In Vitro Digestion and Colonic Fermentation of UHT Treated Faba Protein Emulsions: Effects of Enzymatic Hydrolysis and Thermal Processing on Proteins and Phenolics
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Sample Preparation
2.2.1. Extraction of Faba Bean Phenolics (FBP)
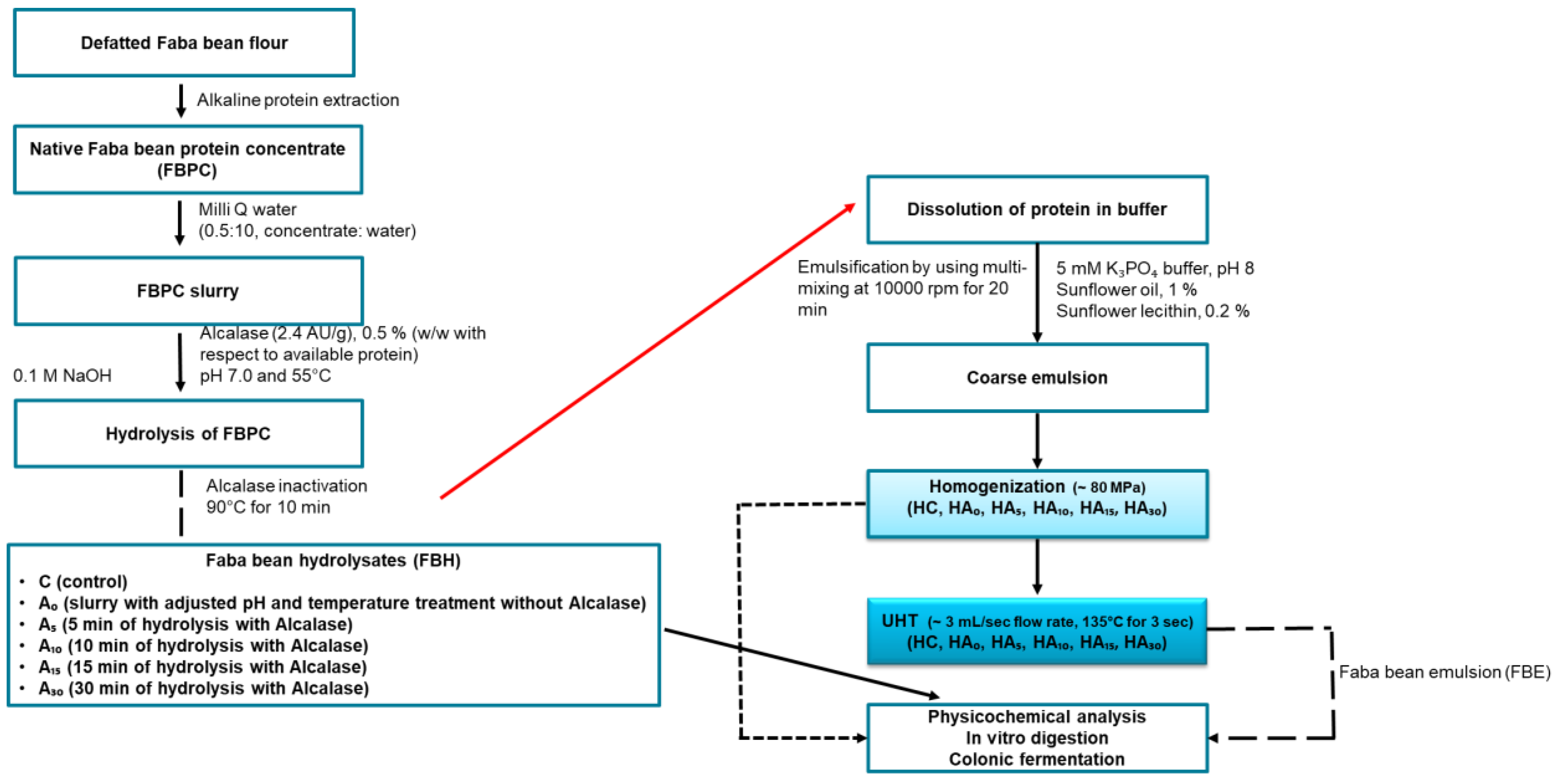
2.2.2. Preparation of Faba Bean Protein Concentrate (FBPC)
2.2.3. Preparation of Faba Bean Hydrolysates (FBH)
2.2.4. Preparation of Faba Bean Emulsions (FBE)
2.3. In Vitro Gastrointestinal Digestion
2.4. In Vitro Colonic Fermentation
2.5. Physicochemical Properties of Hydrolysates and Emulsions
2.5.1. Degree of Hydrolysis (DH)
2.5.2. Protein Solubility
2.5.3. ζ-Potential
2.5.4. Surface Hydrophobicity (S₀)
2.5.5. Particle Size
2.6. Estimation of Phenolics and Their Antioxidant Potential
2.6.1. Determination of Total Phenolic Content (TPC)
2.6.2. Determination of Total Flavonoid Content (TFC)
2.6.3. Determination of Total Condensed Tannin (TCT)
2.6.4. 2,2′-Diphenyl-1-Picrylhydrazyl (DPPH) Assay
2.6.5. Ferric Reducing Antioxidant Power (FRAP) Assay
2.6.6. 2,2′-Azino-Bis-3-Ethylbenzothiazoline-6-Sulfonic Acid (ABTS) Assay
2.7. Short Chain Fatty Acids (SCFAs) Production (GC-FID) Analysis
2.8. Statistical Analysis
3. Results and Discussions
3.1. Changes in the Phenolic Contents and Antioxidant Properties during FBPC Preparation
3.2. Effects of Enzymatic Hydrolysis on the Phenolic Contents and Antioxidant Properties
3.3. Effects of UHT Processing on the Phenolic Content and Antioxidant Properties of Faba Bean Concentrate
3.4. Effects of Enzymatic Hydrolysis Combined with UHT Processing on the Phenolic Contents and Antioxidant Properties
3.5. Molecular Weight Distribution and Physicochemical Properties of Hydrolysates
3.6. Physicochemical Properties of Emulsions
3.7. Phenolic Estimations across In Vitro Digestion
3.8. Antioxidant Activities across the Stages of In Vitro Digestion
3.9. Phenolic Estimations across Colonic Fermentation
3.10. Antioxidant Activities across Colonic Fermentation
3.11. Short Chain Fatty Acids Production
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Maalouf, F.; Hu, J.; O’Sullivan, D.M.; Zong, X.; Hamwieh, A.; Kumar, S.; Baum, M. Breeding and genomics status in faba bean (Vicia faba). Plant Breed. 2019, 138, 465–473. [Google Scholar] [CrossRef]
- Ali, M. Functional properties of faba bean protein and effect of enzymatic hydrolysis on its antioxidant activity. Zagazig J. Agric. Res. 2019, 46, 2019. [Google Scholar] [CrossRef][Green Version]
- Karkanis, A.; Ntatsi, G.; Lepse, L.; Fernández, J.A.; Vågen, I.M.; Rewald, B.; Alsiņa, I.; Kronberga, A.; Balliu, A.; Olle, M.; et al. Faba Bean Cultivation—Revealing Novel Managing Practices for More Sustainable and Competitive European Cropping Systems. Front. Plant Sci. 2018, 9, 1115. [Google Scholar] [CrossRef] [PubMed]
- Siah, S.; Konczak, I.; Wood, J.; Agboola, S.; Blanchard, C. Effects of Roasting on Phenolic Composition and In vitro Antioxidant Capacity of Australian Grown Faba Beans (Vicia faba L.). Plant Foods Hum. Nutr. 2014, 69, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Eckert, E.; Han, J.; Swallow, K.; Tian, Z.; Jarpa-Parra, M.; Chen, L. Effects of enzymatic hydrolysis and ultrafiltration on physicochemical and functional properties of faba bean protein. Cereal Chem. 2019, 96, 725–741. [Google Scholar] [CrossRef]
- Khalil, A.; Mansour, E. The effect of cooking, autoclaving and germination on the nutritional quality of faba beans. Food Chem. 1995, 54, 177–182. [Google Scholar] [CrossRef]
- Apaydin, H.; Ertan, S.; Ozekmekçi, S. Broad bean (Vicia faba)—A natural source of L-dopa--prolongs “on” periods in patients with Parkinson’s disease who have “on-off” fluctuations. Mov. Disord. Off. J. Mov. Disord. Soc. 2000, 15, 164–166. [Google Scholar] [CrossRef]
- Vucenik, I.; Shamsuddin, A.M. Protection Against Cancer by Dietary IP6 and Inositol. Nutr. Cancer 2006, 55, 109–125. [Google Scholar] [CrossRef]
- Laguna, L.; Picouet, P.; Guàrdia, M.D.; Renard, C.M.; Sarkar, A. In vitro gastrointestinal digestion of pea protein isolate as a function of pH, food matrices, autoclaving, high-pressure and re-heat treatments. Lwt 2017, 84, 511–519. [Google Scholar] [CrossRef]
- Zhang, Q.; Cheng, Z.; Wang, Y.; Fu, L. Dietary protein-phenolic interactions: Characterization, biochemical-physiological consequences, and potential food applications. Crit. Rev. Food Sci. Nutr. 2020, 61, 3589–3615. [Google Scholar] [CrossRef]
- Mihaylova, D.; Desseva, I.; Stoyanova, M.; Petkova, N.; Terzyiska, M.; Lante, A. Impact of In Vitro Gastrointestinal Digestion on the Bioaccessibility of Phytochemical Compounds from Eight Fruit Juices. Molecules 2021, 26, 1187. [Google Scholar] [CrossRef] [PubMed]
