GPR-160 Receptor Signaling in the Dorsal Vagal Complex of Male Rats Modulates Meal Microstructure and CART-Mediated Hypophagia
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Endogenous DVC GPR-160 Regulates Light and Dark Phase Meal Microstructure
3.2. DVC Gpr160 KD Does Not Affect Food Intake in Response to a 24-h Fast
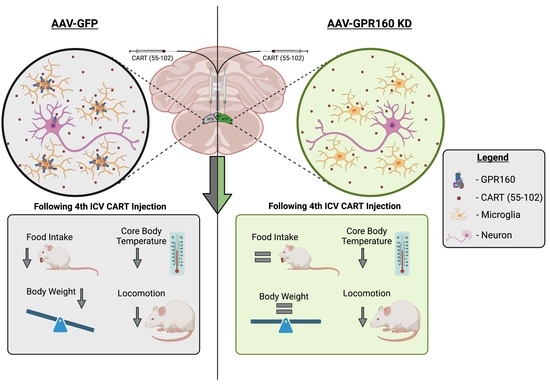
3.3. DVC Gpr160 KD Attenuates 4th ICV CART-Mediated Food Intake without Affecting Locomotor and Thermoregulatory Effects
3.4. DVC Single Nucleus RNAseq Data Reveals Substantial Gpr160 Expression in Microglia, but Not Neurons
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Koylu, E.O.; Couceyro, P.R.; Lambert, P.D.; Kuhar, M.J. Cocaine–and amphetamine-regulated transcript peptide immunohistochemical localization in the rat brain. J. Comp. Neurol. 1998, 391, 115–132. [Google Scholar] [CrossRef]
- Abbott, C.R.; Rossi, M.; Wren, A.M.; Murphy, K.G.; Kennedy, A.R.; Stanley, S.A.; Zollner, A.N.; Morgan, D.G.; Morgan, I.; Ghatei, M.A.; et al. Evidence of an orexigenic role for cocaine–and amphetamine-regulated transcript after administration into discrete hypothalamic nuclei. Endocrinology 2001, 142, 3457–3463. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.; Stanley, S.; Gardiner, J.; Abbott, C.; Murphy, K.; Seth, A.; Connoley, I.; Ghatei, M.; Stephens, D.; Bloom, S. A role for arcuate cocaine and amphetamine-regulated transcript in hyperphagia, thermogenesis, and cold adaptation. FASEB J. 2003, 17, 1688–1690. [Google Scholar] [CrossRef] [Green Version]
- Smith, K.L.; Gardiner, J.V.; Ward, H.L.; Kong, W.M.; Murphy, K.G.; Martin, N.M.; Ghatei, M.A.; Bloom, S.R. Overexpression of CART in the PVN increases food intake and weight gain in rats. Obesity 2008, 16, 2239–2244. [Google Scholar] [CrossRef]
- Hou, J.; Zheng, D.Z.; Zhou, J.Y.; Zhou, S.W. Orexigenic effect of cocaine- and amphetamine-regulated transcript (CART) after injection into hypothalamic nuclei in streptozotocin-diabetic rats. Clin. Exp. Pharmacol. Physiol. 2010, 37, 989–995. [Google Scholar] [CrossRef] [PubMed]
- Lau, J.; Farzi, A.; Qi, Y.; Heilbronn, R.; Mietzsch, M.; Shi, Y.C.; Herzog, H. CART neurons in the arcuate nucleus and lateral hypothalamic area exert differential controls on energy homeostasis. Mol. Metab. 2018, 7, 102–118. [Google Scholar] [CrossRef] [PubMed]
- Skibicka, K.P.; Alhadeff, A.L.; Grill, H.J. Hindbrain cocaine- and amphetamine-regulated transcript induces hypothermia mediated by GLP-1 receptors. J. Neurosci. 2009, 29, 6973–6981. [Google Scholar] [CrossRef] [Green Version]
- Smedh, U.; Moran, T.H. Peptides that regulate food intake: Separable mechanisms for dorsal hindbrain CART peptide to inhibit gastric emptying and food intake. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003, 284, R1418–R1426. [Google Scholar] [CrossRef] [Green Version]
- Smedh, U.; Scott, K.A.; Moran, T.H. Fourth ventricular CART peptide induces c-fos in the area postrema and nucleus of the solitary tract via a CRF-receptor dependent mechanism. Neurosci. Lett. 2015, 609, 124–128. [Google Scholar] [CrossRef]
- Smedh, U.; Scott, K.A.; Moran, T.H. Pretreatment with a CRF antagonist amplifies feeding inhibition induced by fourth ventricular cocaine- and amphetamine-regulated transcript peptide. BMC Neurosci. 2019, 20, 11. [Google Scholar] [CrossRef] [Green Version]
