Differential Orbitofrontal Cortex Responses to Chocolate Images While Performing an Approach–Avoidance Task in the MRI Environment
Abstract
:1. Introduction
2. Methods
2.1. Participants
2.2. Stimuli
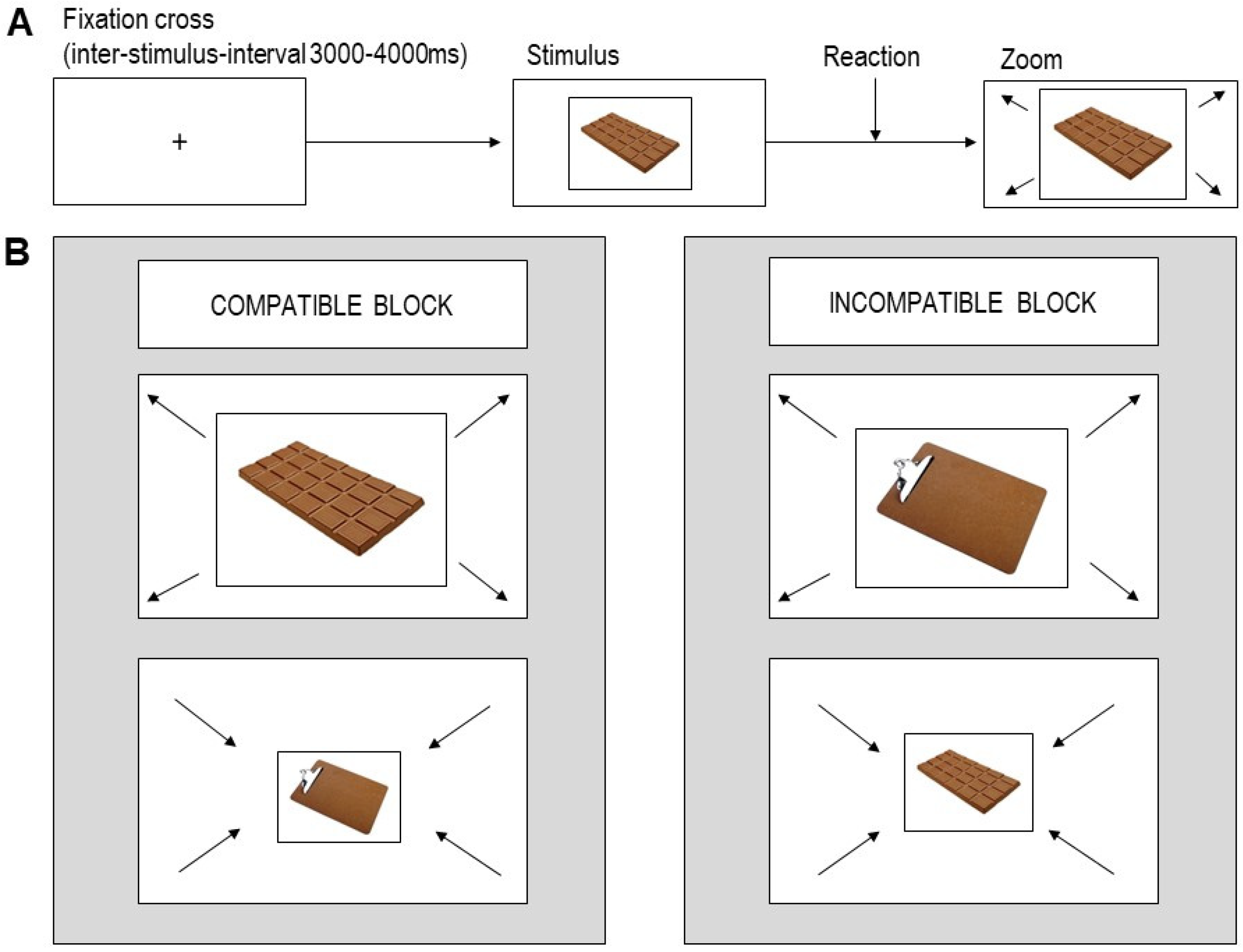
2.3. AAT
2.4. Procedure
2.5. Behavioral Data Analysis
2.6. MRI Data Analysis
3. Results
3.1. Behavioral Results
3.2. MRI Results
4. Discussion
Limitations and Future Studies
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Trial Registration
Appendix A. Comparison of Chocolate vs. Control Items
| Chocolate |  pic: 0137 |  pic: 0107 |  pic: 0163 |  pic: 0465 |  pic: 0286 |  pic: 0173 |  pic: 0289 |  pic: 0189 |
 pic: 0004 |  pic: 0166 |  pic: 0140 |  pic: 0165 |  pic: 0111 |  pic: 0510 |  pic: 0168 |  pic: 0169 | |
| Control |  pic: 1188 |  pic: 1015 |  pic: 1095 |  pic: 1045 |  pic: 1146 |  pic: 1260 |  pic: 1250 |  pic: 1196 |
 pic: 1265 |  pic: 1031 |  pic: 1268 |  pic: 1047 |  pic: 1270 |  pic: 1004 |  pic: 1279 |  pic: 1055 |
| Chocolate | Control | Statistics | ||||
| n = 16 | n = 16 | |||||
| M | SD | M | SD | t(29) | p | |
| Valence 1 | 5.8 | 0.23 | 2.83 | 0.77 | 14.7 | 0.001 |
| Valence 2 | 53.29 | 6.01 | 47.63 | 7.22 | 2.4 | 0.023 |
| Recognizability 2 | 96.53 | 3.72 | 97.46 | 3.21 | −0.76 | 0.455 |
| Familiarity 2 | 97.01 | 2.96 | 98.13 | 2.31 | −1.19 | 0.242 |
| Red 3 | 0.47 | 0.02 | 0.46 | 0.06 | 0.81 | 0.441 |
| Green 3 | 0.31 | 0.02 | 0.31 | 0.03 | −1.20 | 0.241 |
| Blue 3 | 0.22 | 0.02 | 0.23 | 0.05 | −0.26 | 0.798 |
| Size 3 | 0.31 | 0.11 | 0.33 | 0.12 | −0.62 | 0.542 |
| Brightness 3 | 45.91 | 20.77 | 43.52 | 17.67 | 0.35 | 0.728 |
| Contras t3 | 54.24 | 7.9 | 46.92 | 12.79 | 1.95 | 0.061 |
| Complexity 3 | 0.07 | 0.03 | 0.06 | 0.03 | 1.14 | 0.264 |
| 1 Rating data collected from the study sample on valence using a seven-point rating scale from ‘not at all pleasant’ to ‘very pleasant’. 2 Rating data taken from the Food-Pics data base [45], using visual analogue scales from 0 to 100 h to collect data on valence (‘very negative’ to ‘very positive’), recognizability (‘no’ to ‘yes’), and familiarity (‘no’ to ‘yes’). 3 Data on image properties taken from the Food-Pics database [45]. | ||||||
Appendix B. Correlations with Approach Bias-Scores
| All (n = 30) | Compatible Block First (n = 15) | Incompatible Block First (n = 15) | ||||
| r(28) | p | r(13) | p | r(13) | p | |
| State chocolate craving (FCQS) | −0.17 | 0.357 | −0.10 | 0.722 | 0.16 | 0.558 |
| Trait chocolate craving (FCQT) | −0.09 | 0.629 | −0.15 | 0.589 | 0.03 | 0.921 |
| Body-shape concerns (PSRS) | −0.20 | 0.295 | 0.06 | 0.832 | −0.20 | 0.480 |
| Approach motivation (BAS) | −0.11 | 0.581 | 0.21 | 0.452 | −0.06 | 0.839 |
| Chocolate items taken home | 0.08 | 0.698 | 0.02 | 0.958 | 0.06 | 0.852 |