- Hrčková, M.; Rusnáková, M.; Zemanovič, J. Enzymatic hydrolysis of defatted soy flour by three different proteases and their effect on the functional properties of resulting protein hydrolysates. Czech J. Food Sci. 2011, 20, 7–14. [Google Scholar] [CrossRef]
- Ibrahim, E.; Ghani, M. The effect of enzymatic hydrolysis on the antioxidant activities and amino acid profiles of defatted chia (Salvia hispanica L.) flour. Food Res. 2020, 4, 38–50. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, M.A.; Tan, M.; Øiseth, S.; Buckow, R. An Emerging Segment of Functional Legume-Based Beverages: A Review. Food Rev. Int. 2020, 38, 1064–1102. [Google Scholar] [CrossRef]
- Žilić, S.; Akıllıoğlu, G.; Serpen, A.; Barac, M.; Gökmen, V. Effects of isolation, enzymatic hydrolysis, heating, hydratation and Maillard reaction on the antioxidant capacity of cereal and legume proteins. Food Res. Int. 2012, 49, 1–6. [Google Scholar] [CrossRef]
- Nawaz, M.A.; Buckow, R.; Jegasothy, H.; Stockmann, R. Enzymatic hydrolysis improves the stability of UHT treated faba bean protein emulsions. Food Bioprod. Process. 2022, 132, 200–210. [Google Scholar] [CrossRef]
- Liu, C.; Bhattarai, M.; Mikkonen, K.; Heinonen, M. Effects of Enzymatic Hydrolysis of Fava Bean Protein Isolate by Alcalase on the Physical and Oxidative Stability of Oil-in-Water Emulsions. J. Agric. Food Chem. 2019, 67, 6625–6632. [Google Scholar] [CrossRef]
- Ali, A.; Wu, H.; Ponnampalam, E.N.; Cottrell, J.J.; Dunshea, F.R.; Suleria, H.A.R. Comprehensive Profiling of Most Widely Used Spices for Their Phenolic Compounds through LC-ESI-QTOF-MS2 and Their Antioxidant Potential. Antioxidants 2021, 10, 721. [Google Scholar] [CrossRef]
- Ghribi, A.M.; Gafsi, I.M.; Sila, A.; Blecker, C.; Danthine, S.; Attia, H.; Bougatef, A.; Besbes, S. Effects of enzymatic hydrolysis on conformational and functional properties of chickpea protein isolate. Food Chem. 2015, 187, 322–330. [Google Scholar] [CrossRef]
- Sánchez-Velázquez, O.A.; Cuevas-Rodríguez, E.O.; Mondor, M.; Ribéreau, S.; Arcand, Y.; Mackie, A.; Hernández-Álvarez, A.J. Impact of in vitro gastrointestinal digestion on peptide profile and bioactivity of cooked and non-cooked oat protein concentrates. Curr. Res. Food Sci. 2021, 4, 93–104. [Google Scholar] [CrossRef]
- Gu, C.; Suleria, H.A.R.; Dunshea, F.R.; Howell, K. Dietary Lipids Influence Bioaccessibility of Polyphenols from Black Carrots and Affect Microbial Diversity under Simulated Gastrointestinal Digestion. Antioxidants 2020, 9, 762. [Google Scholar] [CrossRef] [PubMed]
- Church, F.C.; Swaisgood, H.E.; Porter, D.H.; Catignani, G.L. Spectrophotometric assay using o-phthaldialdehyde for determination of proteolysis in milk and isolated milk proteins. J. Dairy Sci. 1983, 66, 1219–1227. [Google Scholar] [CrossRef]
- Leong, T.S.; Walter, V.; Gamlath, C.J.; Yang, M.; Martin, G.J.; Ashokkumar, M. Functionalised dairy streams: Tailoring protein functionality using sonication and heating. Ultrason. Sonochem. 2018, 48, 499–508. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Z.; Dai, C.; Wang, Y.; Chen, W.; Ju, X.; Yuan, J.; He, R. Physical stability and microstructure of rapeseed protein isolate/gum Arabic stabilized emulsions at alkaline pH. Food Hydrocoll. 2019, 88, 50–57. [Google Scholar] [CrossRef]
- Nwachukwu, I.D.; Aluko, R.E. Physicochemical and emulsification properties of flaxseed (Linum usitatissimum) albumin and globulin fractions. Food Chem. 2018, 255, 216–225. [Google Scholar] [CrossRef]
- Rahmati, N.F.; Koocheki, A.; Varidi, M.; Kadkhodaee, R. Thermodynamic compatibility and interactions between Speckled Sugar bean protein and xanthan gum for production of multilayer O/W emulsion. J. Food Sci. Technol. 2018, 55, 1143–1153. [Google Scholar] [CrossRef]
- Marsh, K.J.; Wallis, I.R.; Kulheim, C.; Clark, R.; Nicolle, D.; Foley, W.J.; Salminen, J. New approaches to tannin analysis of leaves can be used to explain in vitro biological activities associated with herbivore defence. N. Phytol. 2020, 225, 488–498. [Google Scholar] [CrossRef]
- Nnamezie, A.A.; Famuwagun, A.A.; Gbadamosi, S.O. Characterization of okra seed flours, protein concentrate, protein isolate and enzymatic hydrolysates. Food Prod. Process. Nutr. 2021, 3, 14. [Google Scholar] [CrossRef]
- Delfanian, M.; Kenari, R.E.; Sahari, M.A. Utilization of Jujube Fruit (Ziziphus mauritiana Lam.) Extracts as Natural Antioxidants in Stability of Frying Oil. Int. J. Food Prop. 2015, 19, 789–801. [Google Scholar] [CrossRef]
- Puspita, M.; Déniel, M.; Widowati, I.; Radjasa, O.K.; Douzenel, P.; Marty, C.; Vandanjon, L.; Bedoux, G.; Bourgougnon, N. Total phenolic content and biological activities of enzymatic extracts from Sargassum muticum (Yendo) Fensholt. J. Appl. Phycol. 2017, 29, 2521–2537. [Google Scholar] [CrossRef]
