Kinematic Features of Mandibular Movement during Mastication in Geriatric Individuals Who Are Provided with a Dysphagia Diet at Long-Term Care Facilities
Abstract
:1. Introduction
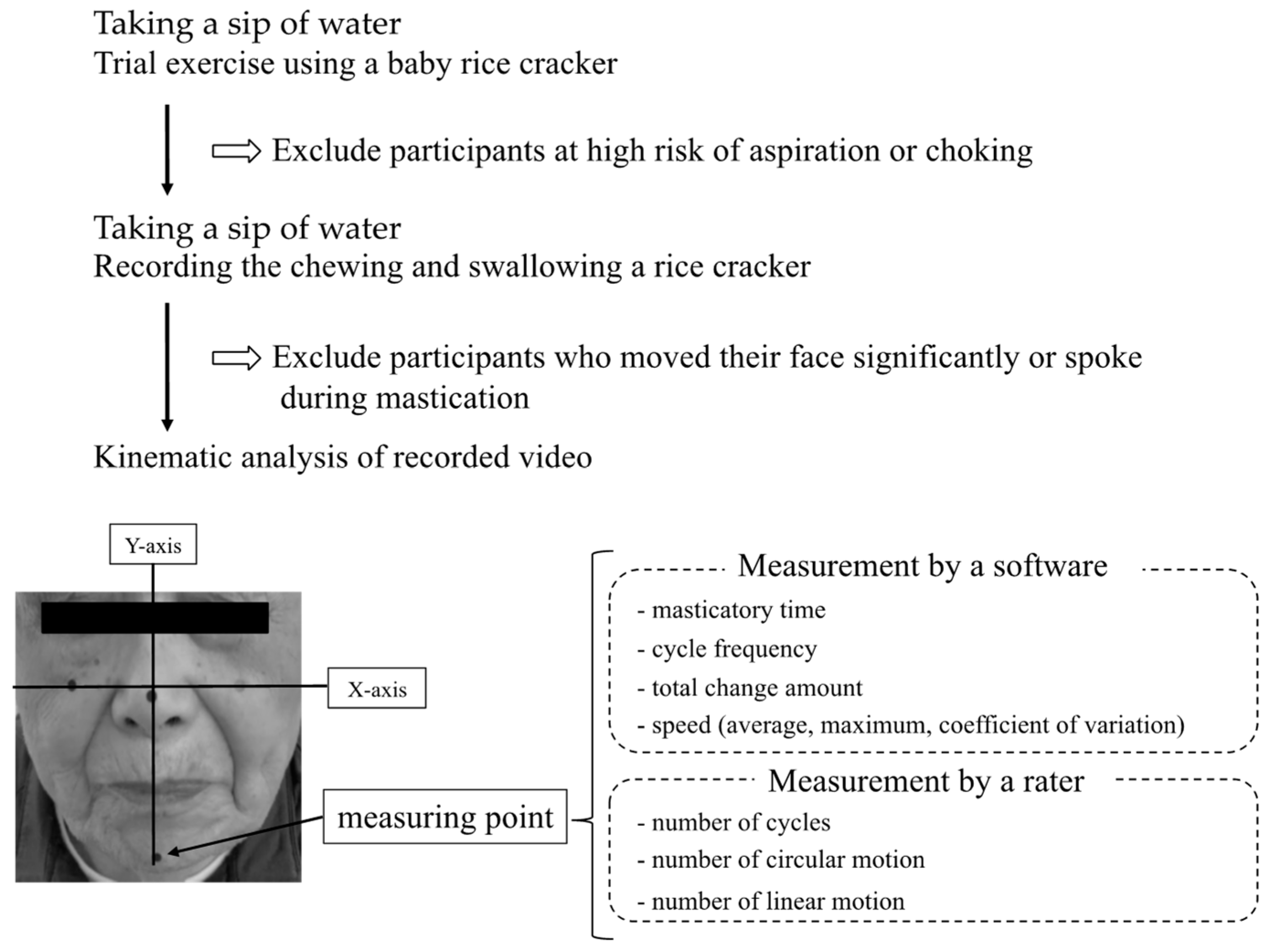
2. Materials and Methods
2.1. Participants
2.2. Observation of Masticatory Motion
2.3. Outcome Measures
2.4. Other Measurements
2.5. Statistical Analysis
2.6. Sample Size Calculation
3. Results
3.1. Characteristics of the Participants
3.2. Kinematic Analysis Results
4. Discussion
4.1. The Evaluation Method of Masticatory Movement
4.2. The Kinematic Differences in Masticatory Movement
4.3. Consideration of Factors Affecting Masticatory Movement
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cichero, J.A.Y. Age-related changes to eating and swallowing impact frailty: Aspiration, choking risk, modified food texture and autonomy of choice. Geriatrics 2018, 3, 69. [Google Scholar] [CrossRef] [PubMed]
- Hase, T.; Miura, Y.; Nakagami, G.; Okamoto, S.; Sanada, H.; Sugama, J. Food bolus-forming ability predicts incidence of aspiration pneumonia in nursing home older adults: A prospective observational study. J. Oral. Rehabil. 2020, 47, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Miura, H.; Yamasaki, K.; Kariyasu, M.; Miura, K.; Sumi, Y. Relationship between cognitive function and mastication in elderly females. J. Oral. Rehabil. 2003, 30, 808–811. [Google Scholar] [CrossRef] [PubMed]
- Murakami, M.; Hirano, H.; Watanabe, Y.; Sakai, K.; Kim, H.; Katakura, A. Relationship between chewing ability and sarcopenia in Japanese community-dwelling older adults. Geriatr. Gerontol. Int. 2015, 15, 1007–1012. [Google Scholar] [CrossRef]
- Magalhães, I.B.; Pereira, L.J.; Marques, L.S.; Gameiro, G.H. The influence of malocclusion on masticatory performance. A systematic review. Angle Orthod. 2010, 80, 981–987. [Google Scholar] [CrossRef] [PubMed]
- Uesugi, H.; Shiga, H. Relationship between masticatory performance using a gummy jelly and masticatory movement. J. Prosthodont. Res. 2017, 61, 419–425. [Google Scholar] [CrossRef]
- Tarkowska, A.; Katzer, L.; Ahlers, M.O. Assessment of masticatory performance by means of a color-changeable chewing gum. J. Prosthodont. Res. 2017, 61, 9–19. [Google Scholar] [CrossRef]
- Huckabee, M.-L.; McIntosh, T.; Fuller, L.; Curry, M.; Thomas, P.; Walshe, M.; McCague, E.; Battel, I.; Nogueira, D.; Frank, U.; et al. The test of masticating and swallowing solids (TOMASS): Reliability, validity and international normative data. Int. J. Lang. Commun. Disord. 2018, 53, 144–156. [Google Scholar] [CrossRef]
- Todaro, F.; Pizzorni, N.; Scarponi, L.; Ronzoni, C.; Huckabee, M.L.; Schindler, A. The test of masticating and swallowing solids (TOMASS): Reliability and validity in patients with dysphagia. Int. J. Lang. Commun. Disord. 2021, 56, 558–566. [Google Scholar] [CrossRef]