- Aja, S.; Schwartz, G.J.; Kuhar, M.J.; Moran, T.H. Intracerebroventricular CART peptide reduces rat ingestive behavior and alters licking microstructure. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001, 280, R1613–R1619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aja, S.; Sahandy, S.; Ladenheim, E.E.; Schwartz, G.J.; Moran, T.H. Intracerebroventricular CART peptide reduces food intake and alters motor behavior at a hindbrain site. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001, 281, R1862–R1867. [Google Scholar] [CrossRef] [Green Version]
- Haddock, C.J.; Almeida-Pereira, G.; Stein, L.M.; Hayes, M.R.; Kolar, G.R.; Samson, W.K.; Yosten, G.L.C. Signaling in rat brainstem via Gpr160 is required for the anorexigenic and antidipsogenic actions of cocaine- and amphetamine-regulated transcript peptide. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2021, 320, R236–R249. [Google Scholar] [CrossRef] [PubMed]
- Larsen, P.J.; Vrang, N.; Petersen, P.C.; Kristensen, P. Chronic intracerebroventricular administration of recombinant CART(42–89) peptide inhibits and causes weight loss in lean and obese Zucker (fa/fa) rats. Obes. Res. 2000, 8, 590–596. [Google Scholar] [CrossRef]
- Vrang, N.; Tang-Christensen, M.; Larsen, P.J.; Kristensen, P. Recombinant CART peptide induces c-Fos expression in central areas involved in control of feeding behaviour. Brain. Res. 1999, 818, 499–509. [Google Scholar] [CrossRef]
- Lee, S.J.; Krieger, J.P.; Vergara, M.; Quinn, D.; McDougle, M.; de Araujo, A.; Darling, R.; Zollinger, B.; Anderson, S.; Pan, A.; et al. Blunted Vagal Cocaine- and Amphetamine-Regulated Transcript Promotes Hyperphagia and Weight Gain. Cell Rep. 2020, 30, 2028–2039.e4. [Google Scholar] [CrossRef] [Green Version]
- Asnicar, M.A.; Smith, D.P.; Yang, D.D.; Heiman, M.L.; Fox, N.; Chen, Y.F.; Hsiung, H.M.; Koster, A. Absence of cocaine- and amphetamine-regulated transcript results in obesity in mice fed a high caloric diet. Endocrinology 2001, 142, 4394–4400. [Google Scholar] [CrossRef]
- Wierup, N.; Richards, W.G.; Bannon, A.W.; Kuhar, M.J.; Ahren, B.; Sundler, F. CART knock out mice have impaired insulin secretion and glucose intolerance, altered beta cell morphology and increased body weight. Regul. Pept. 2005, 129, 203–211. [Google Scholar] [CrossRef] [Green Version]
- Moffett, M.; Stanek, L.; Harley, J.; Rogge, G.; Asnicar, M.; Hsiung, H.; Kuhar, M. Studies of cocaine- and amphetamine-regulated transcript (CART) knockout mice. Peptides 2006, 27, 2037–2045. [Google Scholar] [CrossRef]
- Guerardel, A.; Barat-Houari, M.; Vasseur, F.; Dina, C.; Vatin, V.; Clement, K.; Eberle, D.; Vasseur-Delannoy, V.; Bell, C.G.; Galan, P.; et al. Analysis of sequence variability in the CART gene in relation to obesity in a Caucasian population. BMC Genet. 2005, 6, 19. [Google Scholar] [CrossRef]
- Miraglia del Giudice, E.; Santoro, N.; Fiumani, P.; Dominguez, G.; Kuhar, M.J.; Perrone, L. Adolescents carrying a missense mutation in the CART gene exhibit increased anxiety and depression. Depress. Anxiety 2006, 23, 90–92. [Google Scholar] [CrossRef] [PubMed]
- Yanik, T.; Dominguez, G.; Kuhar, M.J.; Del Giudice, E.M.; Loh, Y.P. The Leu34Phe ProCART mutation leads to cocaine- and amphetamine-regulated transcript (CART) deficiency: A possible cause for obesity in humans. Endocrinology 2006, 147, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Rigoli, L.; Munafo, C.; Di Bella, C.; Salpietro, A.; Procopio, V.; Salpietro, C. Molecular analysis of the CART gene in overweight and obese Italian children using family-based association methods. Acta Paediatr. 2010, 99, 722–726. [Google Scholar] [CrossRef]