- Álvarez, R.; Araya, H.; Navarro-Lisboa, R.; Lopez de Dicastillo, C. Evaluation of polyphenol content and antioxidant capacity of fruits and vegetables using a modified enzymatic extraction. Food Technol. Biotechnol. 2016, 54, 462–467. [Google Scholar] [CrossRef] [PubMed]
- Dangles, O.; Fenger, J.-A. The Chemical Reactivity of Anthocyanins and Its Consequences in Food Science and Nutrition. Molecules 2018, 23, 1970. [Google Scholar] [CrossRef] [PubMed]
- Fossen, T.; Cabrita, L.; Andersen, O.M. Colour and stability of pure anthocyanins influenced by pH including the alkaline region. Food Chem. 1998, 63, 435–440. [Google Scholar] [CrossRef]
- Sbroggio, M.F.; Montilha, M.S.; Figueiredo, V.R.G.D.; Georgetti, S.R.; Kurozawa, L.E. Influence of the degree of hydrolysis and type of enzyme on antioxidant activity of okara protein hydrolysates. Food Sci. Technol. 2016, 36, 375–381. [Google Scholar] [CrossRef]
- Shahi, Z.; Sayyed-Alangi, S.Z.; Najafian, L. Effects of enzyme type and process time on hydrolysis degree, electrophoresis bands and antioxidant properties of hydrolyzed proteins derived from defatted Bunium persicum Bioss. press cake. Heliyon 2020, 6, e03365. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Deng, J.; Shen, S.; Li, T.; Yuan, M.; Yang, R.; Ding, C. Antioxidant activities and functional properties of enzymatic protein hydrolysates from defatted Camellia oleifera seed cake. J. Food Sci. Technol. 2015, 52, 5681–5690. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Chen, J.; Tang, X.; Xiong, Y.L. Reducing, radical scavenging, and chelation properties of in vitro digests of alcalase-treated zein hydrolysate. J. Agric. Food Chem. 2008, 56, 2714–2721. [Google Scholar] [CrossRef]
- Chen, H.-M.; Muramoto, K.; Yamauchi, F.; Fujimoto, K.; Nokihara, K. Antioxidative Properties of Histidine-Containing Peptides Designed from Peptide Fragments Found in the Digests of a Soybean Protein. J. Agric. Food Chem. 1998, 46, 49–53. [Google Scholar] [CrossRef]
- Samaei, S.P.; Ghorbani, M.; Tagliazucchi, D.; Martini, S.; Gotti, R.; Themelis, T.; Tesini, F.; Gianotti, A.; Toschi, T.G.; Babini, E. Functional, nutritional, antioxidant, sensory properties and comparative peptidomic profile of faba bean (Vicia faba, L.) seed protein hydrolysates and fortified apple juice. Food Chem. 2020, 330, 127120. [Google Scholar] [CrossRef]
- Vasconcellos, F.C.S.; Woiciechowski, A.L.; Soccol, V.T.; Mantovani, D.; Soccol, C.R. Antimicrobial and antioxidant properties of-conglycinin and glycinin from soy protein isolate. Int. J. Curr. Microbiol. Appl. Sci. 2014, 3, 144–157. [Google Scholar]
- Xu, B.; Chang, S.K.C. Isoflavones, Flavan-3-ols, Phenolic Acids, Total Phenolic Profiles, and Antioxidant Capacities of Soy Milk as Affected by Ultrahigh-Temperature and Traditional Processing Methods. J. Agric. Food Chem. 2009, 57, 4706–4717. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Leung, C.E.; Corradini, M.; Xiao, H.; Kinchla, A.J. Increasing the nutritional value of strawberry puree by adding xylo-oligosaccharides. Heliyon 2020, 6, e03769. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Chang, S.K.C. Total Phenolics, Phenolic Acids, Isoflavones, and Anthocyanins and Antioxidant Properties of Yellow and Black Soybeans as Affected by Thermal Processing. J. Agric. Food Chem. 2008, 56, 7165–7175. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Zhang, Y.; Zhang, F.; Wang, Y.; Yi, J.; Liao, X. Effects of high hydrostatic pressure on enzymes, phenolic compounds, anthocyanins, polymeric color and color of strawberry pulps. J. Sci. Food Agric. 2011, 91, 877–885. [Google Scholar] [CrossRef]
- Nicoli, M.C.; Anese, M.; Parpinel, M.; Franceschi, S.; Lerici, C.R. Loss and/or formation of antioxidants during food processing and storage. Cancer Lett. 1997, 114, 71–74. [Google Scholar] [CrossRef]
- Dias, F.F.G.; Augusto-Obara, T.R.; Hennebelle, M.; Chantieng, S.; Ozturk, G.; Taha, A.Y.; de Souza Vieira, T.M.F.; de Moura, J.M.L.N. Effects of industrial heat treatments on bovine milk oxylipins and conventional markers of lipid oxidation. Prostaglandins Leukot. Essent. Fat. Acids 2019, 152, 102040. [Google Scholar] [CrossRef]
- Yoo, S.-H.; Chang, Y.H. Volatile Compound, Physicochemical, and Antioxidant Properties of Beany Flavor-Removed Soy Protein Isolate Hydrolyzates Obtained from Combined High Temperature Pre-Treatment and Enzymatic Hydrolysis. Prev. Nutr. Food Sci. 2016, 21, 338–347. [Google Scholar] [CrossRef]
- Voss, G.B.; Osorio, H.; Valente, L.M.; Pintado, M.E. Impact of thermal treatment and hydrolysis by Alcalase and Cynara cardunculus enzymes on the functional and nutritional value of Okara. Process Biochem. 2019, 83, 137–147. [Google Scholar] [CrossRef]
