Eight-Hour Time-Restricted Feeding: A Strong Candidate Diet Protocol for First-Line Therapy in Polycystic Ovary Syndrome
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Plan
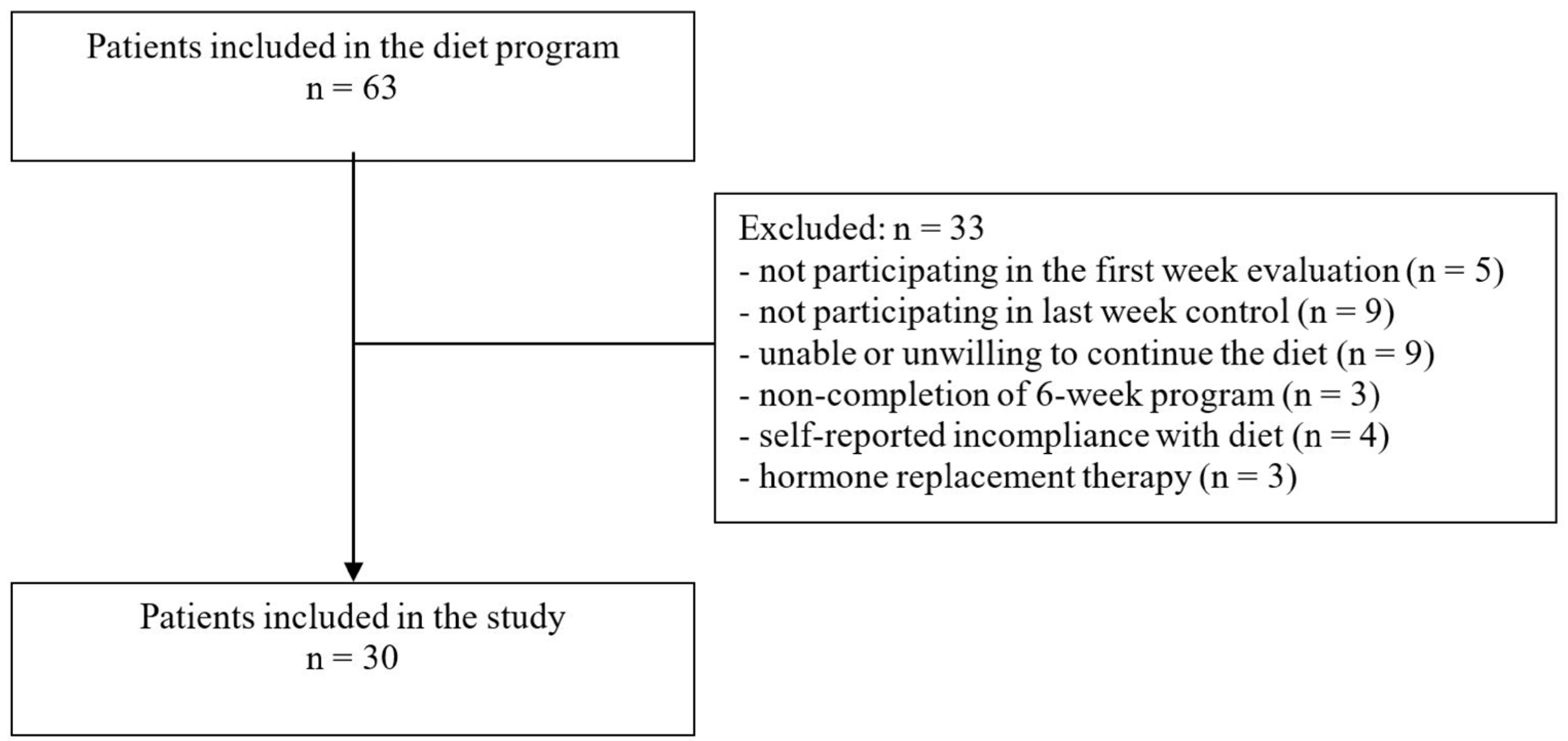
2.2. Participants
2.3. Laboratory Findings
2.4. PCOS Diagnosis and Management
2.5. Time-Restricted Feeding Protocol
2.6. Laboratory Findings
2.7. Anthropometric Measurements
2.8. Insulin Resistance Determination
2.9. Hyperandrogenism Determination
2.10. Statistics
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Maqbool, M.; Dar, M.A.; Gani, I.; Geer, M.I. Insulin resistance and polycystic ovary syndrome: A review. J. Drug Deliv. 2019, 9, 433–436. [Google Scholar] [CrossRef]
- Shang, Y.; Zhou, H.; Hu, M.; Feng, H. Effect of diet on insulin resistance in polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2020, 105, 3346–3360. [Google Scholar] [CrossRef]
- Cooney, L.G.; Dokras, A. Cardiometabolic Risk in Polycystic Ovary Syndrome: Current Guidelines. Endocrinol. Metab. Clin. N. Am. 2021, 50, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Chiofalo, B.; Laganà, A.S.; Palmara, V.; Granese, R.; Corrado, G.; Mancini, E.; Vitale, S.G.; Frangež, H.B.; Vrtačnik-Bokal, E.; Triolo, O. Fasting as possible complementary approach for polycystic ovary syndrome: Hope or hype? Med. Hypotheses 2017, 105, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Jehan, S.; Zizi, F.; Pandi-Perumal, S.R.; Mcfarlane, S.I.; Jean-Louis, G.; Myers, A.K. Energy imbalance: Obesity, associated comorbidities, prevention, management and public health implications. Adv. Obes. Weight Manag. Control 2020, 10, 146–161. [Google Scholar] [CrossRef]
- Wong, J.M.; Gallagher, M.; Gooding, H.; Feldman, H.A.; Gordon, C.M.; Ludwig, D.S.; Ebbeling, C.B. A randomized pilot study of dietary treatments for polycystic ovary syndrome in adolescents. Pediatr. Obes. 2016, 11, 210–220. [Google Scholar] [CrossRef]
- Teede, H.J.; Misso, M.L.; Costello, M.F.; Dokras, A.; Laven, J.; Moran, L.; Piltonen, T.; Norman, R.J.; International PCOS Network. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Hum. Reprod. 2018, 33, 1602–1618. [Google Scholar] [CrossRef]
- Azadi-Yazdi, M.; Karimi-Zarchi, M.; Salehi-Abargouei, A.; Fallahzadeh, H.; Nadjarzadeh, A. Effects of Dietary Approach to Stop Hypertension diet on androgens, antioxidant status and body composition in overweight and obese women with polycystic ovary syndrome: A randomised controlled trial. J. Hum. Nutr. Diet. 2017, 30, 275–283. [Google Scholar] [CrossRef]
- Esfahanian, F.; Zamani, M.M.; Heshmat, R.; Moini Nia, F. Effect of metformin compared with hypocaloric diet on serum C-reactive protein level and insulin resistance in obese and overweight women with polycystic ovary syndrome. J. Obstet. Gynaecol. Res. 2013, 39, 806–813. [Google Scholar] [CrossRef]