| Valence rating (chocolate) | −0.22 | 0.239 | −0.03 | 0.921 | 0.12 | 0.671 |
| Palatability rating (chocolate) | −0.19 | 0.303 | 0.01 | 0.965 | −0.01 | 0.974 |
| Trait chocolate craving (chocolate version of the Food Cravings Questionnaire trait, FCQTr—chocolate*1), dieting and body-shape concerns (German version of the Perceived Self−Regulatory Success in Dieting Scale, PSRS [Meule]), approach motivation (German version of the Behavioral Approach System Scale, BAS−scale [Strobel]), and stimulus ratings were assessed during online survey. State chocolate craving (chocolate version of the Food Cravings Questionnaire state, FCQS—chocolate*1) was assessed right before scanning. | ||||||
Appendix C. Results from Whole-Brain Analysis for Stimulus Type
| Contrast | Brain Area | Voxels | MNI [x, y, z] | Tmax |
| chocolate > objects | ||||
| R L medial occipitotemporal | 1514 | −12, −88, −8 | inf | |
| R hippocampus | 12 | 21, −28, −2 | 6.19 | |
| L hippocampus | 13 | −21, −28, −2 | 6.17 | |
| L middle cingulum | 11 | −9, −19, 43 | 5.69 | |
| objects > chocolate | ||||
| R cuneus | 421 | 9, −73, 28 | 6.96 | |
| L superior temporal | 15 | −45, −31, 7 | 6.47 | |
| R inferior parietal | 81 | 36, −55, 40 | 6.24 | |
| L cerebellum | 31 | −12, −58, −14 | 5.64 | |
| L postcentral | 28 | −18, −31, 67 | 5.62 | |
| L inferior parietal | 22 | −36, −43, 34 | 5.34 | |
| R medial temporal | 19 | 54, −26, −11 | 5.31 | |
| R superior frontal | 14 | 24, 20, 52 | 5.09 | |
| R superior motor | 12 | 0, −10, 67 | 5.08 | |
| L angular | 12 | −33, −58, 34 | 5.04 | |
| Results from whole-brain analysis for stimulus type (statistical threshold: p < 0.05 few-corrected, k > 10 voxels). Labels of brain areas are taken from SPM AAL masks. | ||||
References
- Global Burden of Disease Collaborative Network. Global Burden of Disease Study 2015 (GBD 2015) Disability-Adjusted Life Years and Healthy Life Expectancy 1980–2015; University of Washington: Seattle, WA, USA, 2016. [Google Scholar]
- World Health Organization. Obesity and Overweight. 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 27 January 2021).
- Meule, A.; Lutz, A.; Vögele, C.; Kübler, A. Food cravings discriminate differentially between successful and unsuccessful dieters and non-dieters. Validation of the food cravings questionnaires in German. Appetite 2012, 58, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Hill, A.J. The psychology of food craving: Symposium on molecular mechanisms and psychology of food intake. Proc. Nutr. Soc. 2007, 66, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Davis, C.; Carter, J.C. Compulsive overeating as an addiction disorder. A review of theory and evidence. Appetite 2009, 53, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Meule, A.; Küppers, C.; Harms, L.; Friederich, H.