- Warsame, A.O.; Michael, N.; O’Sullivan, D.M.; Tosi, P. Identification and Quantification of Major Faba Bean Seed Proteins. J. Agric. Food Chem. 2020, 68, 8535–8544. [Google Scholar] [CrossRef]
- Nisov, A.; Ercili-Cura, D.; Nordlund, E. Limited hydrolysis of rice endosperm protein for improved techno-functional properties. Food Chem. 2020, 302, 125274. [Google Scholar] [CrossRef]
- Pan, Y.; Xie, Q.-T.; Zhu, J.; Li, X.-M.; Meng, R.; Zhang, B.; Chen, H.-Q.; Jin, Z.-Y. Study on the fabrication and in vitro digestion behavior of curcumin-loaded emulsions stabilized by succinylated whey protein hydrolysates. Food Chem. 2019, 287, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Akbari, N.; Milani, J.M.; Biparva, P. Functional and conformational properties of proteolytic enzyme-modified potato protein isolate. J. Sci. Food Agric. 2019, 100, 1320–1327. [Google Scholar] [CrossRef] [PubMed]
- Zang, X.; Yue, C.; Wang, Y.; Shao, M.; Yu, G. Effect of limited enzymatic hydrolysis on the structure and emulsifying properties of rice bran protein. J. Cereal Sci. 2018, 85, 168–174. [Google Scholar] [CrossRef]
- Nawaz, M.; Singh, T.; Stockmann, R.; Jegasothy, H.; Buckow, R. Quality Attributes of Ultra-High Temperature-Treated Model Beverages Prepared with Faba Bean Protein Concentrates. Foods 2021, 10, 1244. [Google Scholar] [CrossRef] [PubMed]
- Ahmadian-Kouchaksaraei, Z.; Varidi, M.; Varidi, M.J.; Pourazarang, H. Influence of processing conditions on the physicochemical and sensory properties of sesame milk: A novel nutritional beverage. LWT Food Sci. Technol. 2014, 57, 299–305. [Google Scholar] [CrossRef]
- Gülçin, İ.; Mshvildadze, V.; Gepdiremen, A.; Elias, R. The antioxidant activity of a triterpenoid glycoside isolated from the berries of Hedera colchica: 3-O-(β-d-glucopyranosyl)-hederagenin. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2006, 20, 130–134. [Google Scholar]
- Qamar, S.; Bhandari, B.; Prakash, S. Effect of different homogenisation methods and UHT processing on the stability of pea protein emulsion. Food Res. Int. 2019, 116, 1374–1385. [Google Scholar] [CrossRef]
- Tan, M.; Nawaz, M.A.; Buckow, R. Functional and food application of plant proteins—A review. Food Rev. Int. 2021, 1–29. [Google Scholar] [CrossRef]
- Ribeiro, L.D.O.; Pinheiro, A.C.B.; Brígida, A.I.S.; Genisheva, Z.A.; Vicente, A.A.M.D.O.S.; Teixeira, J.A.C.; de Matta, V.M.; Freitas, S.P. In vitro gastrointestinal evaluation of a juçara-based smoothie: Effect of processing on phenolic compounds bioaccessibility. J. Food Sci. Technol. 2019, 56, 5017–5026. [Google Scholar] [CrossRef]
- Ma, Y.; Yang, Y.; Gao, J.; Feng, J.; Shang, Y.; Wei, Z. Phenolics and antioxidant activity of bamboo leaves soup as affected by in vitro digestion. Food Chem. Toxicol. 2020, 135, 110941. [Google Scholar] [CrossRef]
- Wojtunik-Kulesza, K.; Oniszczuk, A.; Oniszczuk, T.; Combrzyński, M.; Nowakowska, D.; Matwijczuk, A. Influence of in vitro digestion on composition, bioaccessibility and antioxidant activity of food polyphenols—A non-systematic review. Nutrients 2020, 12, 1401. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, J.; Ramos, R.; Luís, A.n.; Rocha, S.; Rosado, T.; Gallardo, E.; Duarte, A.P. Assessment of the Bioaccessibility and Bioavailability of the Phenolic Compounds of Prunus avium L. by in Vitro Digestion and Cell Model. ACS Omega 2019, 4, 7605–7613. [Google Scholar] [CrossRef]
- Zeng, Z.; Liu, C.; Luo, S.; Chen, J.; Gong, E. The Profile and Bioaccessibility of Phenolic Compounds in Cereals Influenced by Improved Extrusion Cooking Treatment. PLoS ONE 2016, 11, e0161086. [Google Scholar] [CrossRef]
- Wang, S.; Amigo-Benavent, M.; Mateos, R.; Bravo, L.; Sarriá, B. Effects of in vitro digestion and storage on the phenolic content and antioxidant capacity of a red grape pomace. Int. J. Food Sci. Nutr. 2016, 68, 188–200. [Google Scholar] [CrossRef]
- Maduwanthi, S.D.T.; Marapana, R. Total phenolics, flavonoids and antioxidant activity following simulated gastro-intestinal digestion and dialysis of banana (Musa acuminata, AAB) as affected by induced ripening agents. Food Chem. 2021, 339, 127909. [Google Scholar] [CrossRef] [PubMed]
- Bouayed, J.; Hoffmann, L.; Bohn, T. Total phenolics, flavonoids, anthocyanins and antioxidant activity following simulated gastro-intestinal digestion and dialysis of apple varieties: Bioaccessibility and potential uptake. Food Chem. 2011, 128, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Sancho, R.A.S.; Pavan, V.; Pastore, G.M. Effect of in vitro digestion on bioactive compounds and antioxidant activity of common bean seed coats. Food Res. Int. 2015, 76, 74–78. [Google Scholar] [CrossRef]
