Probing Folate-Responsive and Stage-Sensitive Metabolomics and Transcriptional Co-Expression Network Markers to Predict Prognosis of Non-Small Cell Lung Cancer Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Cohort and Paired Tumours Tissue Acquisition
2.2. Basic Data and Blood Sample Collection
2.3. Determination of Clinical Folate Markers
2.4. Transcripomics Analysis
2.5. UPLC/MS/MS Quantitative Metabolomics Analysis
2.6. Enrichment Analysis
2.7. Metabolite and Genetic Correlation Network Analysis
2.8. Survival Analysis of Public Data from Cancer Genomics Studies
2.9. General Statistical Analysis
2.10. Metabolomics Statistical Analysis
2.11. Weight Gene Co-Expression Network Analysis
3. Results
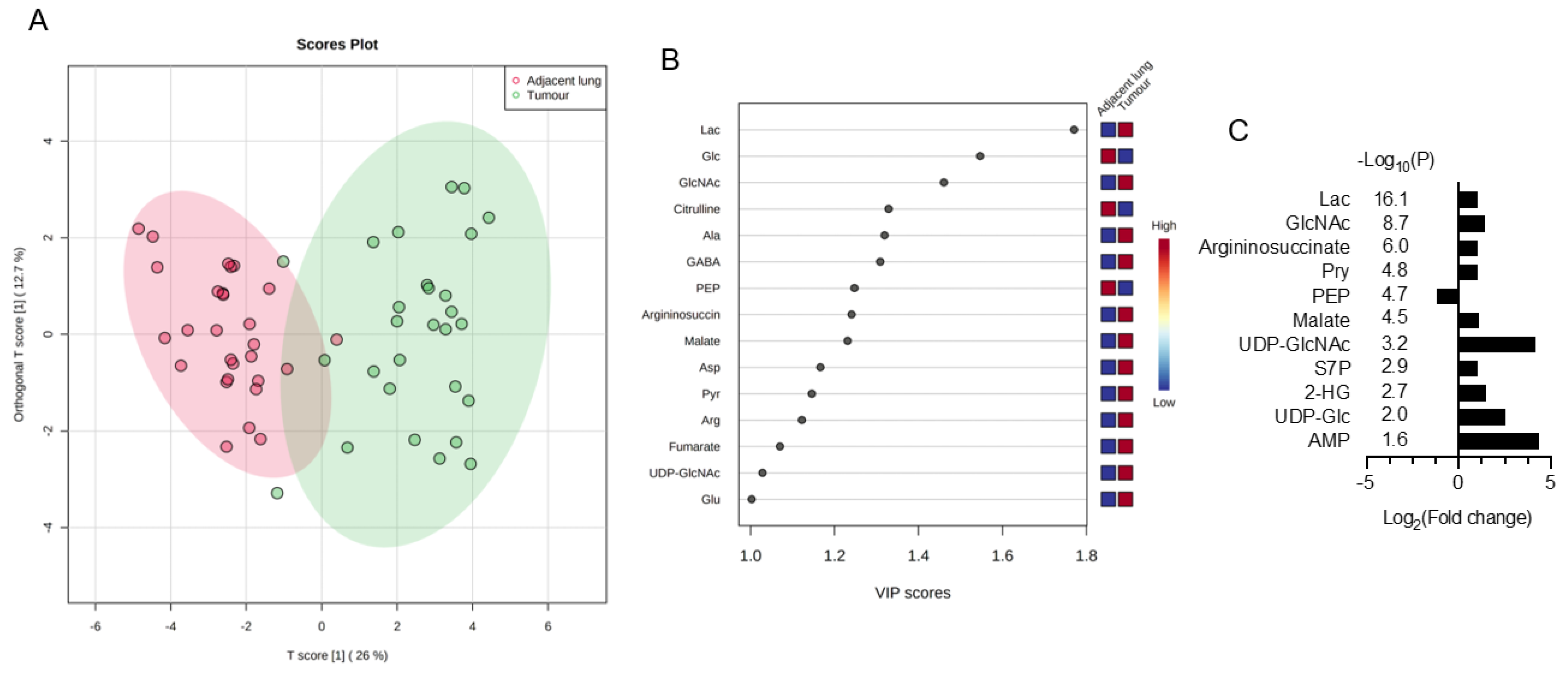
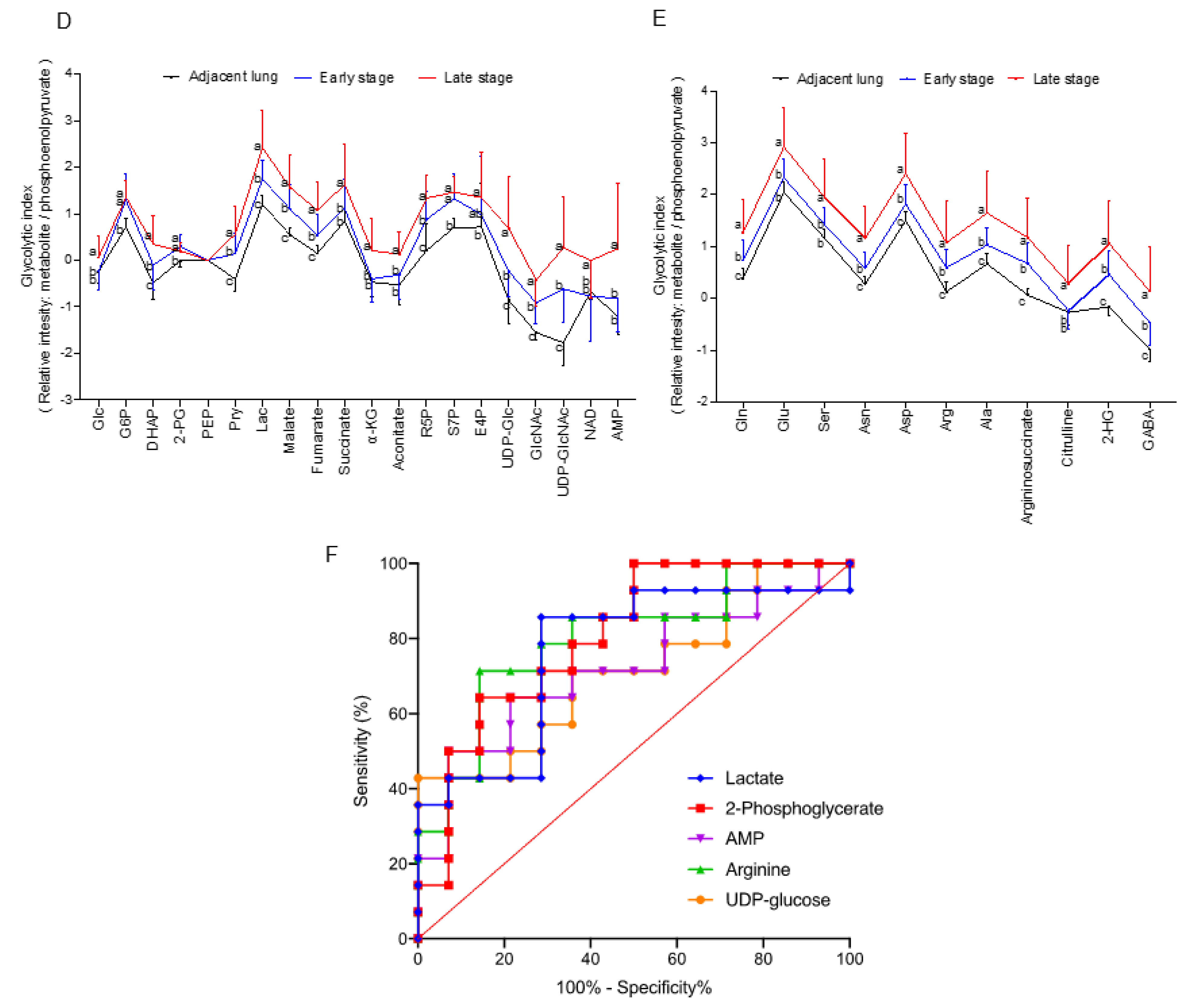
3.1. Discover Stage-Sensitive Tumour Metabolomics Markers in Paired NSCLCs
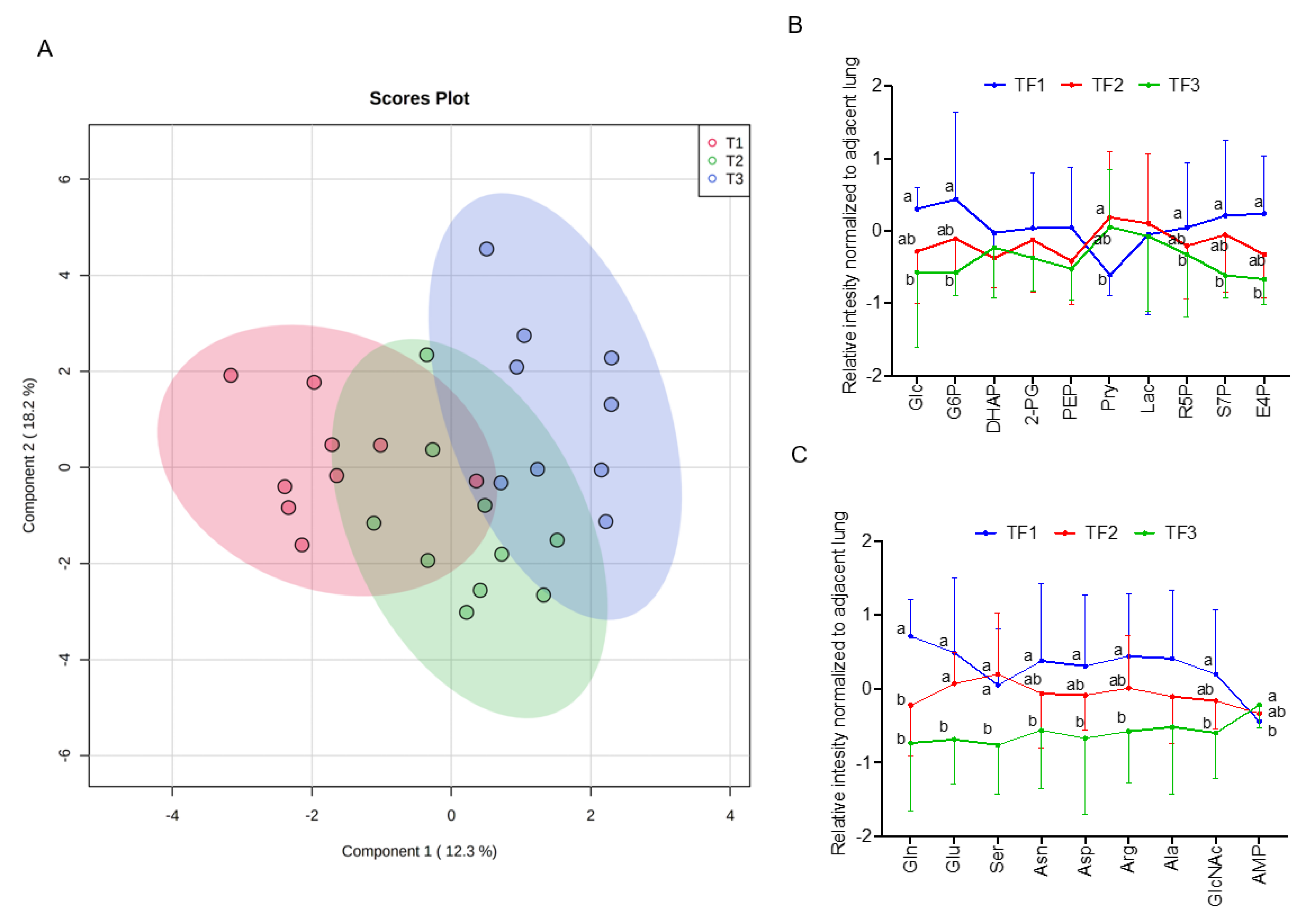
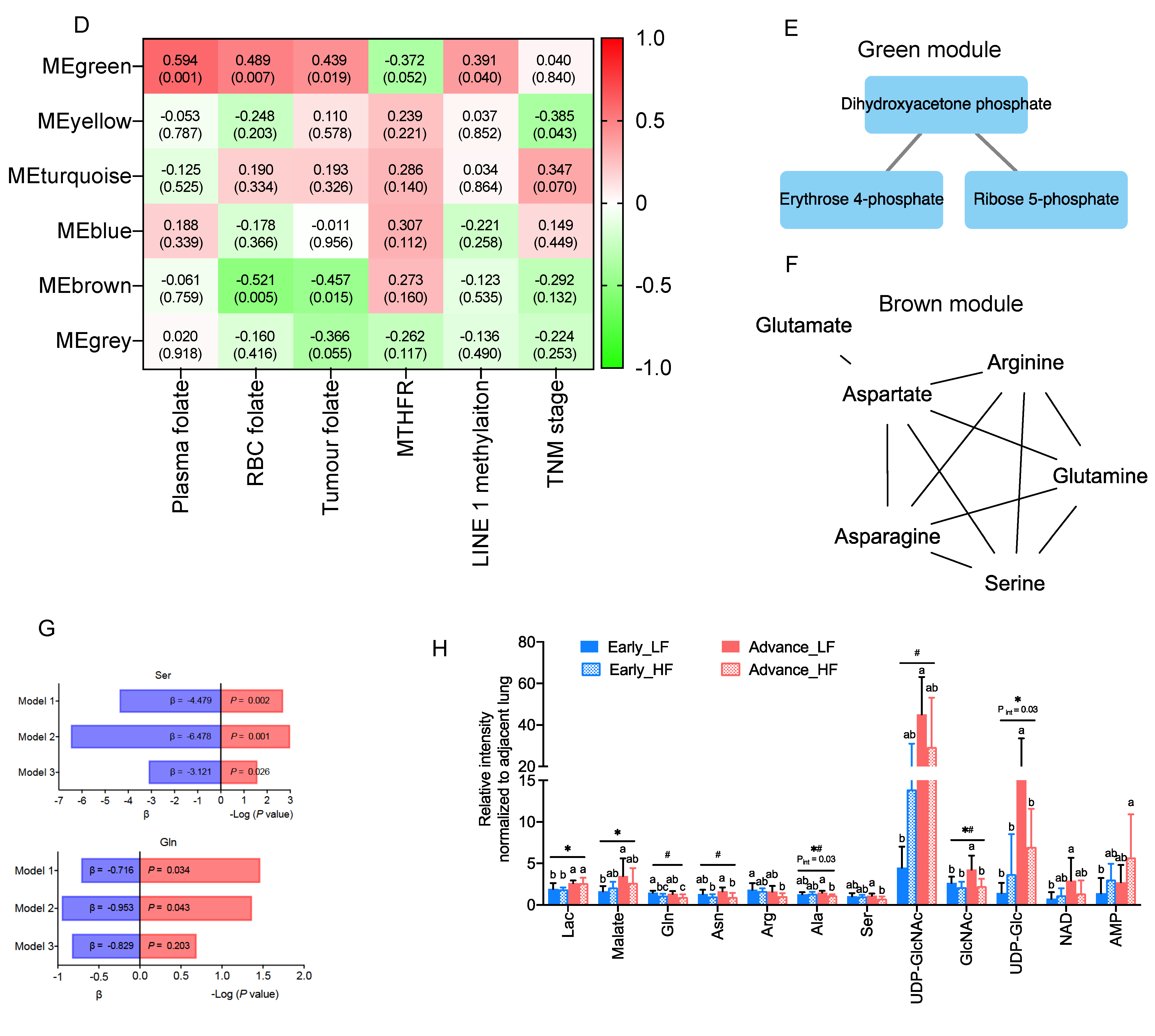
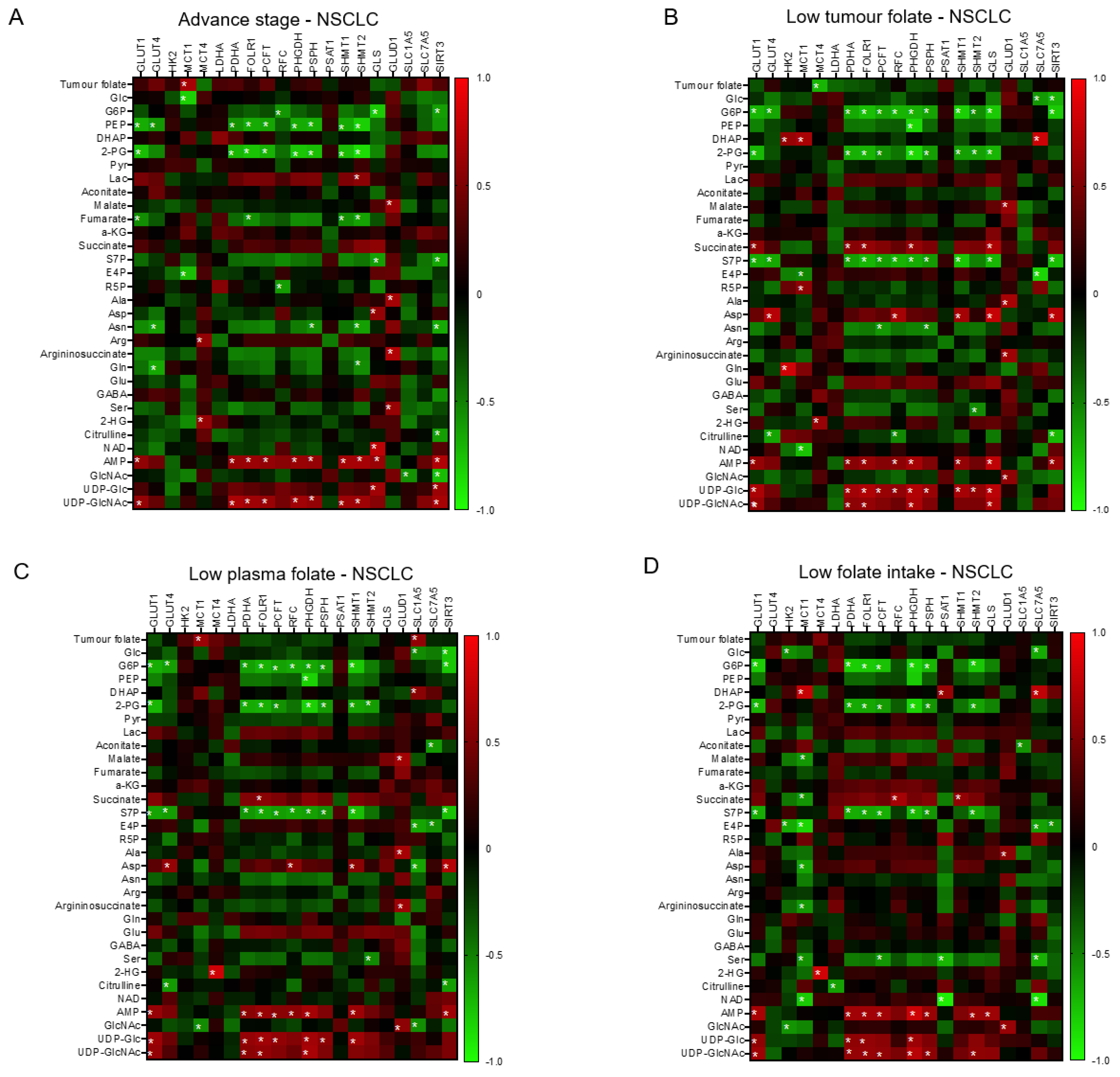
3.2. Association of Clinical Folate Trait with Advance Stage-Sensitive Tumour Markers in NSCLCs
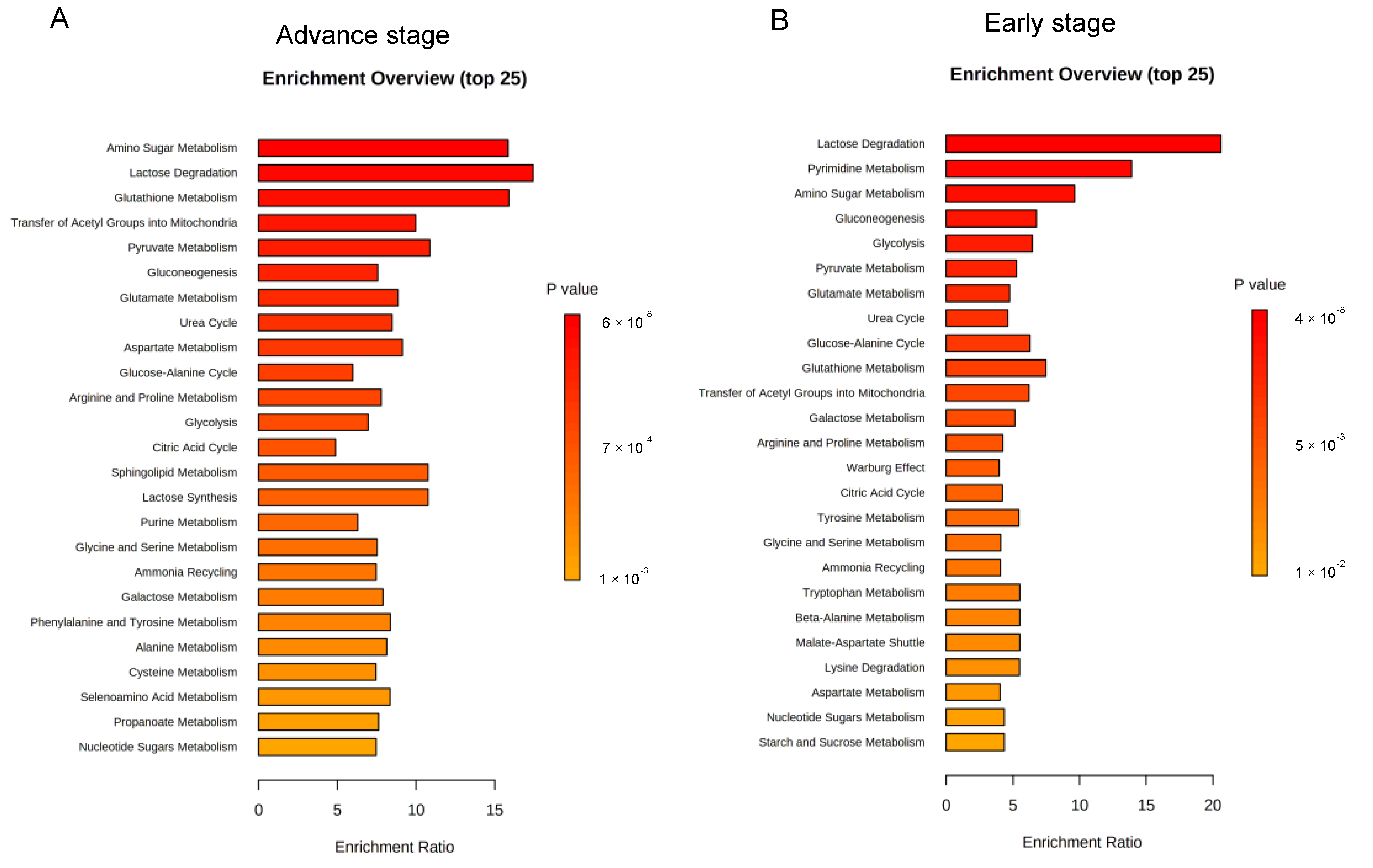
3.3. Enrichment Analysis on Metabolic Pathways by Folate-Responsive and Stage-Sensitive Tumour Metabolomics Markers
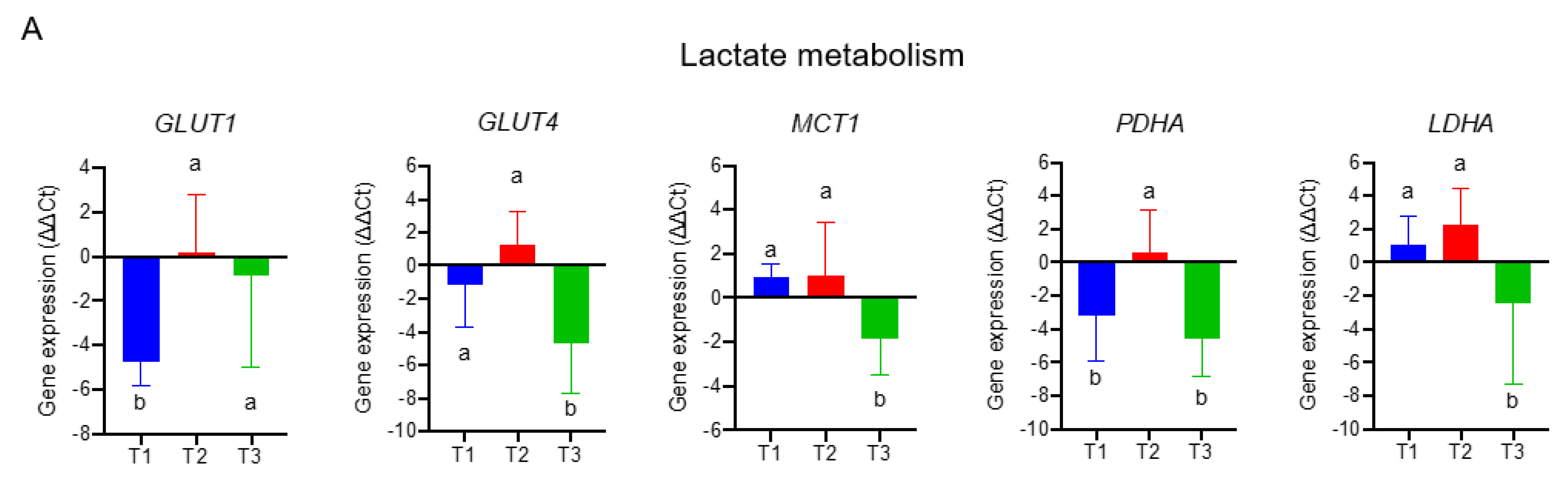
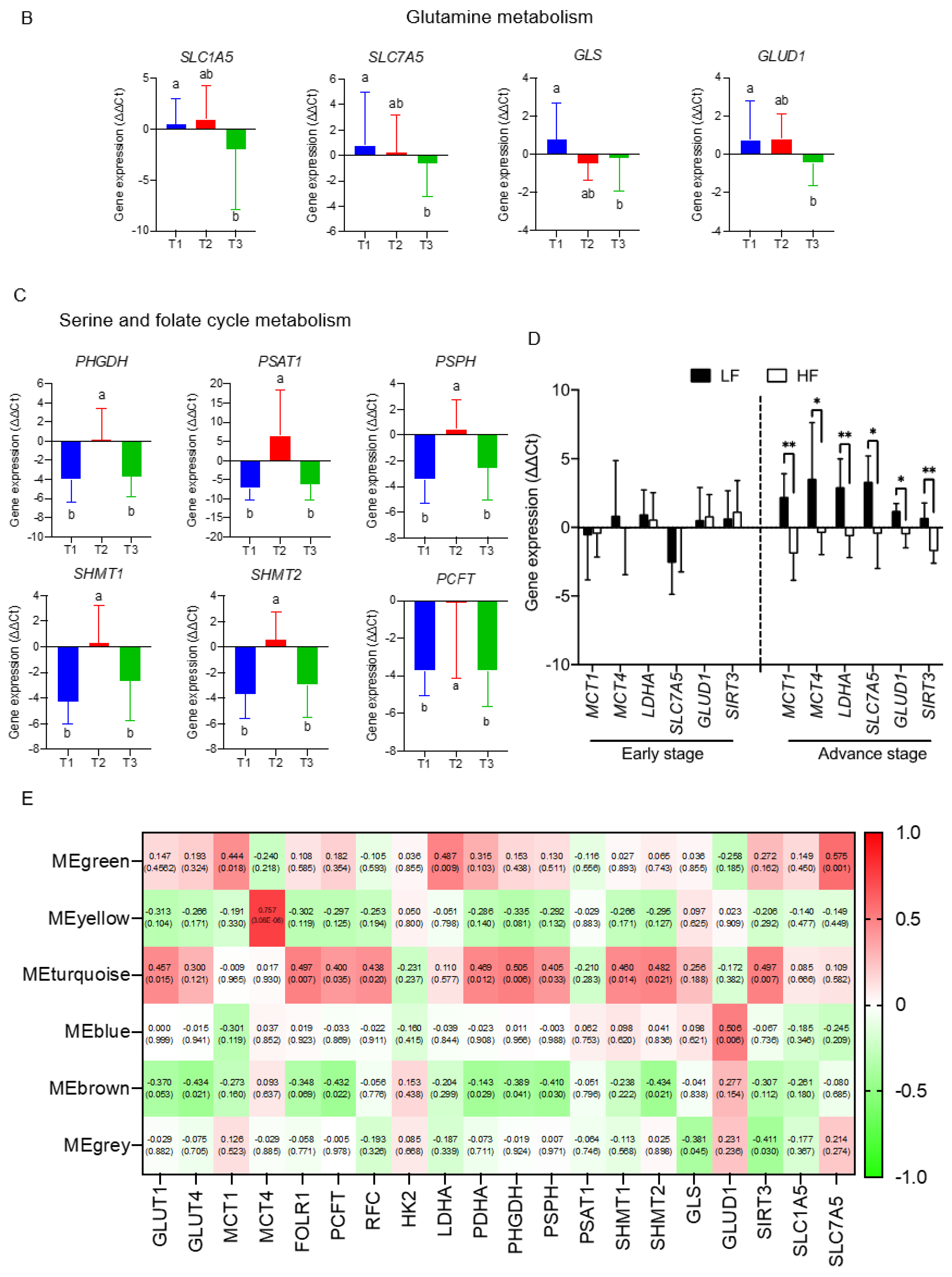
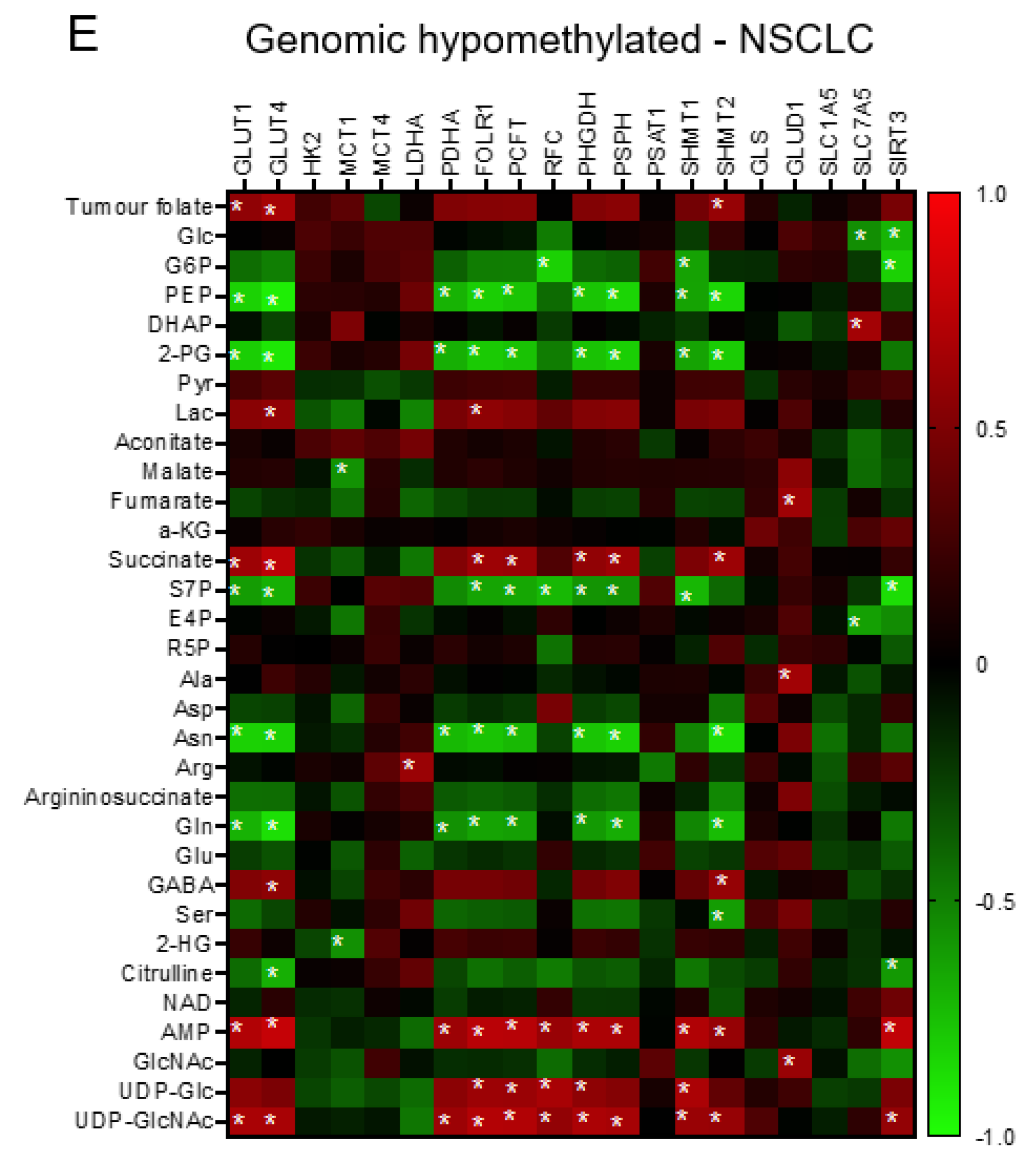
3.4. The Transcriptomics Signatures Associated with Folate-Sensitive and Stage-Responsive Tumour Metabolomics Markers
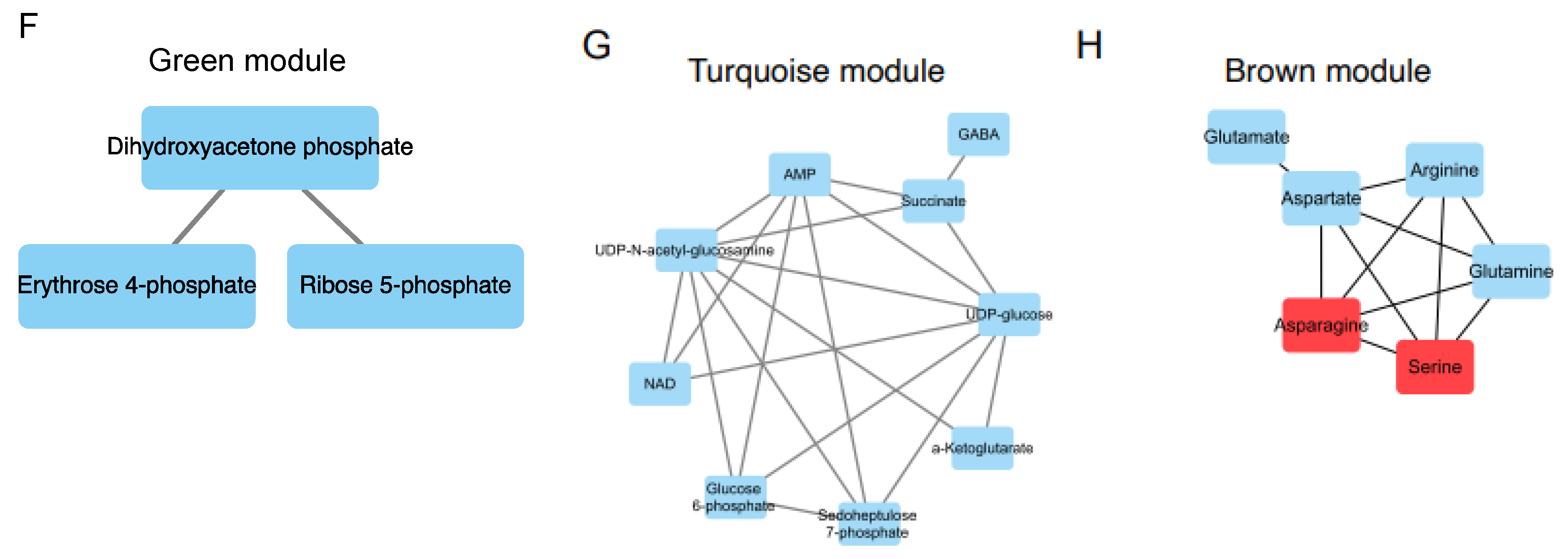
3.5. The Interactive Network of Metabolites and Genetic Signatures Modified by Advance Stage and Clinical Folate Trait in NSCLCs
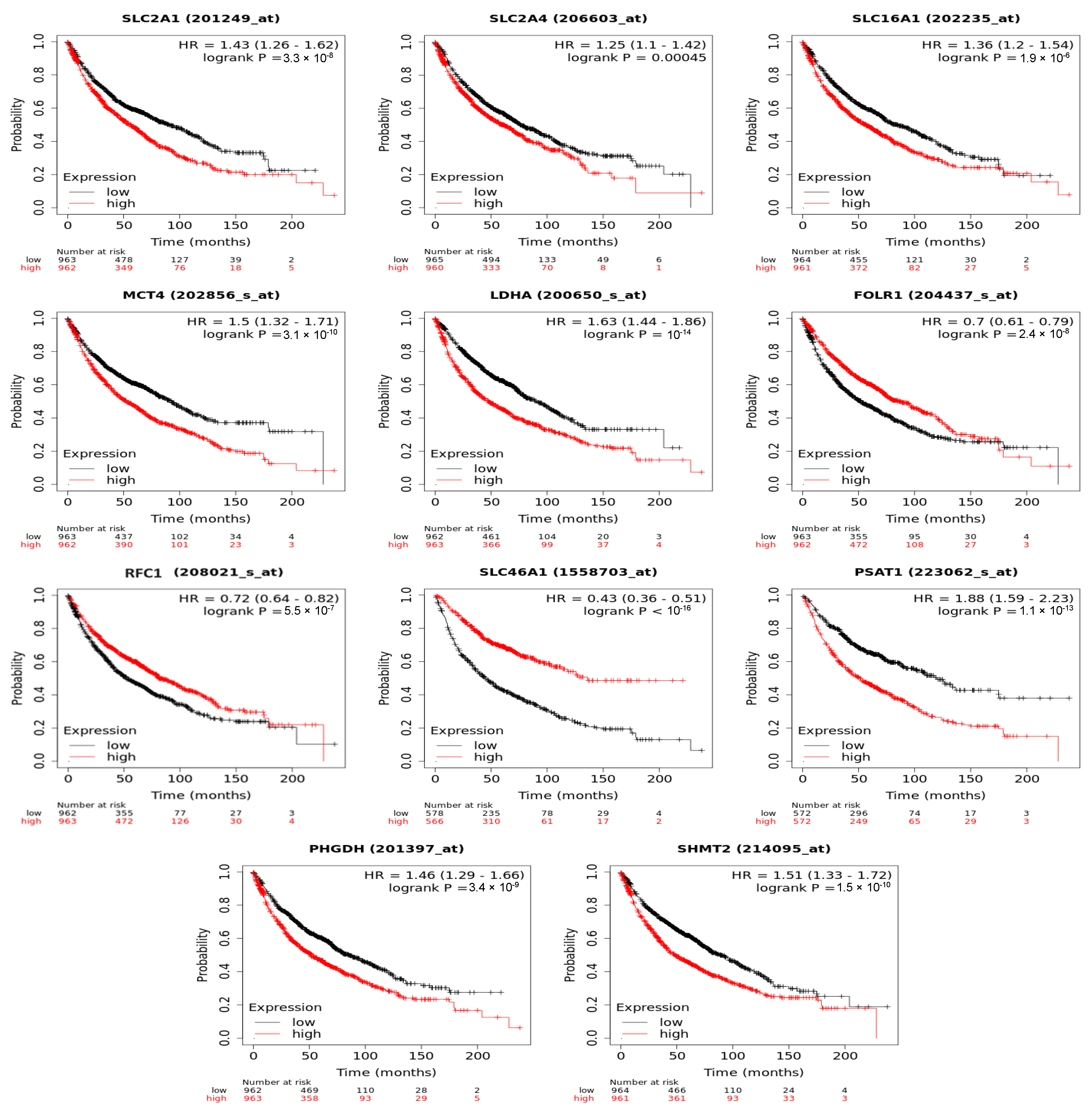
3.6. Folate-Responsive Tumour Signatures Predicted Overall Survival of Patients with Lung Cancers
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ducker, G.S.; Rabinowitz, J.D. One-Carbon Metabolism in Health and Disease. Cell Metab. 2017, 25, 27–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brosnan, M.E.; MacMillan, L.; Stevens, J.R.; Brosnan, J.T. Division of Labour: How Does Folate Metabolism Partition Between One-Carbon Metabolism and Amino Acid Oxidation? Biochem. J. 2015, 472, 135–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.; Zhang, Z.; Hoshino, A.; Zheng, H.D.; Morley, M.; Arany, Z.; Rabinowitz, J.D. NADPH Production by The Oxidative Pentose-Phosphate Pathway Supports Folate Metabolism. Nat. Metab. 2019, 1, 404–415. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Ye, J.; Kamphorst, J.J.; Shlomi, T.; Thompson, C.B.; Rabinowitz, J.D. Quantitative Flux Analysis Reveals Folate-Dependent NADPH Production. Nature 2014, 510, 298–302. [Google Scholar] [CrossRef] [Green Version]
- Newman, A.C.; Maddocks, O.D.K. One-Carbon Metabolism in Cancer. Br. J. Cancer 2017, 116, 1499–1504. [Google Scholar] [CrossRef] [Green Version]