-C.; Schmidt, U.; Blechert, J.; Brockmeyer, T. Food cue-induced craving in individuals with bulimia nervosa and binge-eating disorder. PLoS ONE 2018, 13, e0204151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verzijl, C.L.; Ahlich, E.; Schlauch, R.C.; Rancourt, D. The role of craving in emotional and uncontrolled eating. Appetite 2018, 123, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Meule, A.; Müller, A.; Gearhardt, A.N.; Blechert, J. German version of the Yale Food Addiction Scale 2.0: Prevalence and correlates of ‘food addiction’ in students and obese individuals. Appetite 2017, 115, 54–61. [Google Scholar] [CrossRef] [Green Version]
- Moreno, S.; Warren, C.S.; Rodríguez, S.; Fernández, M.C.; Cepeda-Benito, A. Food cravings discriminate between anorexia and bulimia nervosa. Implications for “success” versus “failure” in dietary restriction. Appetite 2009, 52, 588–594. [Google Scholar] [CrossRef]
- Massey, A.; Hill, A.J. Dieting and food craving. A descriptive, quasi-prospective study. Appetite 2012, 58, 781–785. [Google Scholar] [CrossRef]
- Brockmeyer, T.; Hahn, C.; Reetz, C.; Schmidt, U.; Friederich, H.-C. Approach bias and cue reactivity towards food in people with high versus low levels of food craving. Appetite 2015, 95, 197–202. [Google Scholar] [CrossRef]
- SColles, S.L.; Dixon, J.B.; O’Brien, P.E. Loss of Control Is Central to Psychological Disturbance Associated with Binge Eating Disorder. Obesity 2008, 16, 608–614. [Google Scholar] [CrossRef]
- Horstmann, A.; Dietrich, A.; Mathar, D.; Pössel, M.; Villringer, A.; Neumann, J. Slave to habit? Obesity is associated with decreased behavioural sensitivity to reward devaluation. Appetite 2014, 87, 175–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janssen, L.K.; Duif, I.; van Loon, I.; Wegman, J.; de Vries, J.H.; Cools, R.; Aarts, E. Loss of lateral prefrontal cortex control in food-directed attention and goal-directed food choice in obesity. NeuroImage 2017, 146, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Wiers, R.; Gladwin, T.; Hofmann, W.; Salemink, E.; Ridderinkhof, K.R. Cognitive Bias Modification and Cognitive Control Training in Addiction and Related Psychopathology. Clin. Psychol. Sci. 2013, 1, 192–212. [Google Scholar] [CrossRef] [Green Version]
- Bechara, A. Decision making, impulse control and loss of willpower to resist drugs: A neurocognitive perspective. Nat. Neurosci. 2005, 8, 1458–1463. [Google Scholar] [CrossRef]
- Watson, P.; De Wit, S.; Hommel, B.; Wiers, R.W. Motivational Mechanisms and Outcome Expectancies Underlying the Approach Bias toward Addictive Substances. Front. Psychol. 2012, 3, 440. [Google Scholar] [CrossRef] [Green Version]
- Fridland, E.; Wiers, C.E. Addiction and embodiment. Phenomenol. Cogn. Sci. 2017, 17, 15–42. [Google Scholar] [CrossRef] [Green Version]