- Fernández, K.; Labra, J. Simulated digestion of proanthocyanidins in grape skin and seed extracts and the effects of digestion on the angiotensin I-converting enzyme (ACE) inhibitory activity. Food Chem. 2013, 139, 196–202. [Google Scholar] [CrossRef]
- Seraglio, S.K.T.; Valese, A.C.; Daguer, H.; Bergamo, G.; Azevedo, M.S.; Nehring, P.; Gonzaga, L.V.; Fett, R.; Costa, A.C.O. Effect of in vitro gastrointestinal digestion on the bioaccessibility of phenolic compounds, minerals, and antioxidant capacity of Mimosa scabrella Bentham honeydew honeys. Food Res. Int. 2017, 99, 670–678. [Google Scholar] [CrossRef]
- Bermúdez-Soto, M.J.; Tomás-Barberán, F.A.; García-Conesa, M.T. Stability of polyphenols in chokeberry (Aronia melanocarpa) subjected to in vitro gastric and pancreatic digestion. Food Chem. 2007, 102, 865–874. [Google Scholar] [CrossRef]
- He, Z.; Yuan, B.; Zeng, M.; Chen, J. Effect of thermal processing and digestive protease on the antioxidant capacity of fruit juice-milk beverage model systems under simulated gastrointestinal digestion. Int. J. Food Sci. Technol. 2015, 50, 2306–2315. [Google Scholar] [CrossRef]
- Chen, G.-L.; Chen, S.-G.; Zhao, Y.-Y.; Luo, C.-X.; Li, J.; Gao, Y.-Q. Total phenolic contents of 33 fruits and their antioxidant capacities before and after in vitro digestion. Ind. Crops Prod. 2014, 57, 150–157. [Google Scholar] [CrossRef]
- Boutrou, R.; Henry, G.; Sánchez-Rivera, L. On the trail of milk bioactive peptides in human and animal intestinal tracts during digestion: A review. Dairy Sci. Technol. 2015, 95, 815–829. [Google Scholar] [CrossRef]
- Chen, Y.; Lin, H.; Lin, M.; Zheng, Y.; Chen, J. Effect of roasting and in vitro digestion on phenolic profiles and antioxidant activity of water-soluble extracts from sesame. Food Chem. Toxicol. 2020, 139, 111239. [Google Scholar] [CrossRef] [PubMed]
- Fawole, O.A.; Opara, U.L. Stability of total phenolic concentration and antioxidant capacity of extracts from pomegranate co-products subjected to in vitro digestion. BMC Complement. Altern. Med. 2016, 16, 358. [Google Scholar] [CrossRef]
- Chotphruethipong, L.; Battino, M.; Benjakul, S. Effect of stabilizing agents on characteristics, antioxidant activities and stability of liposome loaded with hydrolyzed collagen from defatted Asian sea bass skin. Food Chem. 2020, 328, 127127. [Google Scholar] [CrossRef]
- Wang, C.; Wu, H.; Liu, Z.; Barrow, C.; Dunshea, F.; Suleria, H.A.R. Bioaccessibility and movement of phenolic compounds from tomato (Solanum lycopersicum) during in vitro gastrointestinal digestion and colonic fermentation. Food Funct. 2022, 13, 4954–4966. [Google Scholar] [CrossRef]
- Quatrin, A.; Rampelotto, C.; Pauletto, R.; Maurer, L.H.; Nichelle, S.M.; Klein, B.; Rodrigues, R.F.; Junior, M.R.M.; Fonseca, B.D.S.; de Menezes, C.R.; et al. Bioaccessibility and catabolism of phenolic compounds from jaboticaba (Myrciaria trunciflora) fruit peel during in vitro gastrointestinal digestion and colonic fermentation. J. Funct. Foods 2019, 65, 103714. [Google Scholar] [CrossRef]
- Campos-Vega, R.; Vázquez-Sánchez, K.; López-Barrera, D.; Loarca-Piña, G.; Mendoza-Díaz, S.; Oomah, B. Simulated gastrointestinal digestion and in vitro colonic fermentation of spent coffee (Coffea arabica L.): Bioaccessibility and intestinal permeability. Food Res. Int. 2015, 77, 156–161. [Google Scholar] [CrossRef]
- Gao, K.; Xu, A.; Krul, C.; Venema, K.; Liu, Y.; Niu, Y.; Lu, J.; Bensoussan, L.; Seeram, N.P.; Heber, D.; et al. Of the Major Phenolic Acids Formed during Human Microbial Fermentation of Tea, Citrus, and Soy Flavonoid Supplements, Only 3,4-Dihydroxyphenylacetic Acid Has Antiproliferative Activity. J. Nutr. 2006, 136, 52–57. [Google Scholar] [CrossRef]
- Rowland, I.; Gibson, G.; Heinken, A.; Scott, K.; Swann, J.; Thiele, I.; Tuohy, K. Gut microbiota functions: Metabolism of nutrients and other food components. Eur. J. Nutr. 2018, 57, 34. [Google Scholar] [CrossRef] [PubMed]
- Kawabata, K.; Yoshioka, Y.; Terao, J. Role of Intestinal Microbiota in the Bioavailability and Physiological Functions of Dietary Polyphenols. Molecules 2019, 24, 370. [Google Scholar] [CrossRef] [PubMed]
- Watrelot, A.A.; Norton, E.L. Chemistry and Reactivity of Tannins in Vitis spp.: A Review. Molecules 2020, 25, 2110. [Google Scholar] [PubMed]
- Cárdenas-Castro, A.P.; Zamora-Gasga, V.M.; Alvarez-Parrilla, E.; Ruíz-Valdiviezo, V.M.; Venema, K.; Sáyago-Ayerdi, S.G. In vitro gastrointestinal digestion and colonic fermentation of tomato (Solanum lycopersicum L.) and husk tomato (Physalis ixocarpa Brot.): Phenolic compounds released and bioconverted by gut microbiota. Food Chem. 2021, 360, 130051. [Google Scholar] [CrossRef] [PubMed]
