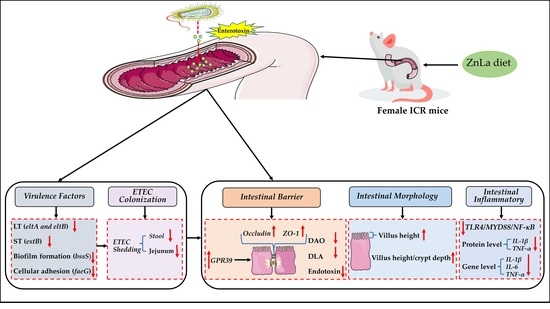
Zinc Laurate Protects against Intestinal Barrier Dysfunction and Inflammation Induced by ETEC in a Mice Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Statement of Ethics
2.2. Experimental Design
2.3. Samples Collection
2.4. Determination of Zinc Content
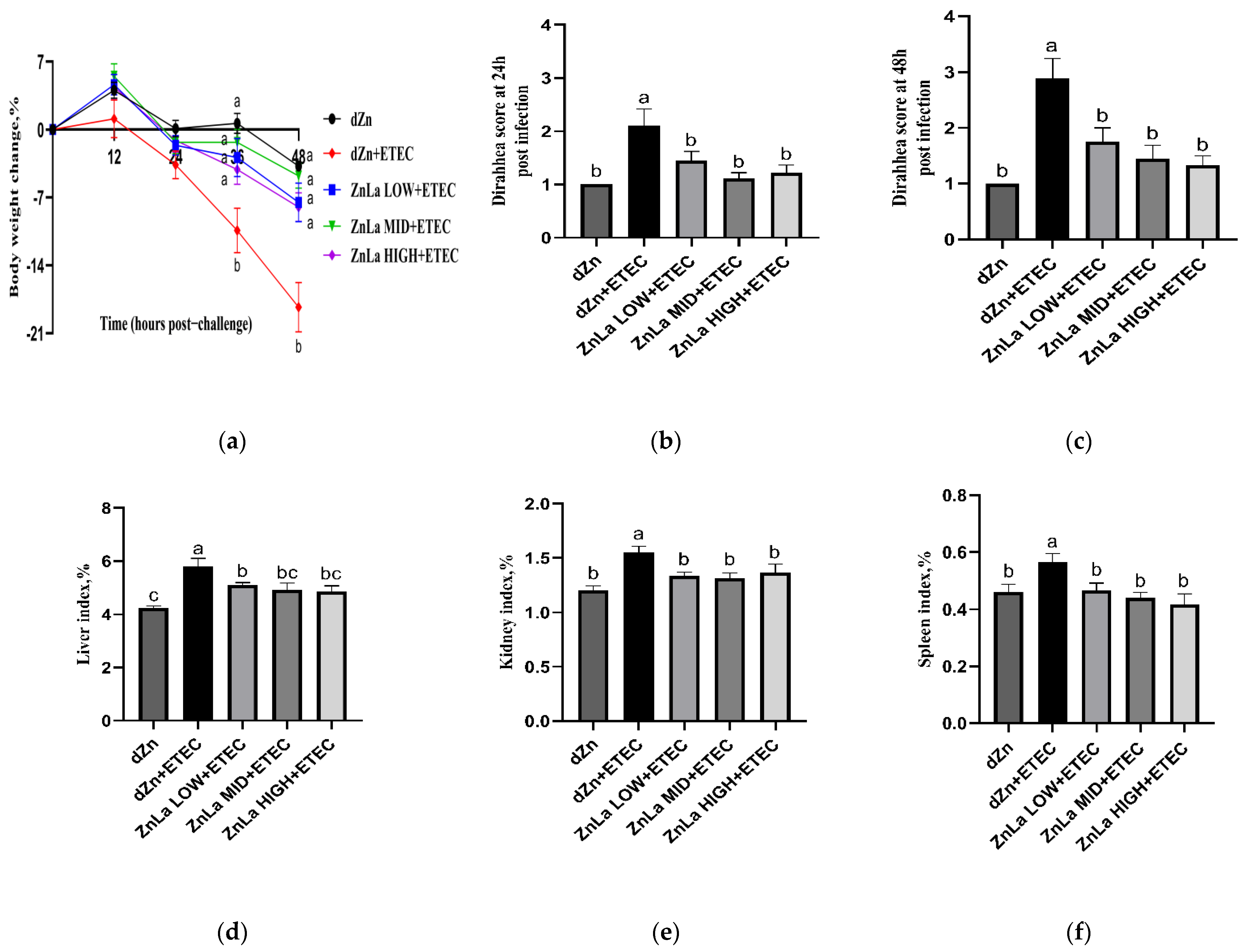
2.5. Organ Index
2.6. Intestinal Morphology
2.7. Inflammatory Cytokines
2.8. Intestinal Permeability
2.9. Bacterial Load
2.10. Effect of Different Source of Zinc on the Growth Curve of ETEC In Vitro
2.11. Gene Expression
2.12. Virulence Factors Assay
2.13. Statistical Analysis
3. Results
3.1. Effects of ZnLa on the Clinical Symptoms of Mice with ETEC Challenge
3.2. Effect of ZnLa on the Intestinal Morphology of Mice with ETEC Infection
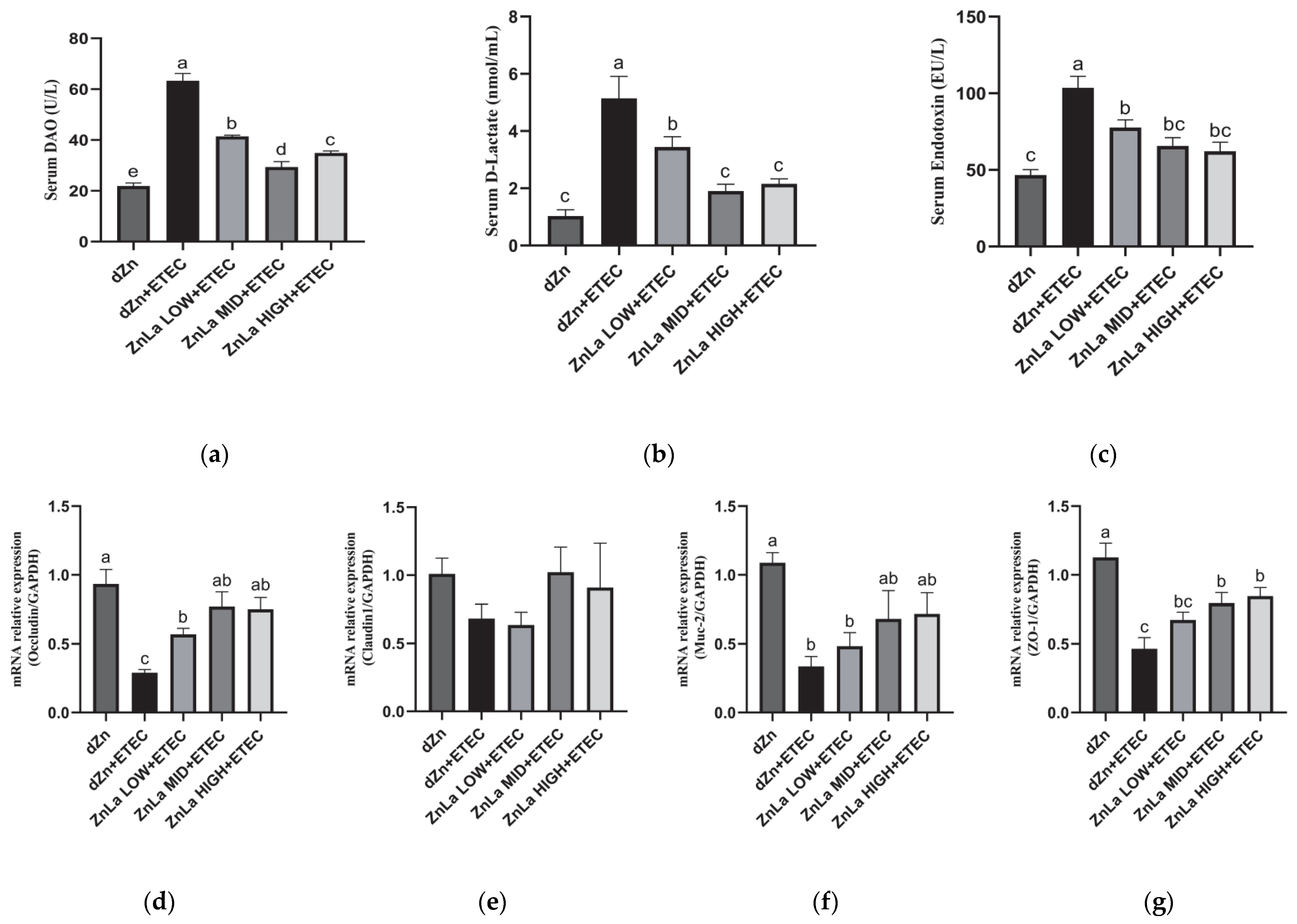
3.3. Effect of ZnLa on Intestinal Barrier Damage Caused by ETEC Infection
3.4. Effect of ZnLa on Intestinal Inflammatory Reaction in ETEC-Challenged Mice
3.5. Effect of ZnLa on the Toll-like Receptor 4 (TLR4)/Myeloiddifferentiation Factor 88 (MYD88)/Nuclear Factor κB (NF-κB) Signaling Pathway in Mice with ETEC Challenge
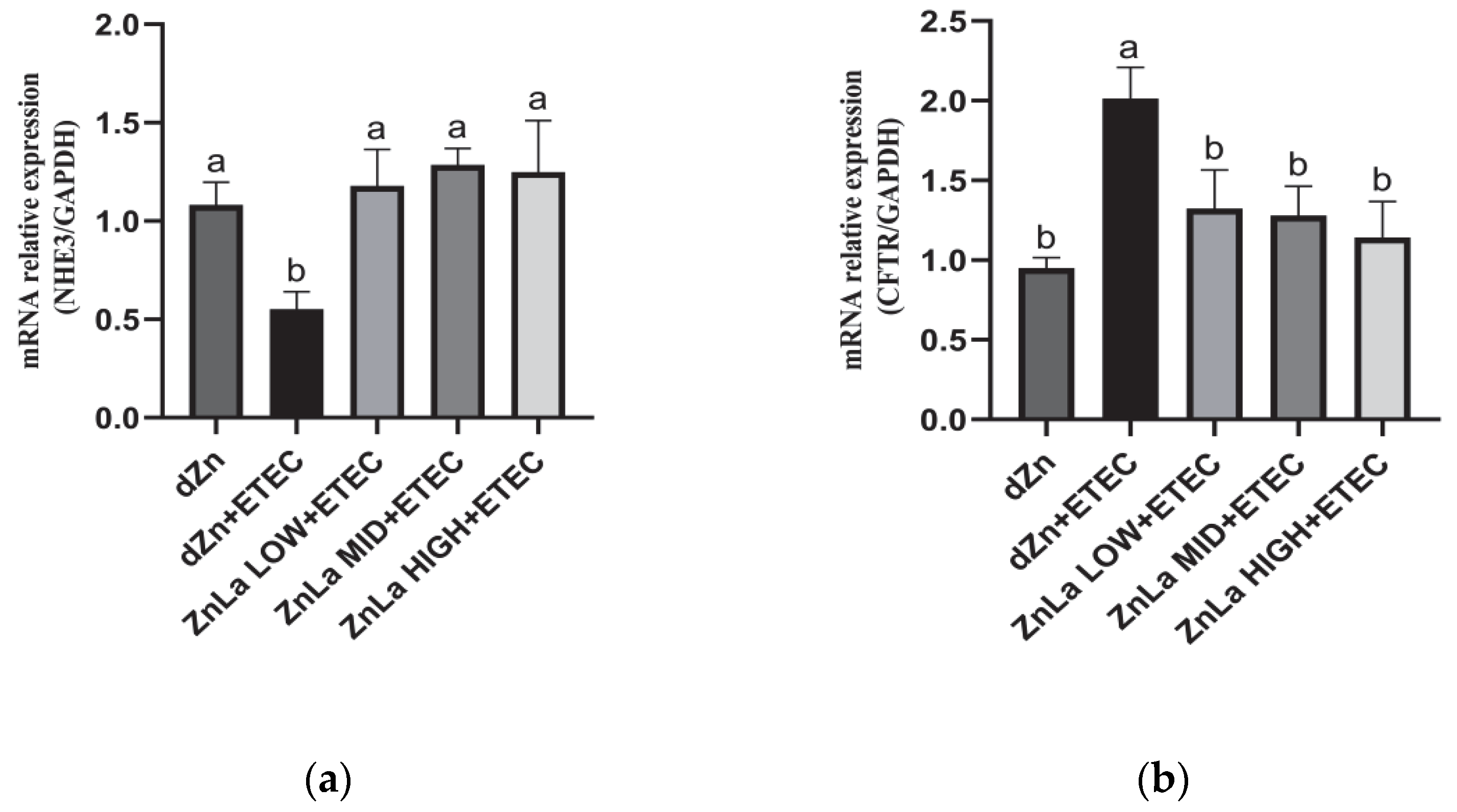
3.6. Effect of ZnLa on Anion Transporters in Mice Challenged with ETEC
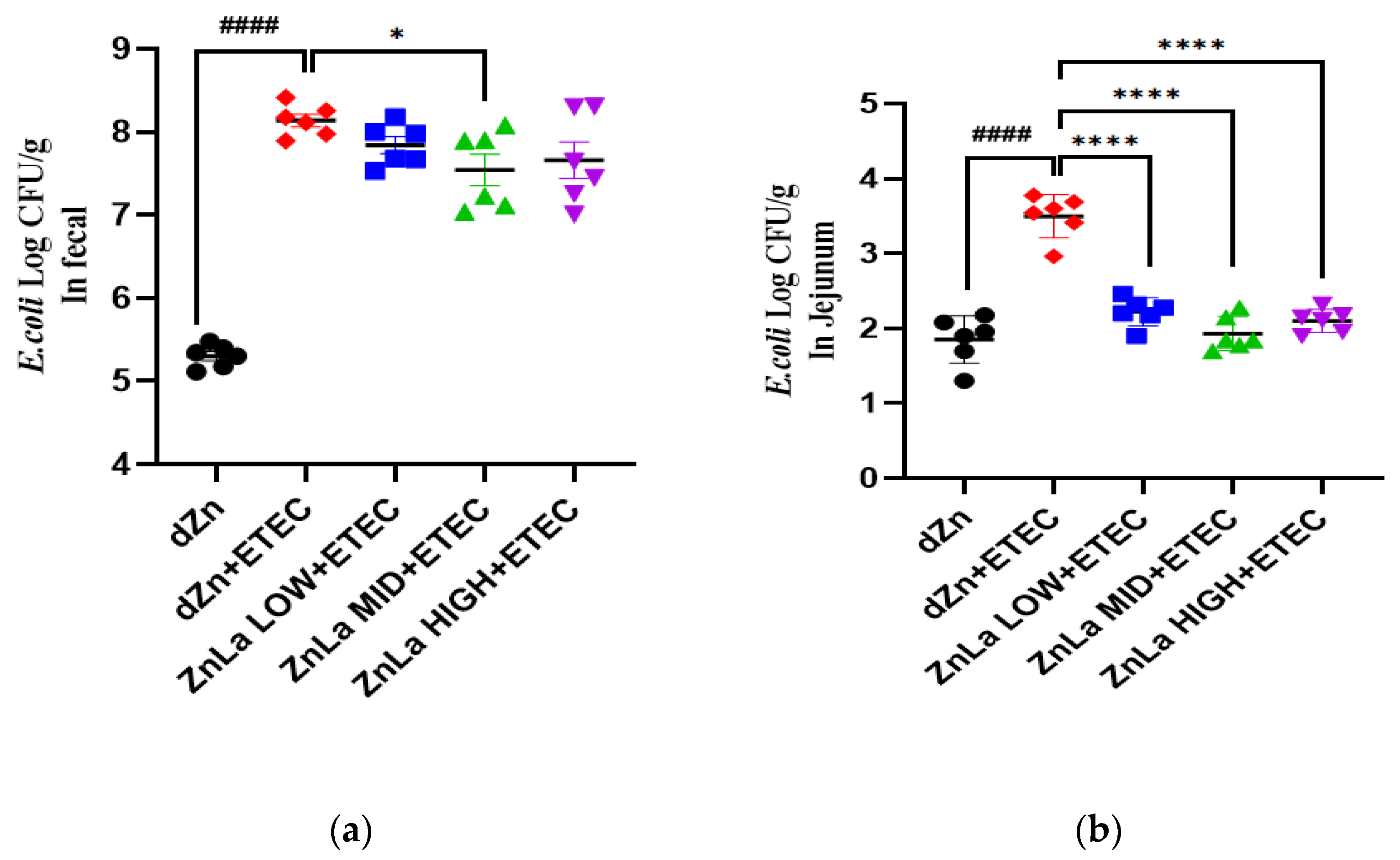
3.7. Effect of ZnLa on ETEC Shedding in the Stool and Intestine
3.8. Effect of ZnLa on the Virulence Factors of Intestinal Content in ETEC-Infected Mice
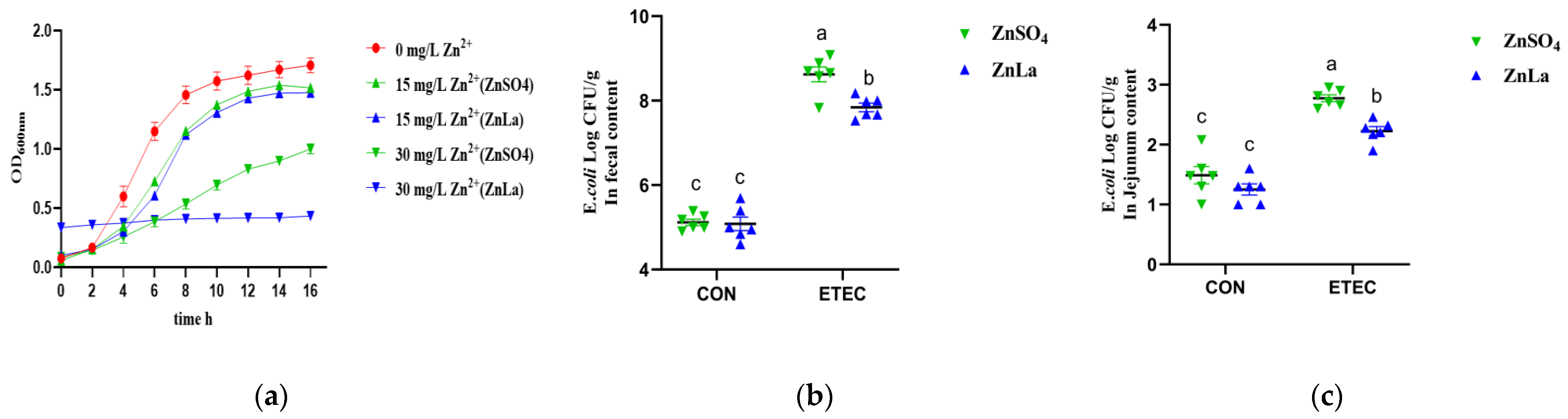
3.9. Effects of Different Zinc Sources on ETEC Inhibition In Vitro and Vivo
3.10. Effects of Different Zinc Sources on the Relative Expression of Virulence Genes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MCFAs | Medium-chain fatty acids |
| ZnLa | Zinc laurate |
| dZn | Marginal zinc deficiency |
| ETEC | Enterotoxigenic Escherichia coli |
| DAO | Diamine oxidase |
| DLA | D-lactic acid |
| TNF-α | Tumor necrosis factor-α |
| IL-1β | Interleukin-1β |
| IL-6 | Interleukin-6 |
| TLR4 | Toll-like receptor 4 |
| MyD88 | Myeloiddifferentiation factor 88 |
| NF-κB | Nuclear factor κB |
| CFTR | Cystic fibrosis transmembrane conductance regulator |
| NHE3 | Na+/H+ exchanger 3 |