- Li, P.; Li, Y.; Li, Y.; Chen, Q.; Zeng, X. Clinical observation of personalized diet intervention and metformin in the treatment of polycystic ovary syndrome. Matern. Child Health Care China 2017, 32, 2535–2539. [Google Scholar]
- Xu, L.; Wang, H.; Gong, J.; Hou, X. Effects of Mediterranean diet on reproductive function in patients with obese polycystic ovary syndrome. Matern. Child Health J. 2017, 32, 122–124. [Google Scholar]
- Paoli, A.; Mancin, L.; Giacona, M.C.; Bianco, A.; Caprio, M. Effects of a ketogenic diet in overweight women with polycystic ovary syndrome. J. Transl. Med. 2020, 18, 104. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Xing, C.; Zhang, J.; Zhao, H.; Shi, W.; He, B. Eight-hour time-restricted feeding improves endocrine and metabolic profiles in women with anovulatory polycystic ovary syndrome. J. Transl. Med. 2021, 19, 148. [Google Scholar] [CrossRef] [PubMed]
- Asemi, Z.; Samimi, M.; Taghizadeh, M.; Esmaillzadeh, A. Effects of ramadan fasting on glucose homeostasis, lipid profiles, ınflammation and oxidative stress in women with polycystic ovary syndrome in Kashan, Iran. Arch. Iran. Med. 2015, 18, 806–810. [Google Scholar]
- Han, Y.; Lin, B.; Lu, W.; Wang, X.; Tang, W.; Tao, X.; Cai, H.; He, C.; Liu, C. Time-restricted feeding improves metabolic and endocrine profiles in mice with polycystic ovary syndrome. Front. Endocrinol. 2022, 13, 1057376. [Google Scholar] [CrossRef]
- Varady, K.A. Impact of intermittent fasting on glucose homeostasis. Curr. Opin. Clin. Nutr. Metab. Care 2016, 19, 300–302. [Google Scholar] [CrossRef]
- Cienfuegos, S.; Corapi, S.; Gabel, K.; Ezpeleta, M.; Kalam, F.; Lin, S.; Pavlou, V.; Varady, K.A. Effect of ıntermittent fasting on reproductive hormone levels in females and males: A review of human trials. Nutrients 2022, 14, 2343. [Google Scholar] [CrossRef]
- Floyd, R.; Gryson, R.; Mockler, D.; Gibney, J.; Duggan, S.N.; Behan, L.A. The effect of time-restricted eating on ınsulin levels and ınsulin sensitivity in patients with polycystic ovarian syndrome: A systematic review. Int. J. Endocrinol. 2022, 2022, 2830545. [Google Scholar] [CrossRef]
- Anson, R.M.; Guo, Z.; De Cabo, R.; Iyun, T.; Rio”s, M.; Hagepanos, A.; Ingram, D.K.; Lane, M.A.; Mattson, M.P. Intermittent fasting dissociates beneficial effects of dietary restriction on glucose metabolism and neuronal resistance to injury from calorie intake. Proc. Natl. Acad. Sci. USA 2003, 100, 6216–6220. [Google Scholar] [CrossRef]
- Samadi, A.; Sabuncuoglu, S.; Samadi, M.; Isikhan, S.Y.; Chirumbolo, S.; Peana, M.; Lay, I.; Yalcinkaya, A.; Bjørklund, G. A comprehensive review on oxysterols and related diseases. Curr. Med. Chem. 2021, 28, 110–136. [Google Scholar] [CrossRef]