- del Giudice, E.M.; Santoro, N.; Cirillo, G.; D’Urso, L.; Di Toro, R.; Perrone, L. Mutational screening of the CART gene in obese children: Identifying a mutation (Leu34Phe) associated with reduced resting energy expenditure and cosegregating with obesity phenotype in a large family. Diabetes 2001, 50, 2157–2160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dominguez, G.; del Giudice, E.M.; Kuhar, M.J. CART peptide levels are altered by a mutation associated with obesity at codon 34. Mol. Psychiatry 2004, 9, 1065–1066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamada, K.; Yuan, X.; Otabe, S.; Koyanagi, A.; Koyama, W.; Makita, Z. Sequencing of the putative promoter region of the cocaine- and amphetamine-regulated-transcript gene and identification of polymorphic sites associated with obesity. Int. J. Obes. Relat. Metab. Disord. 2002, 26, 132–136. [Google Scholar] [CrossRef] [Green Version]
- Yosten, G.L.; Harada, C.M.; Haddock, C.; Giancotti, L.A.; Kolar, G.R.; Patel, R.; Guo, C.; Chen, Z.; Zhang, J.; Doyle, T.M.; et al. GPR160 de-orphanization reveals critical roles in neuropathic pain in rodents. J. Clin. Investig. 2020, 130, 2587–2592. [Google Scholar] [CrossRef] [Green Version]
- Lakatos, A.; Prinster, S.; Vicentic, A.; Hall, R.A.; Kuhar, M.J. Cocaine- and amphetamine-regulated transcript (CART) peptide activates the extracellular signal-regulated kinase (ERK) pathway in AtT20 cells via putative G-protein coupled receptors. Neurosci. Lett. 2005, 384, 198–202. [Google Scholar] [CrossRef]
- Somalwar, A.R.; Choudhary, A.G.; Sharma, P.R.; Nagalakshmi, B.; Sagarkar, S.; Sakharkar, A.J.; Subhedar, N.K.; Kokare, D.M. Cocaine- and amphetamine-regulated transcript peptide (CART) induced reward behavior is mediated via Gi/o dependent phosphorylation of PKA/ERK/CREB pathway. Behav. Brain. Res. 2018, 348, 9–21. [Google Scholar] [CrossRef]
- Borner, T.; Liberini, C.G.; Lutz, T.A.; Riediger, T. Brainstem GLP-1 signalling contributes to cancer anorexia-cachexia syndrome in the rat. Neuropharmacology 2018, 131, 282–290. [Google Scholar] [CrossRef] [Green Version]
- Duffy, S.; Lutz, T.A.; Boyle, C.N. Rodent models of leptin receptor deficiency are less sensitive to amylin. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2018, 315, R856–R865. [Google Scholar] [CrossRef] [PubMed]
- Borner, T.; Geisler, C.E.; Fortin, S.M.; Cosgrove, R.; Alsina-Fernandez, J.; Dogra, M.; Doebley, S.; Sanchez-Navarro, M.J.; Leon, R.M.; Gaisinsky, J.; et al. GIP Receptor Agonism Attenuates GLP-1 Receptor Agonist-Induced Nausea and Emesis in Preclinical Models. Diabetes 2021, 70, 2545–2553. [Google Scholar] [CrossRef] [PubMed]
- Reiner, B.C.; Crist, R.C.; Borner, T.; Doyle, R.P.; Hayes, M.R.; De Jonghe, B.C. Single nuclei RNA sequencing of the rat AP and NTS following GDF15 treatment. Mol. Metab. 2022, 56, 101422. [Google Scholar] [CrossRef]
- Reiner, B.C.; Zhang, Y.; Stein, L.M.; Perea, E.D.; Arauco-Shapiro, G.; Ben Nathan, J.; Ragnini, K.; Hayes, M.R.; Ferraro, T.N.; Berretini, W.H.; et al. Single nucleus transcriptomic analysis of rat nucleus accumbens reveals cell type-specific patterns of gene expression associated with volitional morphine intake. Transl. Psychiatry 2022, 12, 374. [Google Scholar] [CrossRef]