| GPR39 | G protein-coupled receptor39 |
| LT | Heat-labile enterotoxins |
| ST | Heat-stable enterotoxins |
References
- Walker, R.I. An assessment of enterotoxigenic Escherichia coli and shigella vaccine candidates for infants and children. Vaccine 2015, 33, 954–965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dubreuil, J.D.; Isaacson, R.E.; Schifferli, D.M. Animal enterotoxigenic Escherichia coli. EcoSal Plus 2016, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dubreuil, J.D. Enterotoxigenic Escherichia coli targeting intestinal epithelial tight junctions: An effective way to alter the barrier integrity. Microb. Pathog. 2017, 113, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Yuvraj, P. Colibacillosis in poultry: A review. J. Agric. Nat. Resour. 2019, 2, 301–311. [Google Scholar]
- Hanczakowska, E. The use of medium-chain fatty acids in piglet feeding—A review. Ann. Anim. SCI 2017, 17, 967–977. [Google Scholar] [CrossRef] [Green Version]
- Lauridsen, C. Effects of dietary fatty acids on gut health and function of pigs pre-and post-weaning. J. Anim. Sci. 2020, 98, a86. [Google Scholar] [CrossRef]
- Huang, W.; Tsai, T.; Chuang, L.; Li, Y.; Zouboulis, C.C.; Tsai, P. Anti-bacterial and anti-inflammatory properties of capric acid against Propionibacterium acnes: A comparative study with lauric acid. J. Dermatol. Sci. 2014, 73, 232–240. [Google Scholar] [CrossRef]
- Dayrit, F.M. The properties of lauric acid and their significance in coconut oil. J. Am. Oil Chem. Soc. 2015, 92, 1–15. [Google Scholar] [CrossRef]
- Fauser, J.K.; Matthews, G.M.; Cummins, A.G.; Howarth, G.S. Induction of apoptosis by the medium-chain length fatty acid lauric acid in colon cancer cells due to induction of oxidative stress. Chemotherapy 2013, 59, 214–224. [Google Scholar] [CrossRef]
- Rouse, M.S.; Rotger, M.; Piper, K.E.; Steckelberg, J.M.; Scholz, M.; Andrews, J.; Patel, R. In vitro and in vivo evaluations of the activities of lauric acid monoester formulations against Staphylococcus aureus. Antimicrob. Agents Chemother. 2005, 49, 3187–3191. [Google Scholar] [CrossRef] [Green Version]