- Feng, H.C.; Lin, J.Y.; Hsu, S.H.; Lan, W.Y.; Kuo, C.S.; Tian, Y.F.; Sun, D.P.; Huang, R.S. Low Folate Metabolic Stress Reprograms DNA Methylation-activated Sonic Hedgehog Signaling to Mediate Cancer Stem Cell-Like Signatures and Invasive Tumour Stage-specific Malignancy of Human Colorectal Cancers. Int. J. Cancer 2017, 141, 2537–2550. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.P.; Hsu, S.H.; Feng, H.C.; Huang, R.F. Folate Deprivation Enhances Invasiveness of Human Colon Cancer Cells Mediated by Activation of Sonic Hedgehog Signaling Through Promoter Hypomethylation and Cross Action with Transcription Nuclear Factor-Kappa B Pathway. Carcinogenesis 2012, 33, 1158–1168. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.J.; Huang, R.S. Low-folate Stress Reprograms Cancer Stem Cell-like Potentials and Bioenergetics Metabolism Through Activation of mTOR Signaling Pathway to Promote In Vitro Invasion and In Vivo Tumorigenicity of Lung Cancers. J. Nutr. Biochem. 2018, 53, 28–38. [Google Scholar] [CrossRef]
- Huang, A.; Huang, S.Y.; Shah, P.; Ku, W.C.; Huang, K.T.; Liu, Y.F.; Su, C.L.; Huang, R.S. Suboptimal Folic Acid Exposure Rewires Oncogenic Metabolism and Proteomics Signatures to Mediate Human Breast Cancer Malignancy. J. Nutr. Biochem. 2022, 106, 109000. [Google Scholar] [CrossRef]
- Oleinik, N.V.; Helke, K.L.; Kistner-Griffin, E.; Krupenko, N.I.; Krupenko, S.A. Rho GTPases RhoA and Rac1 Mediate Effects of Dietary Folate on Metastatic Potential of A549 Cancer Cells Through the Control of Cofilin Phosphorylation. J. Biol. Chem. 2014, 289, 26383–26394. [Google Scholar] [CrossRef]
- Strickland, K.C.; Krupenko, N.I.; Krupenko, S.A. Molecular Mechanisms Underlying the Potentially Adverse Effects of Folate. Clin. Chem. Lab. Med. 2013, 51, 607–616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Voorrips, L.E.; Goldbohm, R.A.; Brants, H.A.; van Poppel, G.A.; Sturmans, F.; Hermus, R.J.; van den Brandt, P.A. A Prospective Cohort Study on Antioxidant and Folate Intake and Male Lung Cancer Risk. Cancer Epidemiol. Biomark. Prev. 2000, 9, 357–365. [Google Scholar]
- Shen, H.; Wei, Q.; Pillow, P.C.; Amos, C.I.; Hong, W.K.; Spitz, M.R. Dietary Folate Intake and Lung Cancer Risk in Former Smokers: A Case-Control Analysis. Cancer Epidemiol. Biomark. Prev. 2003, 12, 980–986. [Google Scholar]
- Zhang, Y.F.; Zhou, L.; Zhang, H.W.; Hou, A.J.; Gao, H.F.; Zhou, Y.H. Association Between Folate Intake and The Risk of Lung Cancer: A Dose-Response Meta-Analysis of Prospective Studies. PLoS ONE 2014, 9, e93465. [Google Scholar] [CrossRef]
- Durda, K.; Kąklewski, K.; Gupta, S.; Szydłowski, M.; Baszuk, P.; Jaworska-Bieniek, K.; Sukiennicki, G.; Kaczmarek, K.; Waloszczyk, P.; Narod, S.; et al. Serum Folate Concentration and The Incidence of Lung Cancer. PLoS ONE 2017, 12, e0177441. [Google Scholar] [CrossRef] [Green Version]