- Siah, S.D.; Konczak, I.; Agboola, S.; Wood, J.A.; Blanchard, C.L. In vitro investigations of the potential health benefits of Australian-grown faba beans (Vicia faba L.): Chemopreventative capacity and inhibitory effects on the angiotensin-converting enzyme, α-glucosidase and lipase. Br. J. Nutr. 2012, 108, S123–S134. [Google Scholar] [CrossRef] [PubMed]
- Martineau-Côté, D.; Achouri, A.; Karboune, S.; L’Hocine, L. Faba Bean: An Untapped Source of Quality Plant Proteins and Bioactives. Nutrients 2022, 14, 1541. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Li, W.; Deng, Z.; Li, H.; Zhang, B. The Composition and Antioxidant Activity of Bound Phenolics in Three Legumes, and Their Metabolism and Bioaccessibility of Gastrointestinal Tract. Foods 2020, 9, 1816. [Google Scholar] [CrossRef] [PubMed]
- de Cosío-Barrón, A.C.G.; Hernández-Arriaga, A.M.; Campos-Vega, R. Spent coffee (Coffea arabica L.) grounds positively modulate indicators of colonic microbial activity. Innov. Food Sci. Emerg. Technol. 2020, 60, 102286. [Google Scholar] [CrossRef]
- Wu, H.; Liu, Z.; Lu, P.; Barrow, C.; Dunshea, F.R.; Suleria, H.A. Bioaccessibility and bioactivities of phenolic compounds from roasted coffee beans during in vitro digestion and colonic fermentation. Food Chem. 2022, 386, 132794. [Google Scholar] [CrossRef]
- Gullón, P.; Gullón, B.; Tavaria, F.; Vasconcelos, M.; Gomes, A.M. In vitro fermentation of lupin seeds (Lupinus albus) and broad beans (Vicia faba): Dynamic modulation of the intestinal microbiota and metabolomic output. Food Funct. 2015, 6, 3316–3322. [Google Scholar] [CrossRef]
- Karataş, S.; Günay, D.; Sayar, S. In vitro evaluation of whole faba bean and its seed coat as a potential source of functional food components. Food Chem. 2017, 230, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Periago, M.J.; Martín-Pozuelo, G.; González-Barrio, R.; Santaella, M.; Gómez, V.; Vázquez, N.; Navarro-González, I.; García-Alonso, J. Effect of tomato juice consumption on the plasmatic lipid profile, hepatic HMGCR activity, and fecal short chain fatty acid content of rats. Food Funct. 2016, 7, 4460–4467. [Google Scholar] [CrossRef] [PubMed]
- Gniechwitz, D.; Brueckel, B.; Reichardt, N.; Blaut, M.; Steinhart, H.; Bunzel, M. Coffee Dietary Fiber Contents and Structural Characteristics as Influenced by Coffee Type and Technological and Brewing Procedures. J. Agric. Food Chem. 2007, 55, 11027–11034. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Burillo, S.; Mehta, T.; Esteban-Muñoz, A.; Pastoriza, S.; Paliy, O.; Rufián-Henares, J. Effect of in vitro digestion-fermentation on green and roasted coffee bioactivity: The role of the gut microbiota. Food Chem. 2018, 279, 252–259. [Google Scholar] [CrossRef]


| Treatments | Samples | TPC (mg GAE/g) | TFC (µg QE/g) | TCT (mg CE/g) | DPPH (mg TE/g) | FRAP (µg TE/g) | ABTS (mg TE/g) |
|---|---|---|---|---|---|---|---|
| Faba bean flour | FB | 1.78 ± 0.01 b | 119.72 ± 13.44 b | 1.48 ± 0.18 a | 7.18 ± 0.32 b | 1650.74 ± 0.01 b | 28.06 ± 1.08 b |
| Faba bean protein concentrate | FBPC | 2.79 ± 0.14 a | 394.37 ± 28.27 a | 1.74 ± 0.05 a | 15.58 ± 0.55 a | 3271.86 ± 0.19 a | 41.50 ± 0.07 a |
| Hydrolysates (FBH) | HC | 0.23 ± 0.01 cd | 37.65 ± 0.92 c | 0.09 ± 0.01 g | 0.86 ± 0.05 a | 249.11 ± 3.93 b | 5.39 ± 0.61 a |
| HA0 | 0.23 ± 0.01 d | 50.33 ± 3.98 b | 0.09 ± 0.01 fg | 0.61 ± 0.02 bc | 242.01 ± 9.87 b | 4.90 ± 0.53 ab | |
| HA5 | 0.25 ± 0.01 bcd | 63.90 ± 1.13 a | 0.14 ± 0.02 efg | 0.65 ± 0.01 bc | 146.46 ± 2.90 de | 3.72 ± 0.25 c | |
| HA10 | 0.24 ± 0.02 cd | 13.71 ± 0.22 ef | 0.17 ± 0.02 cde | 0.64 ± 0.01 bc | 152.61 ± 2.63 d | 3.92 ± 0.49 bc | |
| HA15 | 0.24 ± 0.01 cd | 15.45 ± 0.41 ef | 0.15 ± 0.02 def | 0.51 ± 0.01 d | 269.37 ± 2.75 a | 4.32 ± 0.25 abc | |
| HA30 | 0.29 ± 0.01 a | 38.69 ± 5.46 c | 0.23 ± 0.02 b | 0.89 ± 0.08 a | 197.51 ± 8.09 c | 5.36 ± 0.61 a | |
| Emulsions (FBE) | EC | 0.23 ± 0.02 d | 30.56 ± 4.15 cd | 0.15 ± 0.01 de | 0.69 ± 0.02 b | 137.63 ± 3.64 ef | 4.60 ± 0.37 abc |
| EA0 | 0.22 ± 0.00 d | 19.34 ± 3.91 e | 0.21 ± 0.01 bc | 0.65 ± 0.01 bc | 143.77 ± 1.88 def | 4.48 ± 0.12 abc | |
| EA5 | 0.25 ± 0.01 bcd | 35.12 ± 4.73 c | 0.20 ± 0.01 bcd | 0.59 ± 0.01 c d | 138.67 ± 2.50 ef | 4.58 ± 0.15 abc | |
| EA10 | 0.23 ± 0.02 cd | 23.52 ± 2.61 de | 0.20 ± 0.01 bcd | 0.60 ± 0.01 cd | 130.35 ± 1.56 f | 4.31 ± 0.26 abc | |
| EA15 | 0.26 ± 0.01 abc | 35.02 ± 3.93 c | 0.23 ± 0.02 b | 0.58 ± 0.01 cd | 135.72 ± 5.90 ef | 4.39 ± 0.36 abc | |
| EA30 | 0.28 ± 0.01 ab | 39.15 ± 4.11 c | 0.29 ± 0.05 a | 0.66 ± 0.03 bc | 146.55 ± 2.48 de | 4.37 ± 0.24 abc |
| Treatment | DH % | Protein Solubility (%) | ζ-Potential (mV) | S₀ | D3,2 (µm) | PDI (%) |
|---|---|---|---|---|---|---|