- Kakoschke, N.; Kemps, E.; Tiggemann, M. Differential effects of approach bias and eating style on unhealthy food consumption in overweight and normal weight women. Psychol. Health 2017, 32, 1371–1385. [Google Scholar] [CrossRef]
- Kakoschke, N.; Kemps, E.; Tiggemann, M. The effect of combined avoidance and control training on implicit food evaluation and choice. J. Behav. Ther. Exp. Psychiatry 2017, 55, 99–105. [Google Scholar] [CrossRef]
- Moore, S.; Rudaizky, D.; MacLeod, C.; Dondzilo, L. Healthiness matters: Approach motivation for healthy food in overweight and obese individuals. Appetite 2021, 168, 105760. [Google Scholar] [CrossRef]
- Becker, D.; Jostmann, N.B.; Wiers, R.W.; Holland, R.W. Approach avoidance training in the eating domain: Testing the effectiveness across three single session studies. Appetite 2015, 85, 58–65. [Google Scholar] [CrossRef]
- Kemps, E.; Tiggemann, M.; Martin, R.; Elliott, M. Implicit approach–avoidance associations for craved food cues. J. Exp. Psychol. Appl. 2013, 19, 30–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meule, A.; Lender, A.; Richard, A.; Dinic, R.; Blechert, J. Approach–avoidance tendencies towards food: Measurement on a touchscreen and the role of attention and food craving. Appetite 2019, 137, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Paslakis, G.; Kühn, S.; Schaubschläger, A.; Schieber, K.; Röder, K.; Rauh, E.; Erim, Y. Explicit and implicit approach vs. avoidance tendencies towards high vs. low calorie food cues in patients with anorexia nervosa and healthy controls. Appetite 2016, 107, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Kahveci, S.; van Alebeek, H.; Berking, M.; Blechert, J. Touchscreen-based assessment of food approach biases: Investigating reliability and item-specific preferences. Appetite 2021, 163, 105190. [Google Scholar] [CrossRef] [PubMed]
- JMaas, J.; Keijsers, G.P.J.; Rinck, M.; Tanis, J.; Becker, E.S. Does a Dieting Goal Affect Automatic Cognitive Processes and Their Trainability? Cogn. Ther. Res. 2014, 39, 378–389. [Google Scholar]
- Richard, A.; Meule, A.; Blechert, J. When and how do explicit measures of food craving predict implicit food evaluation? A moderated mediation model. Food Qual. Preference 2018, 66, 141–147. [Google Scholar] [CrossRef] [Green Version]
- Roelofs, K.; Minelli, A.; Mars, R.; Van Peer, J.; Toni, I. On the neural control of social emotional behavior. Soc. Cogn. Affect. Neurosci. 2008, 4, 50–58. [Google Scholar] [CrossRef]
- Derntl, B.; Seidel, E.-M.; Eickhoff, S.B.; Kellermann, T.; Gur, R.C.; Schneider, F.; Habel, U. Neural correlates of social approach and withdrawal in patients with major depression. Soc. Neurosci. 2011, 6, 482–501. [Google Scholar] [CrossRef] [Green Version]