- Martinez-Outschoorn, U.E.; Peiris-Pagés, M.; Pestell, R.G.; Sotgia, F.; Lisanti, M.P. Cancer Metabolism: A Therapeutic Perspective. Nat. Rev. Clin. Oncol. 2017, 14, 11–31. [Google Scholar] [CrossRef]
- Siegel, D.A.; Henley, S.J.; Wike, J.M.; Ryerson, A.B.; Johnson, C.J.; Rees, J.R.; Pollack, L.A. Capture of Tobacco Use among Population-Based Registries: Findings from 10 National Program of Cancer Registries States. Cancer 2018, 124, 2381–2389. [Google Scholar] [CrossRef] [Green Version]
- Faubert, B.; Li, K.Y.; Cai, L.; Hensley, C.T.; Kim, J.; Zacharias, L.G.; Yang, C.; Do, Q.N.; Doucette, S.; Burguete, D.; et al. Lactate Metabolism in Human Lung Tumors. Cell 2017, 171, 358–371.e9. [Google Scholar] [CrossRef] [Green Version]
- Vlachostergios, P.J.; Oikonomou, K.G.; Gibilaro, E.; Apergis, G. Elevated Lactic Acid Is A Negative Prognostic Factor in Metastatic Lung Cancer. Cancer Biomark. 2015, 15, 725–734. [Google Scholar] [CrossRef]
- Warburg, O. On the Origin of Cancer Cells. Science 1956, 123, 309–314. [Google Scholar] [CrossRef]
- Ortmayr, K.; Dubuis, S.; Zampieri, M. Metabolic Profiling of Cancer Cells Reveals Genome-Wide Crosstalk Between Transcriptional Regulators and Metabolism. Nat. Commun. 2019, 10, 1841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, P.H.; Cai, L.; Huffman, K.; Yang, C.; Kim, J.; Faubert, B.; Boroughs, L.; Ko, B.; Sudderth, J.; McMillan, E.A.; et al. Metabolic Diversity in Human Non-Small Cell Lung Cancer Cells. Mol. Cell 2019, 76, 838–851.e5. [Google Scholar] [CrossRef]
- Hensley, C.T.; Faubert, B.; Yuan, Q.; Lev-Cohain, N.; Jin, E.; Kim, J.; Jiang, L.; Ko, B.; Skelton, R.; Loudat, L.; et al. Metabolic Heterogeneity in Human Lung Tumors. Cell 2016, 164, 681–694. [Google Scholar] [CrossRef] [Green Version]
- Quail, D.F.; Joyce, J.A. Microenvironmental Regulation of Tumor Progression and Metastasis. Nat. Med. 2013, 19, 1423–1437. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, C.M.; Potter, J.D. Folate and Cancer—Timing Is Everything. JAMA 2007, 297, 2408–2409. [Google Scholar] [CrossRef] [PubMed]
- Jemal, A.; Siegel, R.; Ward, E.; Hao, Y.; Xu, J.; Murray, T.; Thun, M.J. Cancer Statistics, 2008. CA Cancer J. Clin. 2008, 58, 71–96. [Google Scholar] [CrossRef]
- Detterbeck, F.C.; Boffa, D.J.; Kim, A.W.; Tanoue, L.T. The Eighth Edition Lung Cancer Stage Classification. Chest 2017, 151, 193–203. [Google Scholar] [CrossRef]
- Lee, C.H.; Wong, J.; Tzeng, M.S.; Huang, R.F.S. Dietary Profile of Folate Intake in Long-term Post-stroke Patients. Nutr. Res. 2005, 25, 465. [Google Scholar] [CrossRef]
- Horne, D.W.; Patterson, D. Lactobacillus Casei Microbiological Assay of Folic Acid Derivatives in 96-Well Microtiter Plates. Clin. Chem. 1988, 34, 2357–2359. [Google Scholar] [CrossRef]