- Oprean, L.; Iancu, R.; Stan, R.; Traşca, C. Comparison between types of feeding on goat milk composition. Sci. Pap. Anim. Sci. Biotechnol./Lucrari Stiintifice Zootehnie si Biotehnologii 2011, 44, 76–79. [Google Scholar]
- Mishima, M.; Wakita, Y.; Nakano, M. Studies on the promoting effects of medium chain fatty acid salts on the nasal absorption of insulin in rats. J. Pharm.-Dyn. 1987, 10, 624–631. [Google Scholar] [CrossRef] [PubMed]
- Kettler, S.I.; Eder, K.; Kettler, A.; Kirchgessner, M. Zinc deficiency and the activities of lipoprotein lipase in plasma and tissues of rats force-fed diets with coconut oil or fish oil. J. Nutr. Biochem. 2000, 11, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Chen, Q.; Gan, L.; Du, X.; Li, Q.; Qiao, H.; Zhao, Y.; Huang, J.; Wang, J. Marginal zinc deficiency aggravated intestinal barrier dysfunction and inflammation through ETEC virulence factors in a mouse model of diarrhea. Vet. Sci. 2022, 9, 507. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Wang, J.; Wang, P.; Qiao, H.; Gan, L.; Liu, C.; Zhang, Y. In vitro inhibitory effect and mechanism of zinc laurate on Staphylococcus aureus and Escherichia coli. Chin. J. Anim. Sci. 2022, 58, 233–240. [Google Scholar] [CrossRef]
- Ko, J.W.; H, E.T.; Lee, I.C.; Park, S.H.; Park, J.L.; Seong, N.W.; Hong, J.S.; Yun, H.I.; Kim, J.C. Evaluation of 2-week repeated oral dose toxicity of 100 nm zinc oxide nanoparticles in rats. Lab. Anim. Res. 2015, 31, 139–147. [Google Scholar] [CrossRef] [Green Version]
- Liu, G.; Ren, W.; Fang, J.; Hu, C.A.; Guan, G.; Al-Dhabi, N.A.; Yin, J.; Duraipandiyan, V.; Chen, S.; Peng, Y.; et al. L-Glutamine and L-arginine protect against enterotoxigenic Escherichia coli infection via intestinal innate immunity in mice. Amino Acids 2017, 49, 1945–1954. [Google Scholar] [CrossRef]
- Ding, X.; Yu, H.; Qiao, S. Lasso peptide microcin J25 effectively enhances gut barrier function and modulates inflammatory response in an enterotoxigenic Escherichia coli-challenged mouse model. Int. J. Mol. Sci. 2020, 21, 6500. [Google Scholar] [CrossRef]