- Kristensen, P.; Judge, M.E.; Thim, L.; Ribel, U.; Christjansen, K.N.; Wulff, B.S.; Clausen, J.T.; Jensen, P.B.; Madsen, O.D.; Vrang, N.; et al. Hypothalamic CART is a new anorectic peptide regulated by leptin. Nature 1998, 393, 72–76. [Google Scholar] [CrossRef]
- Vicentic, A. CART peptide diurnal variations in blood and brain. Peptides 2006, 27, 1942–1948. [Google Scholar] [CrossRef]
- Vicentic, A.; Dominguez, G.; Hunter, R.G.; Philpot, K.; Wilson, M.; Kuhar, M.J. Cocaine- and amphetamine-regulated transcript peptide levels in blood exhibit a diurnal rhythm: Regulation by glucocorticoids. Endocrinology 2004, 145, 4119–4124. [Google Scholar] [CrossRef] [Green Version]
- Vicentic, A.; Lakatos, A.; Hunter, R.; Philpot, K.; Dominguez, G.; Kuhar, M.J. CART peptide diurnal rhythm in brain and effect of fasting. Brain Res. 2005, 1032, 111–115. [Google Scholar] [CrossRef]
- Kastin, A.J.; Akerstrom, V. Entry of CART into brain is rapid but no inhibited by excess CART or leptin. Am. J. Physiol. 1999, 277, E901–E904. [Google Scholar] [CrossRef]
- Page, A.J. Gastrointestinal Vagal Afferents and Food Intake: Relevance of Circadian Rhythms. Nutrients 2021, 13, 844. [Google Scholar] [CrossRef]
- De Luca, S.N.; Sominsky, L.; Soch, A.; Wang, H.; Ziko, I.; Rank, M.M.; Spencer, S.J. Conditional microglial depletion in rats leads to reversible anorexia and weight loss by disrupting gustatory circuitry. Brain. Behav. Immun. 2019, 77, 77–91. [Google Scholar] [CrossRef]
- Badimon, A.; Strasburger, H.J.; Ayata, P.; Chen, X.; Nair, A.; Ikegami, A.; Hwang, P.; Chan, A.T.; Graves, S.M.; Uweru, J.O.; et al. Negative feedback control of neuronal activity by microglia. Nature 2020, 586, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Aja, S.; Ewing, C.; Lin, J.; Hyun, J.; Moran, T.H. Blockade of central GLP-1 receptors prevents CART-induced hypophagia and brain c-Fos expression. Peptides 2006, 27, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Freitas-Lima, L.C.; Pacesova, A.; Stanurova, J.; Sacha, P.; Marek, A.; Hubalek, M.; Kunes, J.; Zelezna, B.; Maletinska, L. GPR160 is not a receptor of anorexigenic cocaine- and amphetamine-regulated transcript peptide. Eur. J. Pharmacol. 2023, 949, 175713. [Google Scholar] [CrossRef] [PubMed]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanchez-Navarro, M.J.; Borner, T.; Reiner, B.C.; Crist, R.C.; Samson, W.K.; Yosten, G.L.C.; Stein, L.; Hayes, M.R. GPR-160 Receptor Signaling in the Dorsal Vagal Complex of Male Rats Modulates Meal Microstructure and CART-Mediated Hypophagia. Nutrients 2023, 15, 2268. https://doi.org/10.3390/nu15102268
Sanchez-Navarro MJ, Borner T, Reiner BC, Crist RC, Samson WK, Yosten GLC, Stein L, Hayes MR. GPR-160 Receptor Signaling in the Dorsal Vagal Complex of Male Rats Modulates Meal Microstructure and CART-Mediated Hypophagia. Nutrients. 2023; 15(10):2268. https://doi.org/10.3390/nu15102268
Chicago/Turabian StyleSanchez-Navarro, Marcos J., Tito Borner, Benjamin C. Reiner, Richard C. Crist, Willis K. Samson, Gina L. C. Yosten, Lauren Stein, and Matthew R. Hayes. 2023. "GPR-160 Receptor Signaling in the Dorsal Vagal Complex of Male Rats Modulates Meal Microstructure and CART-Mediated Hypophagia" Nutrients 15, no. 10: 2268. https://doi.org/10.3390/nu15102268
APA StyleSanchez-Navarro, M. J., Borner, T., Reiner, B. C., Crist, R. C., Samson, W. K., Yosten, G. L. C., Stein, L., & Hayes, M. R. (2023). GPR-160 Receptor Signaling in the Dorsal Vagal Complex of Male Rats Modulates Meal Microstructure and CART-Mediated Hypophagia. Nutrients, 15(10), 2268. https://doi.org/10.3390/nu15102268