- Kou, C.S.; Huang, C.Y.; Kou, H.T.; Cheng, C.P.; Chen, C.H.; Lu, C.L.; Yang, F.L.; Huang, R.F. Interrelationships among Genetic C677T Polymorphism of 5,10-Methylenetetrahydrofolate Reductase, Biochemical Folate Status, and Lymphocytic P53 Oxidative Damage in Association with Tumor Malignancy and Survivals of Patients with Hepatocellular Carcinoma. Mol. Nutr. Food Res. 2014, 58, 329–342. [Google Scholar] [CrossRef]
- Cheng, M.L.; Chang, K.H.; Wu, Y.R.; Chen, C.M. Metabolic Disturbances in Plasma as Biomarkers for Huntington’s Disease. J. Nutr. Biochem. 2016, 31, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; Duren, W.; Evans, C.R.; Burant, C.F.; Michailidis, G.; Karnovsky, A. Sparse Network Modeling and Metscape-Based Visualization Methods for the Analysis ff Large-Scale Metabolomics Data. Bioinformatics 2017, 33, 1545–1553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, W.; Jia, L.; Zhang, J.W.; Wang, D.J.; Ren, Q.; Zhang, W. Andrographolide Against Lung Cancer-New Pharmacological Insights Based on High-Throughput Metabolomics Analysis Combined with Network Pharmacology. Front. Pharmacol. 2021, 12, 596652. [Google Scholar] [CrossRef]
- Qi, Y.S.; Xie, J.B.; Xie, P.; Duan, Y.; Ling, Y.Q.; Gu, Y.L.; Piao, X.L. Uncovering the Anti-NSCLC Effects and Mechanisms of Gypenosides by Metabolomics and Network Pharmacology Analysis. J Ethnopharmacol. 2021, 281, 114506. [Google Scholar] [CrossRef] [PubMed]
- Pei, G.; Chen, L.; Zhang, W. WGCNA Application to Proteomic and Metabolomic Data Analysis. Methods Enzymol. 2017, 585, 135–158. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, L.; Ju, L.; Qian, K.; Liu, X.; Wang, X.; Xiao, Y. Novel Biomarkers Associated With Progression and Prognosis of Bladder Cancer Identified by Co-expression Analysis. Front. Oncol. 2019, 9, 1030. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, L.; Zhao, J.; Chen, Y.; Feng, R. Metabolomics Strategy Assisted By Transcriptomics Analysis to Identify Biomarkers Associated with Schizophrenia. Anal. Chim. Acta 2020, 1140, 18–29. [Google Scholar] [CrossRef]
- Chin, C.H.; Chen, S.H.; Wu, H.H.; Ho, C.W.; Ko, M.T.; Lin, C.Y. cytoHubba: Identifying Hub Objects and Sub-Networks from Complex Interactome. BMC Syst. Biol. 2014, 8 (Suppl. 4), S11. [Google Scholar] [CrossRef] [Green Version]
- Fan, T.W.; Lane, A.N.; Higashi, R.M.; Farag, M.A.; Gao, H.; Bousamra, M.; Miller, D.M. Altered Regulation of Metabolic Pathways in Human Lung Cancer Discerned by (13)C Stable Isotope-resolved Metabolomics (SIRM). Mol. Cancer 2009, 8, 41. [Google Scholar] [CrossRef] [Green Version]
- Sellers, K.; Fox, M.P.; Bousamra, M., 2nd; Slone, S.P.; Higashi, R.M.; Miller, D.M.; Wang, Y.; Yan, J.; Yuneva, M.O.; Deshpande, R.; et al. Pyruvate Carboxylase is Critical for Non-Small-Cell Lung Cancer Proliferation. J. Clin. Investig. 2015, 125, 687–698. [Google Scholar] [CrossRef] [Green Version]