- Yu, H.; Wang, Y.; Zeng, X.; Cai, S.; Wang, G.; Liu, L.; Huang, S.; Li, N.; Liu, H.; Ding, X.; et al. Therapeutic administration of the recombinant antimicrobial peptide microcin J25 effectively enhances host defenses against gut inflammation and epithelial barrier injury induced by enterotoxigenic Escherichia coli infection. FASEB J. 2020, 34, 1018–1037. [Google Scholar] [CrossRef] [Green Version]
- Medeiros, P.; Bolick, D.T.; Roche, J.K.; Noronha, F.; Pinheiro, C.; Kolling, G.L.; Lima, A.; Guerrant, R.L. The micronutrient zinc inhibits EAEC strain 042 adherence, biofilm formation, virulence gene expression, and epithelial cytokine responses benefiting the infected host. Virulence 2013, 4, 624–633. [Google Scholar] [CrossRef] [Green Version]
- Egea, L.; Aguilera, L.; Gimenez, R.; Sorolla, M.A.; Aguilar, J.; Badia, J.; Baldoma, L. Role of secreted glyceraldehyde-3-phosphate dehydrogenase in the infection mechanism of enterohemorrhagic and enteropathogenic Escherichia coli: Interaction of the extracellular enzyme with human plasminogen and fibrinogen. Int. J. Biochem. Cell Biol. 2007, 39, 1190–1203. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Y.; Baek, D.H.; Kim, I.H. Effect of dietary supplemental medium chain fatty acids instead of antibiotics on the growth performance, digestibility and blood profiles in growing pigs. J. Anim. Physiol. Anim. Nutr. 2019, 103, 1946–1951. [Google Scholar] [CrossRef] [PubMed]
- St-Pierre, V.; Vandenberghe, C.; Lowry, C.; Fortier, M.; Castellano, C.; Wagner, R.; Cunnane, S.C. Plasma ketone and medium chain fatty acid response in humans consuming different medium chain triglycerides during a metabolic study day. Front. Nutr. 2019, 6, 46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, C.; Wang, Y.; Lin, X.; Song, H.; Zhou, Q.; Xu, W.; Shi, K.; Chen, J.; Song, J.; Chen, F.; et al. A Combination of Formic Acid and Monolaurin Attenuates Enterotoxigenic Escherichia coli Induced Intestinal Inflammation in Piglets by Inhibiting the NF-κB/MAPK Pathways with Modulation of Gut Microbiota. J. Agric. Food Chem. 2020, 68, 4155–4165. [Google Scholar] [CrossRef]
- Wu, Y.; Li, Q.; Liu, J.; Liu, Y.; Xu, Y.; Zhang, R.; Yu, Y.; Wang, Y.; Yang, C. Integrating serum metabolome and gut microbiome to evaluate the benefits of lauric acid on lipopolysaccharide-challenged broilers. Front. Immunol. 2021, 12, 759323. [Google Scholar] [CrossRef]