- Wiers, C.E.; Stelzel, C.; Park, S.; Gawron, C.K.; Ludwig, V.U.; Gutwinski, S.; Heinz, A.; Lindenmeyer, J.; Wiers, R.; Walter, H.; et al. Neural Correlates of Alcohol-Approach Bias in Alcohol Addiction: The Spirit is Willing but the Flesh is Weak for Spirits. Neuropsychopharmacology 2013, 39, 688–697. [Google Scholar] [CrossRef] [Green Version]
- Mehl, N.; Morys, F.; Villringer, A.; Horstmann, A. Unhealthy yet Avoidable—How Cognitive Bias Modification Alters Behavioral and Brain Responses to Food Cues in Individuals with Obesity. Nutrients 2019, 11, 874. [Google Scholar] [CrossRef] [Green Version]
- Wiers, C.E.; Stelzel, C.; Gladwin, T.E.; Park, S.Q.; Pawelczack, S.; Gawron, C.K.; Stuke, H.; Heinz, A.; Wiers, R.W.; Rinck, M.; et al. Effects of Cognitive Bias Modification Training on Neural Alcohol Cue Reactivity in Alcohol Dependence. Am. J. Psychiatry 2015, 172, 335–343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, D.; Fellows, L.; Small, D.; Dagher, A. Food and drug cues activate similar brain regions: A meta-analysis of functional MRI studies. Physiol. Behav. 2012, 106, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Kemps, E.; Tiggemann, M. Approach bias for food cues in obese individuals. Psychol. Health 2014, 30, 370–380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berridge, K.C.; Ho, C.-Y.; Richard, J.M.; DiFeliceantonio, A.G. The tempted brain eats: Pleasure and desire circuits in obesity and eating disorders. Brain Res. 2010, 1350, 43–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Volkow, N.D.; Wise, R.A. How can drug addiction help us understand obesity? Nat. Neurosci. 2005, 8, 555–560. [Google Scholar] [CrossRef] [PubMed]
- Lender, A.; Meule, A.; Rinck, M.; Brockmeyer, T.; Blechert, J. Measurement of food-related approach–avoidance biases: Larger biases when food stimuli are task relevant. Appetite 2018, 125, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Kersbergen, I.; Woud, M.L.; Field, M. The validity of different measures of automatic alcohol action tendencies. Psychol. Addict. Behav. 2010, 29, 225–230. [Google Scholar] [CrossRef]
- Phaf, R.H.; Mohr, S.E.; Rotteveel, M.; Wicherts, J. Approach, avoidance, and affect: A meta-analysis of approach-avoidance tendencies in manual reaction time tasks. Front. Psychol. 2014, 5, 378. [Google Scholar] [CrossRef] [Green Version]
- Kahveci, S.; Rinck, M.; van Alebeek, H.; Blechert, J. How pre-processing decisions affect the reliability and validity of the Approach-Avoidance Task: Evidence from simulations and multiverse analyses with six datasets. PsyArXiv 2022. [Google Scholar] [CrossRef]
- Lender, A.; Blechert, J.; Kühn, S.; Kronbichler, M.; Wirtz, J. AAT-fMRI. 2019. Available online: https://osf.io/ysbrp (accessed on 27 January 2021).