- Fauser, B.C.; Tarlatzis, B.C.; Rebar, R.W.; Legro, R.S.; Balen, A.H.; Lobo, R.; Carmina, E.; Chang, J.; Yildiz, B.O.; Laven, J.S.E.; et al. Consensus on women’s health aspects of polycystic ovary syndrome (PCOS): The Amsterdam ESHRE/ASRM-Sponsored 3rd PCOS Consensus Workshop Group. Fertil. Steril. 2012, 97, 28–38.e25. [Google Scholar] [CrossRef] [PubMed]
- Martens, C.R.; Rossman, M.J.; Mazzo, M.R.; Jankowski, L.R.; Nagy, E.E.; Denman, B.A.; Richey, J.J.; Johnson, S.A.; Ziemba, B.P.; Wang, Y.; et al. Short-term time-restricted feeding is safe and feasible in non-obese healthy midlife and older adults. GeroScience 2020, 42, 667–686. [Google Scholar] [CrossRef] [PubMed]
- Yalcinkaya, A.; Unal, S.; Oztas, Y. Altered HDL particle in sickle cell disease: Decreased cholesterol content is associated with hemolysis, whereas decreased Apolipoprotein A1 is linked to inflammation. Lipids Health Dis. 2019, 18, 225. [Google Scholar] [CrossRef]
- He, F.F.; Li, Y.M. Role of gut microbiota in the development of insulin resistance and the mechanism underlying polycystic ovary syndrome: A review. J. Ovarian Res. 2020, 13, 73. [Google Scholar] [CrossRef] [PubMed]
- Jiao, N.; Baker, S.S.; Nugent, C.A.; Tsompana, M.; Cai, L.; Wang, Y.; Buck, M.J.; Genco, R.J.; Baker, R.D.; Zhu, R.; et al. Gut microbiome may contribute to insulin resistance and systemic inflammation in obese rodents: A meta-analysis. Physiol. Genom. 2018, 50, 244–254. [Google Scholar] [CrossRef] [PubMed]
- Tremellen, K.; Pearce, K. Dysbiosis of Gut Microbiota (DOGMA)—A novel theory for the development of Polycystic Ovarian Syndrome. Med. Hypotheses 2012, 79, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Rotterdam Eshre/Asrm-Sponsored Pcos Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil. Steril. 2004, 81, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.W.; Kazemi, M.; Jarrett, B.Y.; Vanden Brink, H.; Hoeger, K.M.; Spandorfer, S.D.; Lujan, M.E. Dietary and Physical Activity Behaviors in Women with Polycystic Ovary Syndrome per the New International Evidence-Based Guideline. Nutrients 2019, 11, 2711. [Google Scholar] [CrossRef]
- WHO. Waist Circumference and Waist-Hip Ratio: Report of a WHO Expert Consultation, Geneva, 8–11 December 2008; WHO: Geneva, Switzerland, 2011; 9241501499. [Google Scholar]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef]
- Güngör, O.; Erden, G.; Bal, C.; Uğuz, N.; Sezer, S.; Özdemir, Ş.; Çelik, H.T.; Yıldırımkaya, M.M. The comparison of free androgen index and serum free testosterone levels in women with hirsutism or polycystic ovary syndrome. J. Clin. Exp. Investig. 2011, 2, 152–156. [Google Scholar] [CrossRef]