| HA0 | 0 | 60.95 ± 0.90 a | −30.93 ± 0.45 a | 236.78 ± 3.00 a | - | - |
| HA5 | 1 | 67.78 ± 1.57 b | −34.67 ± 0.41 b | 205.91 ± 7.46 ab | - | - |
| HA10 | 2 | 72.06 ± 0.90 c | −37.53 ± 0.46 c | 193.32 ± 2.83 ab | - | - |
| HA15 | 9 | 77.14 ± 0.90 d | −39.67 ± 0.36 d | 167.23 ± 7.10 ab | - | - |
| HA30 | 16 | 83.65 ± 0.67 e | −43.75 ± 0.47 e | 122.64 ± 3.52 b | - | - |
| EC | - | - | −23.10 ± 0.96 a | 173.07 ± 1.65 b | 14.10 ± 0.46 a | 41.55 ± 3.99 a |
| EA0 | - | - | −21.47 ± 1.10 a | 244.59 ± 13.80 d | 14.73 ± 0.35 a | 30.16 ± 2.17 b |
| EA5 | - | - | −28.45 ± 0.39 b | 167.12 ± 5.30 b | 0.36 ± 0.06 b | 36.99 ± 2.48 a |
| EA10 | - | - | −30.65 ± 0.34 c | 147.90 ± 2.26 a | 0.14 ± 0.01 b | 17.73 ± 0.78 c |
| EA15 | - | - | −33.56 ± 0.40 d | 145.71 ± 1.94 a | 0.10 ± 0.01 b | 16.31 ± 0.79 c |
| EA30 | - | - | −37.60 ± 0.25 e | 135.55 ± 2.69 a | 0.10 ± 0.01 b | 14.31 ± 0.53 c |
| Sample Types | Samples | Phases | TPC (mg GAE/g) | TFC (mg QE/g) | TCT (mg CE/g) | DPPH (mg TE/g) | FRAP (mg TE/g) | ABTS (mg TE/g) |
|---|---|---|---|---|---|---|---|---|
| Hydrolysates (FBH) | HA0 | Oral Gastric Intestinal | 0.53 ± 0.03 c 0.59 ± 0.03 b 1.21 ± 0.15 a | 0.02 ± 0.00 a | 0.41 ± 0.03 a | 0.29 ± 0.02 b | 0.43 ± 0.04 b | 5.12 ± 0.64 b |
| - | - | 0.45 ± 0.06 a | 0.32 ± 0.03 c | 7.61 ± 0.31 b | ||||
| - | - | 0.13 ± 0.01 c | 0.77 ± 0.02 a | 66.36 ± 3.82 a | ||||
| HA5 | Oral Gastric Intestinal | 0.40 ± 0.01 b 0.63 ± 0.02 a 0.39 ± 0.04 b | - | - | 0.16 ± 0.02 b | 0.41 ± 0.01 a | 5.94 ± 0.21 c | |
| - | - | 0.48 ± 0.08 a | 0.10 ± 0.01 c | 7.90 ± 0.12 b | ||||
| - | - | 0.07 ± 0.01 c | 0.24 ± 0.00 b | 22.46 ± 0.39 a | ||||
| HA10 | Oral Gastric Intestinal | 0.46 ± 0.04 c 0.61 ± 0.03 b 0.92 ± 0.14 a | - | 1.16 ± 0.05 b | 0.11 ± 0.00 b | 0.53 ± 0.03 a | 10.52 ± 0.07 b | |
| - | 1.29 ± 0.02 a | 0.39 ± 0.05 a | 0.10 ± 0.01 c | 8.22 ± 0.68 c | ||||
| - | - | - | 0.50 ± 0.01 a | 27.72 ± 0.30 a | ||||
| HA30 | Oral Gastric Intestinal | 0.53 ± 0.03 c 0.79 ± 0.02 b 1.16 ± 0.11 a | - | 0.54 ± 0.08 a | 0.37 ± 0.01 b | 1.10 ± 0.05 a | 12.25 ± 0.09 b | |
| - | - | 0.73 ± 0.06 a | 0.45 ± 0.05 c | 9.62 ± 0.15 b | ||||
| - | - | - | 0.58 ± 0.01 b | 79.79 ± 0.95 a | ||||
| Emulsions (FBE) | EA0 | Oral Gastric Intestinal | 0.49 ± 0.03 c 0.69 ± 0.02 b 1.15 ± 0.13 a | 0.04 ± 0.00 b | 2.04 ± 0.02 a | 0.28 ± 0.02 b | 1.04 ± 0.04 b | 12.79 ± 0.41 b |
| - | - | 0.42 ± 0.05 a | 0.90 ± 0.00 c | 9.10 ± 0.15 b | ||||
| 1.40 ± 0.23 a | - | 0.19 ± 0.01 c | 1.35 ± 0.01 a | 116.13 ± 8.91 a | ||||
| EA5 | Oral Gastric Intestinal | 0.51 ± 0.02 c 0.80 ± 0.05 b 0.65 ± 0.09 a | - | - | 0.24 ± 0.02 a | 0.99 ± 0.04 b | 13.95 ± 0.43 b | |
| - | - | 0.27 ± 0.03 a | 0.96 ± 0.07 b | 9.94 ± 0.53 c | ||||
| 0.80 ± 0.11 a | - | 0.07 ± 0.01 b | 1.10 ± 0.02 a | 22.06 ± 0.88 a | ||||
| EA10 | Oral Gastric Intestinal | 0.50 ± 0.04 c 0.79 ± 0.01 b 1.17 ± 0.08 a | - | 2.28 ± 0.02 a | 0.29 ± 0.00 b | 1.11 ± 0.02 b | 14.80 ± 0.76 b | |
| - | - | 0.40 ± 0.02 a | 0.93 ± 0.04 c | 9.47 ± 0.43 b | ||||
| 2.61 ± 0.10 a | - | 0.14 ± 0.01 c | 2.06 ± 0.09 a | 78.66 ± 6.38 a | ||||
| EA30 | Oral Gastric Intestinal | 0.45 ± 0.04 c 0.86 ± 0.05 b 1.17 ± 0.07 a | - | - | 0.26 ± 0.02 b | 1.00 ± 0.03 b | 14.98 ± 0.86 b | |
| - | - | 0.43 ± 0.06 a | 1.01 ± 0.05 b | 10.00 ± 0.27 b | ||||
| 0.99 ± 0.14 a | - | 0.15 ± 0.01 c | 1.22 ± 0.05 a | 67.46 ± 1.44 a |
| Sample Types | Samples | Phases | TPC (mg GAE/g) | TFC (mg QE/g) | TCT (mg CE/g) | DPPH (mg TE/g) | FRAP (mg TE/g) | ABTS (mg TE/g) |
|---|---|---|---|---|---|---|---|---|
| Hydrolysates (FBH) | HA0 | 0 h 2 h 4 h | 3.39 ± 0.04 b | - | - | 2.36 ± 0.01 f | 2.91 ± 0.07 d | 26.55 ± 1.37 d |
| 3.44 ± 0.03 b | 0.14 ± 0.00 c | - | 3.88 ± 0.00 d | 3.99 ± 0.02 b | 16.15 ± 0.01 f | |||
| 2.40 ± 0.07 e | 1.06 ± 0.08 a | - | 3.45 ± 0.03 e | 4.14 ± 0.00 a | 33.89 ± 0.12 c | |||
| 8 h | 3.63 ± 0.06 a | 0.45 ± 0.01 b | - | 5.78 ± 0.03 c | 2.77 ± 0.00 e | 19.25 ± 0.00 e | ||
| 16 h | 2.80 ± 0.00 c | - | - | 6.40 ± 0.01 b | 3.76 ± 0.00 c | 92.52 ± 0.06 b | ||
| 24 h | 2.52 ± 0.01 d | - | - | 6.60 ± 0.03 a | 4.09 ± 0.03 a | 116.94 ± 0.05 a | ||
| HA5 | 0 h 2 h 4 h | 3.17 ± 0.00 c | 0.03 ± 0.01 d | 5.44 ± 0.13 a | 1.13 ± 0.02 f | 3.30 ± 0.08 d | 33.98 ± 0.35 d | |
| 3.64 ± 0.01 b | 0.17 ± 0.00 c | - | 4.32 ± 0.00 d | 3.45 ± 0.00 c | 16.18 ± 0.01 f | |||
| 2.94 ± 0.04 d | 0.26 ± 0.01 b | - | 3.66 ± 0.00 e | 4.61 ± 0.01 a | 36.17 ± 0.06 c | |||
| 8 h | 4.07 ± 0.03 a | 0.60 ± 0.02 a | - | 6.08 ± 0.01 c | 1.30 ± 0.00 f | 19.35 ± 0.00 e | ||
| 16 h | 2.52 ± 0.02 e | - | - | 7.88 ± 0.26 b | 3.05 ± 0.00 e | 92.38 ± 0.03 b | ||
| 24 h | 2.00 ± 0.02 f | - | - | 8.48 ± 0.09 a | 3.64 ± 0.01 b | 116.72 ± 0.06 a | ||
| HA10 | 0 h 2 h 4 h | 3.15 ± 0.01 c | - | - | 4.27 ± 0.09 d | 2.10 ± 0.00 f | 23.59 ± 0.37 d | |
