyman, S.N.; Abassi, Z.; Frajewicki, V. Neutrophil gelatinase-associated lipocalin in a triphasic rat model of adenine-induced kidney injury. Ren. Fail. 2016, 38, 1194164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hojs, N.V.; Bevc, S.; Ekart, R.; Hojs, R. Oxidative Stress Markers in Chronic Kidney Disease with Emphasis on Diabetic Nephropathy. Antioxidants 2020, 9, 925. [Google Scholar] [CrossRef] [PubMed]
- Kuo, H.-T.; Kuo, M.-C.; Chiu, Y.-W.; Chang, J.-M.; Guh, J.-Y.; Chen, H.-C. Increased glomerular and extracellular malondialdehyde levels in patients and rats with focal segmental glomerulosclerosis. Eur. J. Clin. Investig. 2005, 35, 245–250. [Google Scholar] [CrossRef]
- Nemmar, A.; Yuvaraju, P.; Beegam, S.; Fahim, M.A.; Ali, B.H. Cerium Oxide Nanoparticles in Lung Acutely Induce Oxidative Stress, Inflammation, and DNA Damage in Various Organs of Mice. Oxidative Med. Cell. Longev. 2017, 2017, 9639035. [Google Scholar] [CrossRef] [Green Version]
- Sung, C.-C.; Hsu, Y.-C.; Chen, C.-C.; Lin, Y.-F.; Wu, C.-C. Oxidative Stress and Nucleic Acid Oxidation in Patients with Chronic Kidney Disease. Oxidative Med. Cell. Longev. 2013, 2013, 301982. [Google Scholar] [CrossRef] [Green Version]
- Hoeijmakers, J.H.J. DNA Damage, Aging, and Cancer. N. Engl. J. Med. 2009, 361, 1475–1485. [Google Scholar] [CrossRef]
- Yousefzadeh, M.; Henpita, C.; Vyas, R.; Soto-Palma, C.; Robbins, P.; Niedernhofer, L. DNA damage—How and why we age? Elife 2021, 10, e62852. [Google Scholar] [CrossRef]
- Schupp, N.; Stopper, H.; Heidland, A. DNA Damage in Chronic Kidney Disease: Evaluation of Clinical Biomarkers. Oxidative Med. Cell. Longev. 2016, 2016, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Stoyanova, E.; Sandoval, S.B.; Zúñiga, L.A.; El-Yamani, N.; Coll, E.; Pastor, S.; Reyes, J.; Andrés, E.; Ballarin, J.; Xamena, N.; et al. Oxidative DNA damage in chronic renal failure patients. Nephrol. Dial. Transplant. 2010, 25, 879–885. [Google Scholar] [CrossRef]
- Nemmar, A.; Al-Salam, S.; Beegam, S.; Yuvaraju, P.; Hamadi, N.; Ali, B.H. In vivo protective effects of nootkatone against particles-induced lung injury caused by diesel exhaust is mediated via the Nf-κB pathway. Nutrients 2018, 10, 263. [Google Scholar] [CrossRef] [Green Version]
- Song, N.; Thaiss, F.; Guo, L. NFκB and kidney injury. Front Immunol. 2019, 10, 815. [Google Scholar] [CrossRef] [Green Version]
- Yeung, F.; Hoberg, J.E.; Ramsey, C.S.; Keller, M.D.; Jones, D.R.; Frye, R.A.; Mayo, M.W. Modulation of NF-κB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 2004, 23, 2369–2380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nemmar, A.; Yuvaraju, P.; Beegam, S.; John, A.; Raza, H.; Ali, B.H. Cardiovascular effects of nose-only water-pipe smoking exposure in mice. Am. J. Physiol. Heart Circ. Physiol. 2013, 305, H740–H746. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Shi, Z.; You, L.; Du, Y.; Ni, J.; Yan, D. Neuroprotective Effect of Catalpol via Anti-Oxidative, Anti-Inflammatory, and Anti-Apoptotic Mechanisms. Front. Pharmacol. 2020, 11, 690. [Google Scholar] [CrossRef] [PubMed]
- Salminen, A.; Kaarniranta, K.; Kauppinen, A. Crosstalk between Oxidative Stress and SIRT1: Impact on the Aging Process. Int. J. Mol. Sci. 2013, 14, 3834–3859. [Google Scholar] [CrossRef] [Green Version]