- Dynia, D.W.; Steinmetz, A.G.; Kocinsky, H.S. Nhe3 function and phosphorylation are regulated by a calyculin a-sensitive phosphatase. Am. J. Physiol.-Renal Physiol. 2010, 298, F745–F753. [Google Scholar] [CrossRef] [Green Version]
- Rommens, J.M.; Iannuzzi, M.C.; Kerem, B.; Drumm, M.L.; Melmer, G.; Dean, M.; Rozmahel, R.; Cole, J.L.; Kennedy, D.; Hidaka, N. Identification of the cystic fibrosis gene: Chromosome walking and jumping. Science 1989, 245, 1059–1065. [Google Scholar] [CrossRef]
- Xue, J.; Thomas, L.; Tahmasbi, M.; Valdez, A.; Rieg, J.A.D.; Fenton, R.A.; Rieg, T. An inducible intestinal epithelial cell-specific nhe3 knockout mouse model mimicking congenital sodium diarrhea. Clin. Sci. 2020, 134, 941–953. [Google Scholar] [CrossRef] [Green Version]
- Schultheis, P.J.; Clarke, L.L.; Meneton, P.; Miller, M.L.; Soleimani, M.; Gawenis, L.R.; Riddle, T.M.; Duffy, J.J.; Doetschman, T.; Wang, T.; et al. Renal and intestinal absorptive defects in mice lacking the nhe3 Na+/H+ exchanger. Nat. Genet. 1998, 19, 282–285. [Google Scholar] [CrossRef]
- Li, X.C.; Zhu, D.; Chen, X.; Zheng, X.; Zhao, C.; Zhang, J.; Soleimani, M.; Rubera, I.; Tauc, M.; Zhou, X.; et al. Proximal tubule-specific deletion of the NHE3 (Na+/H+ exchanger 3) in the kidney attenuates ANG II (Angiotensin II)-induced hypertension in mice. Hypertension 2019, 74, 526–535. [Google Scholar] [CrossRef]
- Casellas, J.; Casas, X.; Piedrafita, J.; Manteca, X. Effect of medium- and long-chain triglyceride supplementation on small newborn-pig survival. Prev. Vet. Med. 2005, 67, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Rebucci, R.; Comi, M.; Ghiringhelli, M.; Giorgi, S.; Cheli, F.; Bontempo, V. Lauric acid saponified with calcium ameliorates indices of intestinal function and gut health in weaned piglets. Ital. J. Anim. Sci. 2021, 20, 1479–1490. [Google Scholar] [CrossRef]
- Chwen, L.T.; Foo, H.L.; Nguyen, T.T.; Choe, D.W. Growth performance, plasma fatty acids, villous height and crypt depth of preweaning piglets fed with medium chain triacylglycerol. Asian Austral. J. Anim. 2013, 26, 700–704. [Google Scholar] [CrossRef] [PubMed]