- Meule, A.; Hermann, T.; Kã¼Bler, A. A short version of the Food Cravings Questionnaire—Trait: The FCQ-T-reduced. Front. Psychol. 2014, 5, 190. [Google Scholar] [CrossRef] [Green Version]
- Wegman, J.; van Loon, I.; Smeets, P.A.M.; Cools, R.; Aarts, E. Top-down expectation effects of food labels on motivation. NeuroImage 2018, 173, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Blechert, J.; Lender, A.; Polk, S.; Busch, N.A.; Ohla, K. Food-Pics_Extended—An Image Database for Experimental Research on Eating and Appetite: Additional Images, Normative Ratings and an Updated Review. Front. Psychol. 2019, 10, 307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spunt, B. Easy-optimize-x: Formal Release for Archiving on Zenodo (1.0). Zenodo.
- Blechert, J.; Klackl, J.; Miedl, S.F.; Wilhelm, F.H. To eat or not to eat: Effects of food availability on reward system activity during food picture viewing. Appetite 2016, 99, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Polivy, J.; Heatherton, T.F.; Herman, C.P. Self-esteem, restraint, and eating behavior. J. Abnorm. Psychol. 1988, 97, 354–356. [Google Scholar] [CrossRef] [PubMed]
- Wiers, R.W.; Eberl, C.; Rinck, M.; Becker, E.S.; Lindenmeyer, J. Retraining Automatic Action Tendencies Changes Alcoholic Patients’ Approach Bias for Alcohol and Improves Treatment Outcome. Psychol. Sci. 2011, 22, 490–497. [Google Scholar] [CrossRef] [Green Version]
- Kahveci, S. AATtools: Tools for Analyzing the Approach-Avoidance Task. R package version 0.0.1. 2020. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
- The Functional Imaging Laboratory Methods Group. Statistical Parametric Mapping (SPM) 12; Wellcome Trust Centre for Neuroimaging: London, UK, 2014. [Google Scholar]
- Tierney, T.M.; Weiss-Croft, L.J.; Centeno, M.; Shamshiri, E.A.; Perani, S.; Baldeweg, T.; Clark, C.A.; Carmichael, D.W. FIACH: A biophysical model for automatic retrospective noise control in fMRI. Neuroimage 2016, 124, 1009–1020. [Google Scholar] [CrossRef] [Green Version]
- Brett, M.; Anton, J.L.; Valabregue, R.; Poline, J.B. Region of interest analysis using an SPM toolbox. Neuroimage 2002, 16, 497. [Google Scholar]
- Wittekind, C.E.; Blechert, J.; Schiebel, T.; Lender, A.; Kahveci, S.; Kühn, S. Comparison of different response devices to assess behavioral tendencies towards chocolate in the approach-avoidance task. Appetite 2021, 165, 105294. [Google Scholar] [CrossRef]