| 3.93 ± 0.00 b | 0.36 ± 0.00 c | - | 5.14 ± 0.01 c | 4.53 ± 0.01 b | 15.92 ± 0.03 f | |||
| 2.81 ± 0.04 e | 0.93 ± 0.00 b | - | 3.64 ± 0.09 e | 4.79 ± 0.00 a | 34.52 ± 0.22 c | |||
| 8 h | 4.67 ± 0.02 a | 1.02 ± 0.00 a | - | 7.43 ± 0.05 b | 3.39 ± 0.00 c | 19.16 ± 0.00 e | ||
| 16 h | 2.95 ± 0.05 d | - | 0.91 ± 0.02 b | 7.61 ± 0.10 a | 3.11 ± 0.01 d | 94.60 ± 0.03 b | ||
| 24 h | 2.38 ± 0.02 f | - | 1.22 ± 0.03 a | 7.67 ± 0.06 a | 3.02 ± 0.01 e | 119.75 ± 0.05 a | ||
| HA30 | 0 h 2 h 4 h | 3.75 ± 0.00 a | 0.20 ± 0.00 c | - | 3.11 ± 0.01 f | 2.22 ± 0.00 e | 56.29 ± 0.26 c | |
| 3.17 ± 0.02 d | 0.20 ± 0.00 c | - | 5.59 ± 0.00 c | 4.26 ± 0.04 a | 16.89 ± 0.00 f | |||
| 3.15 ± 0.03 d | 0.89 ± 0.01 a | - | 3.86 ± 0.01 e | 4.13 ± 0.00 b | 33.69 ± 0.10 d | |||
| 8 h | 3.70 ± 0.07 a | 0.29 ± 0.01 b | - | 4.33 ± 0.02 d | 3.12 ± 0.00 c | 18.99 ± 0.00 e | ||
| 16 h | 3.43 ± 0.06 b | 0.15 ± 0.02 d | 0.63 ± 0.01 b | 7.19 ± 0.09 b | 3.04 ± 0.04 d | 97.71 ± 0.04 b | ||
| 24 h | 3.33 ± 0.03 c | 0.11 ± 0.01 e | 0.83 ± 0.02 a | 8.14 ± 0.04 a | 3.01 ± 0.02 d | 123.95 ± 0.03 a | ||
| Emulsions (FBE) | EA0 | 0 h 2 h 4 h | 3.17 ± 0.02 d | - | - | 3.00 ± 0.03 f | 3.52 ± 0.05 c | 19.65 ± 0.05 d |
| 3.32 ± 0.03 c | 0.38 ± 0.00 b | - | 4.87 ± 0.05 d | 3.72 ± 0.00 b | 15.33 ± 0.03 f | |||
| 2.45 ± 0.02 e | 0.96 ± 0.02 a | - | 3.77 ± 0.05 e | 4.38 ± 0.00 a | 50.07 ± 0.09 c | |||
| 8 h | 3.85 ± 0.01 a | 0.29 ± 0.00 c | - | 6.10 ± 0.00 c | 2.51 ± 0.00 f | 18.69 ± 0.00 e | ||
| 16 h | 3.63 ± 0.04 b | - | 5.51 ± 0.00 b | 6.84 ± 0.05 b | 3.15 ± 0.06 e | 97.17 ± 0.07 b | ||
| 24 h | 3.56 ± 0.02 b | - | 7.35 ± 0.02 a | 7.08 ± 0.03 a | 3.36 ± 0.04 d | 123.33 ± 0.06 a | ||
| EA5 | 0 h 2 h 4 h | 2.31 ± 0.01 f | 0.18 ± 0.00 c | - | 3.31 ± 0.02 e | 2.56 ± 0.01 e | 61.22 ± 0.01 c | |
| 3.71 ± 0.01 b | 0.26 ± 0.00 b | - | 5.01 ± 0.00 c | 3.57 ± 0.00 b | 15.88 ± 0.01 e | |||
| 2.83 ± 0.13 e | 0.25 ± 0.02 b | - | 3.45 ± 0.04 de | 4.70 ± 0.00 a | 57.03 ± 0.19 d | |||
| 8 h | 4.20 ± 0.01 a | 2.61 ± 0.00 a | - | 3.58 ± 0.03 d | 2.39 ± 0.00 f | 61.11 ± 0.01 c | ||
| 16 h | 3.25 ± 0.01 c | - | 2.35 ± 0.01 b | 5.99 ± 0.13 b | 3.02 ± 0.01 d | 88.35 ± 0.01 b | ||
| 24 h | 2.93 ± 0.02 d | - | 3.13 ± 0.01 a | 6.80 ± 0.05 a | 3.23 ± 0.01 c | 97.43 ± 0.08 a | ||
| EA10 | 0 h 2 h 4 h | 3.51 ± 0.00 b | 0.10 ± 0.00 b | - | 2.23 ± 0.10 e | 2.32 ± 0.01 f | 61.28 ± 0.02 c | |
| 3.40 ± 0.06 c | 0.06 ± 0.00 c | - | 6.31 ± 0.02 a | 4.09 ± 0.00 a | 16.67 ± 0.00 f | |||
| 3.55 ± 0.01 b | 0.22 ± 0.02 a | - | 5.29 ± 0.05 d | 3.94 ± 0.00 b | 36.02 ± 0.06 d | |||
| 8 h | 3.93 ± 0.01 a | 0.10 ± 0.00 b | - | 5.66 ± 0.05 c | 2.42 ± 0.00 e | 19.33 ± 0.00 e | ||
| 16 h | 3.43 ± 0.01 c | - | 2.20 ± 0.01 b | 5.87 ± 0.01 b | 2.86 ± 0.03 d | 87.43 ± 0.03 b | ||
| 24 h | 3.26 ± 0.01 d | - | 2.93 ± 0.02 a | 5.95 ± 0.02 b | 3.01 ± 0.02 c | 110.13 ± 0.05 a | ||
| EA30 | 0 h 2 h 4 h | 2.12 ± 0.02 d | - | 0.53 ± 0.01 c | 1.85 ± 0.01 f | 2.51 ± 0.00 e | 76.29 ± 1.26 c | |
| 3.80 ± 0.03 a | 0.15 ± 0.00 b | - | 5.92 ± 0.00 c | 3.41 ± 0.00 b | 17.02 ± 0.00 f | |||
| 3.19 ± 0.02 b | 0.88 ± 0.00 a | - | 4.46 ± 0.05 e | 4.55 ± 0.01 a | 32.61 ± 0.24 d | |||
| 8 h | 2.90 ± 0.06 c | - | - | 4.96 ± 0.02 d | 2.41 ± 0.00 f | 18.95 ± 0.01 e | ||
| 16 h | 1.93 ± 0.00 e | - | 4.13 ± 0.01 b | 7.03 ± 0.16 b | 2.70 ± 0.00 d | 84.57 ± 0.18 b | ||
| 24 h | 1.61 ± 0.02 f | - | 5.51 ± 0.02 a | 7.72 ± 0.06 a | 2.79 ± 0.02 c | 106.45 ± 0.09 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, J.; Li, M.; Nawaz, M.A.; Stockmann, R.; Buckow, R.; Suleria, H.A.R. In Vitro Digestion and Colonic Fermentation of UHT Treated Faba Protein Emulsions: Effects of Enzymatic Hydrolysis and Thermal Processing on Proteins and Phenolics. Nutrients 2023, 15, 89. https://doi.org/10.3390/nu15010089
Gu J, Li M, Nawaz MA, Stockmann R, Buckow R, Suleria HAR. In Vitro Digestion and Colonic Fermentation of UHT Treated Faba Protein Emulsions: Effects of Enzymatic Hydrolysis and Thermal Processing on Proteins and Phenolics. Nutrients. 2023; 15(1):89. https://doi.org/10.3390/nu15010089
Chicago/Turabian StyleGu, Jingyu, Minhao Li, Malik Adil Nawaz, Regine Stockmann, Roman Buckow, and Hafiz A. R. Suleria. 2023. "In Vitro Digestion and Colonic Fermentation of UHT Treated Faba Protein Emulsions: Effects of Enzymatic Hydrolysis and Thermal Processing on Proteins and Phenolics" Nutrients 15, no. 1: 89. https://doi.org/10.3390/nu15010089
APA StyleGu, J., Li, M., Nawaz, M. A., Stockmann, R., Buckow, R., & Suleria, H. A. R. (2023). In Vitro Digestion and Colonic Fermentation of UHT Treated Faba Protein Emulsions: Effects of Enzymatic Hydrolysis and Thermal Processing on Proteins and Phenolics. Nutrients, 15(1), 89. https://doi.org/10.3390/nu15010089