- De Keyser, K.; Dierick, N.; Kanto, U.; Hongsapak, T.; Buyens, G.; Kuterna, L.; Vanderbeke, E. Medium-chain glycerides affect gut morphology, immune- and goblet cells in post-weaning piglets: In vitro fatty acid screening with Escherichia coli and in vivo consolidation with LPS challenge. J. Anim. Physiol. Anim. Nutr. 2019, 103, 221–230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Camilleri, M.; Madsen, K.; Spiller, R.; Greenwood-Van Meerveld, B.; Verne, G.N. Intestinal barrier function in health and gastrointestinal disease. Neurogastroenterol. Motil. 2012, 24, 503–512. [Google Scholar] [CrossRef]
- Chang, Y.Q.; Mei, J.; Yang, T.; Zhang, Z.Y.; Liu, G.M.; Zhao, H.; Chen, X.L.; Tian, G.; Cai, J.Y.; Wu, B.; et al. Effect of dietary zinc methionine supplementation on growth performance, immune function and intestinal health of cherry valley ducks challenged with avian pathogenic Escherichia coli. Front. Microbiol. 2022, 13, 849067. [Google Scholar] [CrossRef]
- Tian, M.; Chen, J.; Wu, Z.; Song, H.; Yang, F.; Cui, C.; Chen, F.; Zhang, S.; Guan, W. Fat encapsulation reduces diarrhea in piglets partially by repairing the intestinal barrier and improving fatty acid transport. Animals 2021, 11, 28. [Google Scholar] [CrossRef]
- Lee, S.I.; Kang, K.S. Function of capric acid in cyclophosphamide-induced intestinal inflammation, oxidative stress, and barrier function in pigs. Sci. Rep. 2017, 7, 10653. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.; Guo, Y. Supplemental zinc reduced intestinal permeability by enhancing occludin and zonula occludens protein-1 (ZO-1) expression in weaning piglets. Br. J. Nutr. 2009, 102, 687–693. [Google Scholar] [CrossRef] [Green Version]
- Capaldo, C.T.; Powell, D.N.; Kalman, D. Layered defense: How mucus and tight junctions seal the intestinal barrier. J. Mol. Med. 2017, 95, 927–934. [Google Scholar] [CrossRef] [Green Version]
- Kuo, W.; Zuo, L.; Odenwald, M.A.; Madha, S.; Singh, G.; Gurniak, C.B.; Abraham, C.; Turner, J.R. The tight junction protein zo-1 is dispensable for barrier function but critical for effective mucosal repair. Gastroenterology 2021, 161, 1924–1939. [Google Scholar] [CrossRef] [PubMed]