- Puchades-Carrasco, L.; Jantus-Lewintre, E.; Pérez-Rambla, C.; García-García, F.; Lucas, R.; Calabuig, S.; Blasco, A.; Dopazo, J.; Camps, C.; Pineda-Lucena, A. Serum Metabolomic Profiling Facilitates the Non-Invasive Identification of Metabolic Biomarkers Associated with The Onset and Progression of Non-small Cell Lung Cancer. Oncotarget 2016, 7, 12904–12916. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De la Cruz-López, K.G.; Castro-Muñoz, L.J.; Reyes-Hernández, D.O.; García-Carrancá, A.; Manzo-Merino, J. Lactate in the Regulation of Tumor Microenvironment and Therapeutic Approaches. Front. Oncol. 2019, 9, 1143. [Google Scholar] [CrossRef] [PubMed]
- Yokota, H.; Guo, J.; Matoba, M.; Higashi, K.; Tonami, H.; Nagao, Y. Lactate, Choline, and Creatine Levels Measured by Vitro 1H-MRS as Prognostic Parameters in Patients with Non-Small-Cell Lung Cancer. J. Magn. Reson. Imaging 2007, 25, 992–999. [Google Scholar] [CrossRef]
- Moreno, P.; Jiménez-Jiménez, C.; Garrido-Rodríguez, M.; Calderón-Santiago, M.; Molina, S.; Lara-Chica, M.; Priego-Capote, F.; Salvatierra, Á.; Muñoz, E.; Calzado, M.A. Metabolomic Profiling of Human Lung Tumor Tissues-Nucleotide Metabolism as a Candidate for Therapeutic Interventions and Biomarkers. Mol. Oncol. 2018, 12, 1778–1796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rocha, C.M.; Barros, A.S.; Goodfellow, B.J.; Carreira, I.M.; Gomes, A.; Sousa, V.; Bernardo, J.; Carvalho, L.; Gil, A.M.; Duarte, I.F. NMR Metabolomics of Human Lung Tumours Reveals Distinct Metabolic Signatures for Adenocarcinoma and Squamous Cell Carcinoma. Carcinogenesis 2015, 36, 68–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wikoff, W.R.; Grapov, D.; Fahrmann, J.F.; DeFelice, B.; Rom, W.N.; Pass, H.I.; Kim, K.; Nguyen, U.; Taylor, S.L.; Gandara, D.R.; et al. Metabolomic Markers of Altered Nucleotide Metabolism in Early Stage Adenocarcinoma. Cancer Prev. Res. 2015, 8, 410–418. [Google Scholar] [CrossRef] [Green Version]
- Ashkavand, Z.; O’Flanagan, C.; Hennig, M.; Du, X.; Hursting, S.D.; Krupenko, S.A. Metabolic Reprogramming by Folate Restriction Leads to a Less Aggressive Cancer Phenotype. Mol. Cancer Res. 2017, 15, 189–200. [Google Scholar] [CrossRef] [Green Version]
- Rinaldi, G.; Pranzini, E.; Van Elsen, J.; Broekaert, D.; Funk, C.M.; Planque, M.; Doglioni, G.; Altea-Manzano, P.; Rossi, M.; Geldhof, V.; et al. In Vivo Evidence for Serine Biosynthesis-Defined Sensitivity of Lung Metastasis, but Not of Primary Breast Tumors, to mTORC1 Inhibition. Mol. Cell 2021, 81, 386–397.e7. [Google Scholar] [CrossRef]
- Altman, B.J.; Stine, Z.E.; Dang, C.V. From Krebs to Clinic: Glutamine Metabolism to Cancer Therapy. Nat. Rev. Cancer 2016, 16, 619–634. [Google Scholar] [CrossRef] [Green Version]
- Davidson, S.M.; Papagiannakopoulos, T.; Olenchock, B.A.; Heyman, J.E.; Keibler, M.A.; Luengo, A.; Bauer, M.R.; Jha, A.K.; O’Brien, J.P.; Pierce, K.A.; et al. Environment Impacts the Metabolic Dependencies of Ras-Driven Non-Small Cell Lung Cancer. Cell Metab. 2016, 23, 517–528. [Google Scholar] [CrossRef] [Green Version]