- Martínez-Bermejo, E.; Luque-Ramírez, M.; Escobar-Morreale, H.F. Obesity and the polycystic ovary syndrome. Minerva Endocrinol. 2007, 32, 129–140. [Google Scholar] [PubMed]
- Panidis, D.; Farmakiotis, D.; Rousso, D.; Kourtis, A.; Katsikis, I.; Krassas, G. Obesity, weight loss, and the polycystic ovary syndrome: Effect of treatment with diet and orlistat for 24 weeks on insulin resistance and androgen levels. Fertil. Steril. 2008, 89, 899–906. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, M.; Cioffi, I.; Evangelista, A.; Ponzo, V.; Goitre, I.; Ciccone, G.; Ghigo, E.; Bo, S. Effects of time-restricted feeding on body weight and metabolism. A systematic review and meta-analysis. Rev. Endocr. Metab. Disord. 2020, 21, 17–33. [Google Scholar] [CrossRef] [PubMed]
- Sadiya, A.; Ahmed, S.; Siddieg, H.H.; Babas, I.J.; Carlsson, M. Effect of Ramadan fasting on metabolic markers, body composition, and dietary intake in Emiratis of Ajman (UAE) with metabolic syndrome. Diabetes Metab. Syndr. Obes. 2011, 4, 409–416. [Google Scholar] [CrossRef]
- Harvie, M.N.; Pegington, M.; Mattson, M.P.; Frystyk, J.; Dillon, B.; Evans, G.; Cuzick, J.; Jebb, S.A.; Martin, B.; Cutler, R.G.; et al. The effects of intermittent or continuous energy restriction on weight loss and metabolic disease risk markers: A randomized trial in young overweight women. Int. J. Obes. 2011, 35, 714–727. [Google Scholar] [CrossRef]
- Kiddy, D.S.; Hamilton-Fairley, D.; Bush, A.; Short, F.; Anyaoku, V.; Reed, M.J.; Franks, S. Improvement in endocrine and ovarian function during dietary treatment of obese women with polycystic ovary syndrome. Clin. Endocrinol. 1992, 36, 105–111. [Google Scholar] [CrossRef]
- Polak, A.M.; Adamska, A.; Krentowska, A.; Łebkowska, A.; Hryniewicka, J.; Adamski, M.; Kowalska, I. Body Composition, Serum Concentrations of Androgens and Insulin Resistance in Different Polycystic Ovary Syndrome Phenotypes. J. Clin. Med. 2020, 9, 732. [Google Scholar] [CrossRef]
- Polak, A.M.; Krentowska, A.; Łebkowska, A.; Buczyńska, A.; Adamski, M.; Adamska-Patruno, E.; Fiedorczuk, J.; Krętowski, A.J.; Kowalska, I.; Adamska, A. The Association of Serum Levels of Leptin and Ghrelin with the Dietary Fat Content in Non-Obese Women with Polycystic Ovary Syndrome. Nutrients 2020, 12, 2753. [Google Scholar] [CrossRef]
- Stepto, N.K.; Cassar, S.; Joham, A.E.; Hutchison, S.K.; Harrison, C.L.; Goldstein, R.F.; Teede, H.J. Women with polycystic ovary syndrome have intrinsic insulin resistance on euglycaemic-hyperinsulaemic clamp. Hum. Reprod. 2013, 28, 777–784. [Google Scholar] [CrossRef]