- Singh, C.K.; Chhabra, G.; Ndiaye, M.A.; Garcia-Peterson, L.M.; Mack, N.J.; Ahmad, N. The Role of Sirtuins in Antioxidant and Redox Signaling. Antioxid. Redox Signal. 2018, 28, 643–661. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.-W.; Yao, H.; Caito, S.; Sundar, I.K.; Rahman, I. Redox regulation of SIRT1 in inflammation and cellular senescence. Free. Radic. Biol. Med. 2013, 61, 95–110. [Google Scholar] [CrossRef] [Green Version]
- Liu, A.; Zhang, B.; Zhao, W.; Tu, Y.; Wang, Q.; Li, J. Catalpol ameliorates psoriasis-like phenotypes via SIRT1 mediated suppression of NF-κB and MAPKs signaling pathways. Bioengineered 2021, 12, 183–195. [Google Scholar] [CrossRef]
- Zhao, J.; Tan, Y.; Feng, Z.; Zhou, Y.; Wang, F.; Zhou, G.; Yan, J.; Nie, X. Catalpol attenuates polycystic ovarian syndrome by regulating sirtuin 1 mediated NF-κB signaling pathway. Reprod. Biol. 2022, 22, 100671. [Google Scholar] [CrossRef]
- Xiong, Y.; Shi, L.; Wang, L.; Zhou, Z.; Wang, C.; Lin, Y.; Luo, D.; Qiu, J.; Chen, D. Activation of sirtuin 1 by catalpol-induced down-regulation of microRNA-132 attenuates endoplasmic reticulum stress in colitis. Pharmacol. Res. 2017, 123, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.-Z.; Wen, D.; Zhang, M.; Xie, Q.; Ma, L.; Guan, Y.; Ren, Y.; Chen, J.; Hao, C.-M. Sirt1 Activation Ameliorates Renal Fibrosis by Inhibiting the TGF-β/Smad3 Pathway. J. Cell. Biochem. 2014, 115, 996–1005. [Google Scholar] [CrossRef] [PubMed]


| Parameters/Treatment | Control | Adenine | CAT | CAT + Adenine |
|---|---|---|---|---|
| Body weight change (%) | 5.56 ± 1.23 | −28.35 ± 0.98 *** | 2.68 ± 0.69 | −15.84 ± 2.93 Δ ᴏᴏᴏ |
| Kidney weight change (%) | 1.07 ± 0.01 | 2.88 ± 0.13 *** | 1.06 ± 0.02 | 1.57 ± 0.06 ΔΔΔ ᴏᴏ |
| Water intake | 8 ± 1.06 | 28.67 ± 1.35 *** | 11.67 ± 0.61 | 23.17 ± 1.55 ΔΔΔ ᴏᴏᴏ |
| Urine volume | 3.61 ± 0.28 | 17.72 ± 1.56 *** | 4.08 ± 0.21 | 9.83 ± 0.47 ΔΔΔ ᴏᴏ |
| Parameters/Treatment | Control | Adenine | CAT | CAT + Adenine |
|---|---|---|---|---|
| Urea (mmol/L) | 4.12 ± 0.09 | 16.67 ± 0.52 *** | 4.70 ± 0.23 | 7.28 ± 0.33 ΔΔΔ ᴏᴏᴏ |
| Creatinine (µmol/L) | 7.60 ± 0.48 | 19.42 ± 1.06 *** | 7.83 ± 0.18 | 7.45 ± 0.63 ΔΔΔ |
| Creatinine clearance (ml/min) | 0.92 ± 0.11 | 0.20 ± 0.03 ** | 1.03 ± 0.13 | 0.62 ± 0.05 Δ ᴏ |
| Albumin/creatinine (mg/mmol) | 0.87 ± 0.12 | 20.87 ± 1.86 *** | 1.03 ± 0.23 | 1.09 ± 0.06 ΔΔΔ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaaba, N.E.; Al-Salam, S.; Beegam, S.; Elzaki, O.; Yasin, J.; Nemmar, A. Catalpol Attenuates Oxidative Stress and Inflammation via Mechanisms Involving Sirtuin-1 Activation and NF-κB Inhibition in Experimentally-Induced Chronic Kidney Disease. Nutrients 2023, 15, 237. https://doi.org/10.3390/nu15010237
Zaaba NE, Al-Salam S, Beegam S, Elzaki O, Yasin J, Nemmar A. Catalpol Attenuates Oxidative Stress and Inflammation via Mechanisms Involving Sirtuin-1 Activation and NF-κB Inhibition in Experimentally-Induced Chronic Kidney Disease. Nutrients. 2023; 15(1):237. https://doi.org/10.3390/nu15010237
Chicago/Turabian StyleZaaba, Nur Elena, Suhail Al-Salam, Sumaya Beegam, Ozaz Elzaki, Javed Yasin, and Abderrahim Nemmar. 2023. "Catalpol Attenuates Oxidative Stress and Inflammation via Mechanisms Involving Sirtuin-1 Activation and NF-κB Inhibition in Experimentally-Induced Chronic Kidney Disease" Nutrients 15, no. 1: 237. https://doi.org/10.3390/nu15010237
APA StyleZaaba, N. E., Al-Salam, S., Beegam, S., Elzaki, O., Yasin, J., & Nemmar, A. (2023). Catalpol Attenuates Oxidative Stress and Inflammation via Mechanisms Involving Sirtuin-1 Activation and NF-κB Inhibition in Experimentally-Induced Chronic Kidney Disease. Nutrients, 15(1), 237. https://doi.org/10.3390/nu15010237