- Hensley, C.T.; Wasti, A.T.; DeBerardinis, R.J. Glutamine and Cancer: Cell Biology, Physiology, and Clinical Opportunities. J. Clin. Investig. 2013, 123, 3678–3684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kerr, E.M.; Gaude, E.; Turrell, F.K.; Frezza, C.; Martins, C.P. Mutant Kras Copy Number Defines Metabolic Reprogramming and Therapeutic Susceptibilities. Nature 2016, 531, 110–113. [Google Scholar] [CrossRef] [PubMed]
- Sayin, V.I.; Ibrahim, M.X.; Larsson, E.; Nilsson, J.A.; Lindahl, P.; Bergo, M.O. Antioxidants Accelerate Lung Cancer Progression in Mice. Sci. Transl. Med. 2014, 6, 221ra215. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.M.; Yu, C.C.; Lu, H.T.; Chou, Y.F.; Huang, R.F. Folate Deprivation Promotes Mitochondrial Oxidative Decay: DNA Large Deletions, Cytochrome C Oxidase Dysfunction, Membrane Depolarization and Superoxide Overproduction in Rat Liver. Br. J. Nutr. 2007, 97, 855–863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chou, Y.F.; Yu, C.C.; Huang, R.F. Changes in Mitochondrial DNA Deletion, Content, and Biogenesis in Folate-Deficient Tissues of Young Rats Depend on Mitochondrial Folate and Oxidative DNA Injuries. J. Nutr. 2007, 137, 2036–2042. [Google Scholar] [CrossRef] [Green Version]
- Locasale, J.W. Serine, Glycine and One-Carbon Units: Cancer Metabolism in Full Circle. Nat. Rev. Cancer 2013, 13, 572–583. [Google Scholar] [CrossRef] [Green Version]
- Mahmood, K.; Emadi, A. 1-C Metabolism-Serine, Glycine, Folates-In Acute Myeloid Leukemia. Pharmaceuticals 2021, 14, 190. [Google Scholar] [CrossRef]
- Tedeschi, P.M.; Markert, E.K.; Gounder, M.; Lin, H.; Dvorzhinski, D.; Dolfi, S.C.; Chan, L.L.; Qiu, J.; DiPaola, R.S.; Hirshfield, K.M.; et al. Contribution of Serine, Folate and Glycine Metabolism to The ATP, NADPH and Purine Requirements of Cancer Cells. Cell Death Dis. 2013, 4, e877. [Google Scholar] [CrossRef] [Green Version]
- Sun, L.; Song, L.; Wan, Q.; Wu, G.; Li, X.; Wang, Y.; Wang, J.; Liu, Z.; Zhong, X.; He, X.; et al. cMyc-Mediated Activation of Serine Biosynthesis Pathway Is Critical for Cancer Progression under Nutrient Deprivation Conditions. Cell Res. 2015, 25, 429–444. [Google Scholar] [CrossRef] [Green Version]
- Commisso, C.; Davidson, S.M.; Soydaner-Azeloglu, R.G.; Parker, S.J.; Kamphorst, J.J.; Hackett, S.; Grabocka, E.; Nofal, M.; Drebin, J.A.; Thompson, C.B.; et al. Macropinocytosis of Protein Is an Amino Acid Supply Route in Ras-Transformed Cells. Nature 2013, 497, 633–637. [Google Scholar] [CrossRef] [Green Version]
- Kamphorst, J.J.; Nofal, M.; Commisso, C.; Hackett, S.R.; Lu, W.; Grabocka, E.; Vander Heiden, M.G.; Miller, G.; Drebin, J.A.; Bar-Sagi, D.; et al. Human Pancreatic Cancer Tumors Are Nutrient Poor and Tumor Cells Actively Scavenge Extracellular Protein. Cancer Res. 2015, 75, 544–553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tibbetts, A.S.; Appling, D.R. Compartmentalization Of Mammalian Folate-Mediated One-Carbon Metabolism. Annu Rev. Nutr. 2010, 30, 57–81. [Google Scholar] [CrossRef] [PubMed]