- Doerfel, M.J.; Huber, O. Modulation of tight junction structure and function by kinases and phosphatases targeting occludin. J. Biomed. Biotechnol. 2012, 2012, 807356. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Xie, W.; Zan, G.; Gao, C.; Yan, H.; Zhou, J.; Wang, X. Lauric acid alleviates deoxynivalenol-induced intestinal stem cell damage by potentiating the Akt/mTORC1/S6K1 signaling axis. Chem.-Biol. Interact. 2021, 348, 109640. [Google Scholar] [CrossRef] [PubMed]
- Cohen, L.; Sekler, I.; Hershfinkel, M. The zinc sensing receptor, ZnR/GPR39, controls proliferation and differentiation of colonocytes and thereby tight junction formation in the colon. Cell Death. Dis. 2014, 5, e1307. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Wang, W.; Wang, L.; Yu, C.; Zhang, G.; Zhu, H.; Wang, C.; Zhao, S.; Hu, C.A.; Liu, Y. Lentinan modulates intestinal microbiota and enhances barrier integrity in a piglet model challenged with lipopolysaccharide. Food Funct. 2019, 10, 479–489. [Google Scholar] [CrossRef]
- Carlson, S.J.; Nandivada, P.; Chang, M.I.; Mitchell, P.D.; O’Loughlin, A.; Cowan, E.; Gura, K.M.; Nose, V.; Bistrian, B.R.; Puder, M. The addition of medium-chain triglycerides to a purified fish oil-based diet alters inflammatory profiles in mice. Metabolism 2015, 64, 274–282. [Google Scholar] [CrossRef] [Green Version]
- Khan, H.U.; Aamir, K.; Jusuf, P.R.; Sethi, G.; Sisinthy, S.P.; Ghildyal, R.; Arya, A. Lauric acid ameliorates lipopolysaccharide (LPS)-induced liver inflammation by mediating TLR4/MyD88 pathway in Sprague Dawley (SD) rats. Life Sci. 2021, 265, 118750. [Google Scholar] [CrossRef]
- Kong, L.; Wang, Z.; Xiao, C.; Zhu, Q.; Song, Z. Glycerol monolaurate attenuated immunological stress and intestinal mucosal injury by regulating the gut microbiota and activating AMPK/Nrf2 signaling pathway in lipopolysaccharide-challenged broilers. Anim. Nutr. 2022, 10, 347–359. [Google Scholar] [CrossRef]