- Daghestani, M.H. Evaluation of biochemical, endocrine, and metabolic biomarkers for the early diagnosis of polycystic ovary syndrome among non-obese Saudi women. Int. J. Gynaecol. Obstet. 2018, 142, 162–169. [Google Scholar] [CrossRef]
- Mattson, M.P. Energy intake and exercise as determinants of brain health and vulnerability to injury and disease. Cell Metab. 2012, 16, 706–722. [Google Scholar] [CrossRef] [PubMed]
- Thankamony, A.; Capalbo, D.; Marcovecchio, M.L.; Sleigh, A.; Jørgensen, S.W.; Hill, N.R.; Mooslehner, K.; Yeo, G.S.H.; Bluck, L.; Juul, A.; et al. Low circulating levels of IGF-1 in healthy adults are associated with reduced β-cell function, increased intramyocellular lipid, and enhanced fat utilization during fasting. J. Clin. Endocrinol. Metab. 2014, 99, 2198–2207. [Google Scholar] [CrossRef] [PubMed]
- Gnanou, J.V.; Caszo, B.A.; Khalil, K.M.; Abdullah, S.L.; Knight, V.F.; Bidin, M.Z. Effects of Ramadan fasting on glucose homeostasis and adiponectin levels in healthy adult males. J. Diabetes Metab. Disord. 2015, 14, 55. [Google Scholar] [CrossRef] [PubMed]
- Kiyani, M.M.; Memon, A.R.; Amjad, M.I.; Ameer, M.R.; Sadiq, M.; Mahmood, T. Study of Human Biochemical Parameters during and After Ramadan. J. Relig. Health 2017, 56, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Templeman, I.; Smith, H.A.; Chowdhury, E.; Chen, Y.C.; Carroll, H.; Johnson-Bonson, D.; Hengist, A.; Smith, R.; Creighton, J.; Clayton, D.; et al. A randomized controlled trial to isolate the effects of fasting and energy restriction on weight loss and metabolic health in lean adults. Sci. Transl. Med. 2021, 13, eabd8034. [Google Scholar] [CrossRef]
- Masi, D.; Spoltore, M.E.; Rossetti, R.; Watanabe, M.; Tozzi, R.; Caputi, A.; Risi, R.; Balena, A.; Gandini, O.; Mariani, S.; et al. The Influence of Ketone Bodies on Circadian Processes Regarding Appetite, Sleep and Hormone Release: A Systematic Review of the Literature. Nutrients 2022, 14, 1410. [Google Scholar] [CrossRef] [PubMed]
- Lindheim, L.; Bashir, M.; Münzker, J.; Trummer, C.; Zachhuber, V.; Leber, B.; Horvath, A.; Pieber, T.R.; Gorkiewicz, G.; Stadlbauer, V.; et al. Alterations in gut microbiome composition and barrier function are associated with reproductive and metabolic defects in women with Polycystic Ovary Syndrome (PCOS): A pilot study. PLoS ONE 2017, 12, e0168390. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Jiang, M.; Ding, T.; Wang, J.; Long, P. Calprotectin is a potential prognostic marker for polycystic ovary syndrome. Ann. Clin. Biochem. 2017, 54, 253–257. [Google Scholar] [CrossRef]
- Łoniewski, I.; Szulińska, M.; Kaczmarczyk, M.; Podsiadło, K.; Styburski, D.; Skonieczna-Żydecka, K.; Bogdański, P. Analysis of correlations between gut microbiota, stool short chain fatty acids, calprotectin and cardiometabolic risk factors in postmenopausal women with obesity: A cross-sectional study. J. Transl. Med. 2022, 20, 585. [Google Scholar] [CrossRef]