- Labuschagne, C.F.; van den Broek, N.J.; Mackay, G.M.; Vousden, K.H.; Maddocks, O.D. Serine, but Not Glycine, Supports One-Carbon Metabolism and Proliferation of Cancer Cells. Cell Rep. 2014, 7, 1248–1258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ducker, G.S.; Chen, L.; Morscher, R.J.; Ghergurovich, J.M.; Esposito, M.; Teng, X.; Kang, Y.; Rabinowitz, J.D. Reversal of Cytosolic One-Carbon Flux Compensates for Loss of the Mitochondrial Folate Pathway. Cell Metab. 2016, 23, 1140–1153. [Google Scholar] [CrossRef] [Green Version]
- Pike, S.T.; Rajendra, R.; Artzt, K.; Appling, D.R. Mitochondrial C1-Tetrahydrofolate Synthase (MTHFD1L) Supports the Flow of Mitochondrial One-Carbon Units into the Methyl Cycle in Embryos. J. Biol. Chem. 2010, 285, 4612–4620. [Google Scholar] [CrossRef] [Green Version]
- Li Petri, G.; El Hassouni, B.; Sciarrillo, R.; Funel, N.; Mantini, G.; Zeeuw van der Laan, E.A.; Cascioferro, S.; Avan, A.; Zucali, P.A.; Zaffaroni, N.; et al. Impact of Hypoxia on Chemoresistance of Mesothelioma Mediated by the Proton-Coupled Folate Transporter, and Preclinical Activity of New Anti-LDH-A Compounds. Br. J. Cancer 2020, 123, 644–656. [Google Scholar] [CrossRef]
- Gazdar, A.F.; Minna, J.D. Molecular Detection of Early Lung Cancer. J. Natl. Cancer Inst. 1999, 91, 299–301. [Google Scholar] [CrossRef] [Green Version]
- Hou, X.M.; Yuan, S.Q.; Zhao, D.; Liu, X.J.; Wu, X.A. LDH-A Promotes Malignant Behavior via Activation of Epithelial-to-Mesenchymal Transition in Lung Adenocarcinoma. Biosci. Rep. 2019, 39, BSR20181476. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Li, Y.; Yan, X.; Song, Q.; Wang, G.; Hu, Y.; Jiao, S.; Wang, J. Pretreatment Lactate Dehydrogenase May Predict Outcome of Advanced Non Small-Cell Lung Cancer Patients Treated with Immune Checkpoint Inhibitors: A Meta-Analysis. Cancer Med. 2019, 8, 1467–1473. [Google Scholar] [CrossRef]
- Izumi, H.; Takahashi, M.; Uramoto, H.; Nakayama, Y.; Oyama, T.; Wang, K.Y.; Sasaguri, Y.; Nishizawa, S.; Kohno, K. Monocarboxylate Transporters 1 and 4 Are Involved in the Invasion Activity of Human Lung Cancer Cells. Cancer Sci. 2011, 102, 1007–1013. [Google Scholar] [CrossRef]
- Sandforth, L.; Ammar, N.; Dinges, L.A.; Röcken, C.; Arlt, A.; Sebens, S.; Schäfer, H. Impact of the Monocarboxylate Transporter-1 (MCT1)-Mediated Cellular Import of Lactate on Stemness Properties of Human Pancreatic Adenocarcinoma Cells. Cancers 2020, 12, 581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kasiappan, R.; Shih, H.J.; Chu, K.L.; Chen, W.T.; Liu, H.P.; Huang, S.F.; Choy, C.O.; Shu, C.L.; Din, R.; Chu, J.S.; et al. Loss of P53 and MCT-1 Overexpression Synergistically Promote Chromosome Instability and Tumorigenicity. Mol. Cancer Res. 2009, 7, 536–548. [Google Scholar] [CrossRef] [PubMed]