- Ren, W.; Yin, J.; Xiao, H.; Chen, S.; Liu, G.; Tan, B.; Li, N.; Peng, Y.; Li, T.; Zeng, B.; et al. Intestinal microbiota-derived GABA mediates interleukin-17 expression during enterotoxigenic Escherichia coli infection. Front. Immunol. 2017, 7, 685. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.A.; Rhee, M.S. Highly enhanced bactericidal effects of medium chain fatty acids (caprylic, capric, and lauric acid) combined with edible plant essential oils (carvacrol, eugenol, β-resorcylic acid, trans-cinnamaldehyde, thymol, and vanillin) against Escherichia coli o157:h7. Food Control 2016, 60, 447–454. [Google Scholar]
- Xia, P.; Song, Y.; Zou, Y.; Yang, Y.; Zhu, G. F4+ enterotoxigenic Escherichia coli (ETEC) adhesion mediated by the major fimbrial subunit FaeG. J. Basic Microb. 2015, 55, 1118–1124. [Google Scholar] [CrossRef] [PubMed]
- Mukiza, C.N.; Dubreuil, J.D. Escherichia coli heat-stable toxin b impairs intestinal epithelial barrier function by altering tight junction proteins. Infect. Immun. 2013, 81, 2819–2827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sauer, A.K.; Grabrucker, A.M. Zinc Deficiency during Pregnancy Leads to Altered Microbiome and Elevated Inflammatory Markers in Mice. Front. Neurosci. 2019, 13, 1295. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Q.; Wang, P.; Wang, J.; Xu, J.; Liu, C.; Qiao, H.; Gan, L.; Duan, E.; Zhang, Y.; Wang, M.; et al. Zinc Laurate Protects against Intestinal Barrier Dysfunction and Inflammation Induced by ETEC in a Mice Model. Nutrients 2023, 15, 54. https://doi.org/10.3390/nu15010054
Chen Q, Wang P, Wang J, Xu J, Liu C, Qiao H, Gan L, Duan E, Zhang Y, Wang M, et al. Zinc Laurate Protects against Intestinal Barrier Dysfunction and Inflammation Induced by ETEC in a Mice Model. Nutrients. 2023; 15(1):54. https://doi.org/10.3390/nu15010054
Chicago/Turabian StyleChen, Qianqian, Peng Wang, Jinrong Wang, Jilong Xu, Cen Liu, Hanzhen Qiao, Liping Gan, Erzhen Duan, Yihui Zhang, Meiying Wang, and et al. 2023. "Zinc Laurate Protects against Intestinal Barrier Dysfunction and Inflammation Induced by ETEC in a Mice Model" Nutrients 15, no. 1: 54. https://doi.org/10.3390/nu15010054
APA StyleChen, Q., Wang, P., Wang, J., Xu, J., Liu, C., Qiao, H., Gan, L., Duan, E., Zhang, Y., Wang, M., Wu, X., Du, X., & Li, L. (2023). Zinc Laurate Protects against Intestinal Barrier Dysfunction and Inflammation Induced by ETEC in a Mice Model. Nutrients, 15(1), 54. https://doi.org/10.3390/nu15010054