- Rolls, E.T.; Cheng, W.; Feng, J. The orbitofrontal cortex: Reward, emotion and depression. Brain Commun. 2020, 2, fcaa196. [Google Scholar] [CrossRef]
- De Araujo, I.E.T.; Rolls, E.T.; Kringelbach, M.; McGlone, F.; Phillips, N. Taste-olfactory convergence, and the representation of the pleasantness of flavour, in the human brain. Eur. J. Neurosci. 2003, 18, 2059–2068. [Google Scholar] [CrossRef] [Green Version]
- Kringelbach, M.; O’Doherty, J.; Rolls, E.; Andrews, C. Activation of the Human Orbitofrontal Cortex to a Liquid Food Stimulus is Correlated with its Subjective Pleasantness. Cereb. Cortex 2003, 13, 1064–1071. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rolls, E.T.; McCabe, C. Enhanced affective brain representations of chocolate in cravers vs. non-cravers. Eur. J. Neurosci. 2007, 26, 1067–1076. [Google Scholar] [CrossRef] [PubMed]
- Siep, N.; Roefs, A.; Roebroeck, A.; Havermans, R.; Bonte, M.L.; Jansen, A. Hunger is the best spice: An fMRI study of the effects of attention, hunger and calorie content on food reward processing in the amygdala and orbitofrontal cortex. Behav. Brain Res. 2009, 198, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Mehta, S.; Melhorn, S.J.; Smeraglio, A.; Tyagi, V.; Grabowski, T.; Schwartz, M.W.; Schur, E.A. Regional brain response to visual food cues is a marker of satiety that predicts food choice. Am. J. Clin. Nutr. 2012, 96, 989–999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robinson, T.E.; Berridge, K.C. The neural basis of drug craving: An incentive-sensitization theory of addiction. Brain Res. Rev. 1993, 18, 247–291. [Google Scholar] [CrossRef] [PubMed]
- Robinson, T.E.; Berridge, K.C. Addiction. Annu. Rev. Psychol. 2003, 54, 25–53. [Google Scholar] [CrossRef]
- Benton, D.; Young, H. A meta-analysis of the relationship between brain dopamine receptors and obesity: A matter of changes in behavior rather than food addiction? Int. J. Obes. 2016, 40, S12–S21. [Google Scholar] [CrossRef] [Green Version]
- Veling, H.; Holland, R.W.; van Knippenberg, A. When approach motivation and behavioral inhibition collide: Behavior regulation through stimulus devaluation. J. Exp. Soc. Psychol. 2008, 44, 1013–1019. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Veling, H.; de Vries, S.P.; Bijvank, B.O.; Janssen, I.M.C.; Dijksterhuis, A.; Holland, R.W. Go/no-go training changes food evaluation in both morbidly obese and normal-weight individuals. J. Consult. Clin. Psychol. 2018, 86, 980–990. [Google Scholar] [CrossRef]