- Bovenzi, C.D.; Hamilton, J.; Tassone, P.; Johnson, J.; Cognetti, D.M.; Luginbuhl, A.; Keane, W.M.; Zhan, T.; Tuluc, M.; Bar-Ad, V.; et al. Prognostic Indications of Elevated MCT4 and CD147 across Cancer Types: A Meta-Analysis. Biomed. Res. Int. 2015, 2015, 242437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rumsey, S.C.; Kwon, O.; Xu, G.W.; Burant, C.F.; Simpson, I.; Levine, M. Glucose Transporter Isoforms GLUT1 and GLUT3 Transport Dehydroascorbic Acid. J. Biol. Chem. 1997, 272, 18982–18989. [Google Scholar] [CrossRef] [Green Version]
- Giatromanolaki, A.; Sivridis, E.; Arelaki, S.; Koukourakis, M.I. Expression of Enzymes Related to Glucose Metabolism in Non-Small Cell Lung Cancer and Prognosis. Exp. Lung Res. 2017, 43, 167–174. [Google Scholar] [CrossRef]
- Zhang, B.; Xie, Z.; Li, B. The Clinicopathologic Impacts and Prognostic Significance of GLUT1 Expression in Patients With Lung Cancer: A Meta-Analysis. Gene 2019, 689, 76–83. [Google Scholar] [CrossRef]
- Meijer, T.W.H.; Schuurbiers, O.C.J.; Kaanders, J.H.A.M.; Looijen-Salamon, M.G.; de Geus-Oei, L.F.; Verhagen, A.F.T.M.; Lok, J.; van der Heijden, H.F.M.; Rademakers, S.E.; Span, P.N.; et al. Differences In Metabolism between Adeno- and Squamous Cell Non-Small Cell Lung Carcinomas: Spatial Distribution and Prognostic Value of GLUT1 and MCT4. Lung Cancer 2012, 76, 316–323. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Y.-S.; Chen, Y.-C.; Chen, T.-E.; Cheng, M.-L.; Lynn, K.-S.; Shah, P.; Chen, J.-S.; Huang, R.-F.S. Probing Folate-Responsive and Stage-Sensitive Metabolomics and Transcriptional Co-Expression Network Markers to Predict Prognosis of Non-Small Cell Lung Cancer Patients. Nutrients 2023, 15, 3. https://doi.org/10.3390/nu15010003
Lin Y-S, Chen Y-C, Chen T-E, Cheng M-L, Lynn K-S, Shah P, Chen J-S, Huang R-FS. Probing Folate-Responsive and Stage-Sensitive Metabolomics and Transcriptional Co-Expression Network Markers to Predict Prognosis of Non-Small Cell Lung Cancer Patients. Nutrients. 2023; 15(1):3. https://doi.org/10.3390/nu15010003
Chicago/Turabian StyleLin, Yu-Shun, Yen-Chu Chen, Tzu-En Chen, Mei-Ling Cheng, Ke-Shiuan Lynn, Pramod Shah, Jin-Shing Chen, and Rwei-Fen S. Huang. 2023. "Probing Folate-Responsive and Stage-Sensitive Metabolomics and Transcriptional Co-Expression Network Markers to Predict Prognosis of Non-Small Cell Lung Cancer Patients" Nutrients 15, no. 1: 3. https://doi.org/10.3390/nu15010003
APA StyleLin, Y.-S., Chen, Y.-C., Chen, T.-E., Cheng, M.-L., Lynn, K.-S., Shah, P., Chen, J.-S., & Huang, R.-F. S. (2023). Probing Folate-Responsive and Stage-Sensitive Metabolomics and Transcriptional Co-Expression Network Markers to Predict Prognosis of Non-Small Cell Lung Cancer Patients. Nutrients, 15(1), 3. https://doi.org/10.3390/nu15010003