| Before | After | p | |
|---|---|---|---|
| Age | 25.57 ± 2.67 | - | - |
| Body mass index (kg/m2) | 25.12 ± 3.17 | 22.13 ± 2.06 | <0.001 |
| Waist to hip ratio | 0.85 (0.82–0.88) | 0.82 (0.82–0.83) | 0.001 |
| Calprotectin (µg/g) | 81.5 (13–267) | 31 (9–78) | <0.001 |
| AMH (ng/mL) | 4.17 (2.76–5.72) | 2.66 (2.34–3.3) | <0.001 |
| FSH (mlU/mL) | 5.11 (4.1–6.45) | 4.52 (3.6–5.18) | 0.002 |
| LH (mlU/mL) | 9.95 (6.7–11.7) | 5.23 (4.32–6.33) | <0.001 |
| E2 (mlU/mL) | 43 (35–52) | 33 (30–39) | <0.001 |
| TSH mlU/mL | 1.11 (0.8–1.76) | 1.68 (1.23–1.93) | 0.001 |
| Prolactin (ng/mL) | 21 (17.3–24.9) | 19 (17–21) | 0.038 |
| Fasting insulin (μU/mL) | 20.17 (11.3–26.4) | 13.5 (10.3–17.8) | <0.001 |
| Fasting blood glucose (mg/dL) | 89.33 ± 8.39 | 83.17 ± 5.93 | <0.001 |
| HOMA-IR | 4.29 ± 1.82 | 2.87 ± 0.98 | <0.001 |
| Insulin resistance (>2.4) | 25 (83.3%) | 19 (63.3%) | 0.070 |
| HbA1c | 5.27 ± 0.42 | 4.96 ± 0.34 | <0.001 |
| Total testosterone (ng/dL) | 53.34 (25.48–77.15) | 32.98 (13.18–41.57) | <0.001 |
| Free testosterone (pg/mL) | 1.75 (0.86–2.12) | 0.78 (0.58–1.05) | <0.001 |
| SHBG (nmol/L) | 43.6 (33.5–66.9) | 76.4 (57.4–86.7) | <0.001 |
| Free androgen index | 3.92 (1.32–7.56) | 1.46 (0.53–2.58) | <0.001 |
| Hyperandrogenism (≥8) | 7 (23.3%) | 0 (0.0%) | 0.016 |
| DHEAS (µg/dL) | 252.95 (186.3–296.1) | 188.05 (167.8–213.4) | <0.001 |
| HDL-C (mg/dL) | 47.2 (41.9–62.2) | 67.25 (56.3–73.7) | <0.001 |
| LDL-C (mg/dL) | 136 (84–158) | 97 (77–117) | <0.001 |
| Triglyceride (mg/dL) | 170.5 (135–212) | 120 (94–143) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feyzioglu, B.S.; Güven, C.M.; Avul, Z. Eight-Hour Time-Restricted Feeding: A Strong Candidate Diet Protocol for First-Line Therapy in Polycystic Ovary Syndrome. Nutrients 2023, 15, 2260. https://doi.org/10.3390/nu15102260
Feyzioglu BS, Güven CM, Avul Z. Eight-Hour Time-Restricted Feeding: A Strong Candidate Diet Protocol for First-Line Therapy in Polycystic Ovary Syndrome. Nutrients. 2023; 15(10):2260. https://doi.org/10.3390/nu15102260
Chicago/Turabian StyleFeyzioglu, Bihter Senem, Cenk Mustafa Güven, and Zerrin Avul. 2023. "Eight-Hour Time-Restricted Feeding: A Strong Candidate Diet Protocol for First-Line Therapy in Polycystic Ovary Syndrome" Nutrients 15, no. 10: 2260. https://doi.org/10.3390/nu15102260
APA StyleFeyzioglu, B. S., Güven, C. M., & Avul, Z. (2023). Eight-Hour Time-Restricted Feeding: A Strong Candidate Diet Protocol for First-Line Therapy in Polycystic Ovary Syndrome. Nutrients, 15(10), 2260. https://doi.org/10.3390/nu15102260