- Quandt, J.; Holland, R.W.; Chen, Z.; Veling, H. The role of attention in explaining the no-go devaluation effect: Effects on appetitive food items. J. Exp. Psychol. Hum. Percept. Perform. 2019, 45, 1119–1133. [Google Scholar] [CrossRef]
- Yang, Y.; Shields, G.S.; Wu, Q.; Liu, Y.; Chen, H.; Guo, C. Cognitive training on eating behaviour and weight loss: A meta-analysis and systematic review. Obes. Rev. 2019, 20, 1628–1641. [Google Scholar] [CrossRef] [PubMed]
- Veling, H.; Lawrence, N.S.; Chen, Z.; van Koningsbruggen, G.M.; Holland, R.W. What Is Trained During Food Go/No-Go Training? A Review Focusing on Mechanisms and a Research Agenda. Curr. Addict. Rep. 2017, 4, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Messner, C.; Vosgerau, J. Cognitive Inertia and the Implicit Association Test. J. Mark. Res. 2010, 47, 374–386. [Google Scholar] [CrossRef]
- van Alebeek, H.; Kahveci, S.; Blechert, J. Improving the touchscreen-based food approach-avoidance task: Remediated block-order effects and initial findings regarding validity. Open Res. Eur. 2021, 1, 15. [Google Scholar] [CrossRef]
- Stevenson, R.J.; Francis, H.M. The hippocampus and the regulation of human food intake. Psychol. Bull. 2017, 143, 1011–1032. [Google Scholar] [CrossRef]


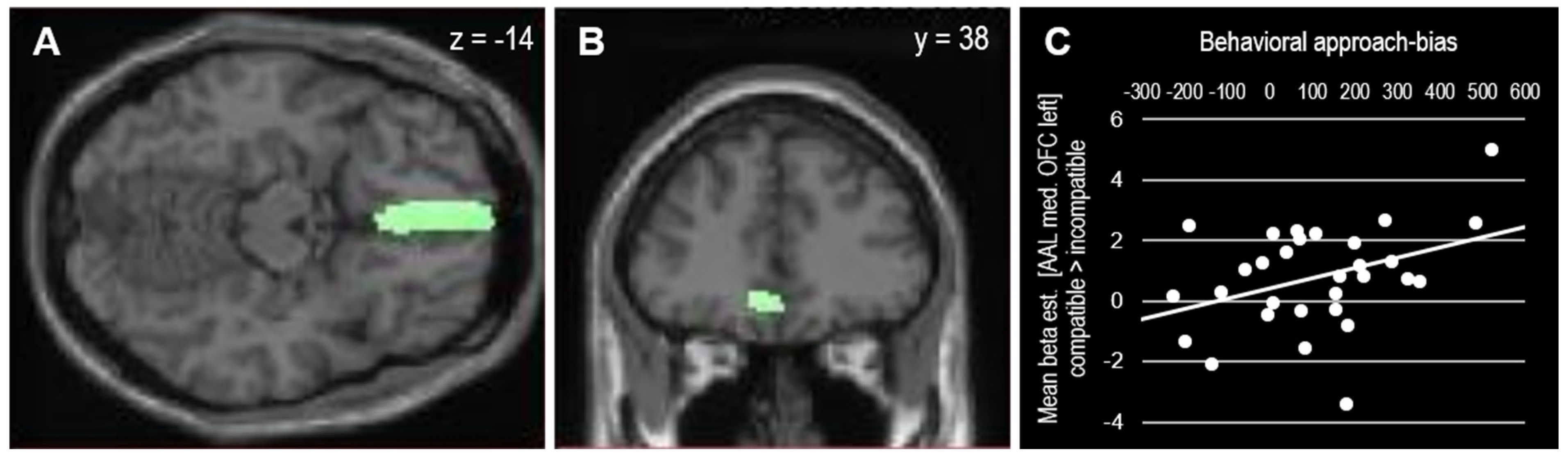
| Contrast | Brain Area | Voxels | MNI [x, y, z] | Tmax |
|---|---|---|---|---|
| Compatible > incompatible | ||||
| R medial occipitotemporal | 6 | 24, −76, −2 | 3.53 | |
| L medial orbitofrontal | 7 | −6, 38, −14 | 3.42 | |
| Incompatible > compatible | ||||
| L caudate nucleus | 7 | −6, −17, 7 | 4.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lender, A.; Wirtz, J.; Kronbichler, M.; Kahveci, S.; Kühn, S.; Blechert, J. Differential Orbitofrontal Cortex Responses to Chocolate Images While Performing an Approach–Avoidance Task in the MRI Environment. Nutrients 2023, 15, 244. https://doi.org/10.3390/nu15010244
Lender A, Wirtz J, Kronbichler M, Kahveci S, Kühn S, Blechert J. Differential Orbitofrontal Cortex Responses to Chocolate Images While Performing an Approach–Avoidance Task in the MRI Environment. Nutrients. 2023; 15(1):244. https://doi.org/10.3390/nu15010244
Chicago/Turabian StyleLender, Anja, Janina Wirtz, Martin Kronbichler, Sercan Kahveci, Simone Kühn, and Jens Blechert. 2023. "Differential Orbitofrontal Cortex Responses to Chocolate Images While Performing an Approach–Avoidance Task in the MRI Environment" Nutrients 15, no. 1: 244. https://doi.org/10.3390/nu15010244