- Tagashira, I.; Tohara, H.; Wakasugi, Y.; Hara, K.; Nakane, A.; Yamazaki, Y.; Matsubara, M.; Minakuchi, S. A new evaluation of masticatory ability in patients with dysphagia: The Saku-Saku Test. Arch. Gerontol. Geriatr. 2018, 74, 106–111. [Google Scholar] [CrossRef]
- Hashimoto, H.; Hirata, M.; Takahashi, K.; Kameda, S.; Katsuta, Y.; Yoshida, F.; Hattori, N.; Yanagisawa, T.; Palmer, J.; Oshino, S.; et al. Non-invasive quantification of human swallowing using a simple motion tracking system. Sci. Rep. 2018, 8, 5095. [Google Scholar] [CrossRef] [PubMed]
- Arai, Y. Insurance for long-term care planned in Japan. Lancet 1997, 350, 1831. [Google Scholar] [CrossRef]
- Kondrup, J.; Allison, S.P.; Elia, M.; Vellas, B.; Plauth, M.; Educational and Clinical Practice Committee, European Society of Parenteral and Enteral Nutrition (ESPEN). ESPEN guidelines for nutrition screening 2002. Clin. Nutr. 2003, 22, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Umeda-Kameyama, Y.; Mori, T.; Wada-Isoe, K.; Kikuchi, T.; Kojima, S.; Ueki, A.; Watabe, T.; Kudoh, C.; Akishita, M.; Nakamura, Y.; et al. Development of a novel convenient Alzheimer’s disease assessment scale, the ABC Dementia Scale, using item response theory. Geriatr. Gerontol. Int. 2019, 19, 18–23. [Google Scholar] [CrossRef]
- Mori, T.; Kikuchi, T.; Umeda-Kameyama, Y.; Wada-Isoe, K.; Kojima, S.; Kagimura, T.; Kudoh, C.; Uchikado, H.; Ueki, A.; Yamashita, M.; et al. ABC Dementia Scale: A Quick Assessment Tool for Determining Alzheimer’s Disease Severity. Dement. Geriatr. Cogn. Dis. Extra 2018, 8, 85–97. [Google Scholar] [CrossRef]
- Eichner, K. Über eine Gruppeneinteilung der Lückengebisse für die Prothetik. Dtsch Zahnartzl Z 1955, 10, 1831–1834. [Google Scholar]
- Tanaka, Y.; Nakano, Y.; Yokoo, M.; Takeda, Y.; Yamada, K.; Kayashita, J. Examination about the relation of meal form, tongue pressure, grip and walking state in inpatient and elderly residents. Jpn. J. Dysphagia Rehabil. 2015, 19, 52–62. [Google Scholar]
- Peyron, M.A.; Mishellany, A.; Woda, A. Particle size distribution of food boluses after mastication of six natural foods. J. Dent. Res. 2004, 83, 578–582. [Google Scholar] [CrossRef]
- Kato, Y.; Kikutani, T.; Tohara, T.; Takahashi, N.; Tamura, F. Masticatory movements and food textures in older patients with eating difficulties. Gerodontology 2022, 39, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Unno, M.; Shiga, H.; Kobayashi, Y. The relationship between masticatory path pattern and masticatory efficiency in gumi-jelly chewing. Nihon Hotetsu Shika Gakkai Zasshi 2005, 49, 65–73. [Google Scholar] [CrossRef]
- Fischer, J.E.; Bachmann, L.M.; Jaeschke, R. A readers’ guide to the interpretation of diagnostic test properties: Clinical example of sepsis. Intensive Care Med. 2003, 29, 1043–1051. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, M.; Takada, K.; Shinde, M.; Matsumoto, N.; Tanaka, K.; Kiriya, Y.; Nishimoto, E.; Kuzuya, M. National survey of the prevalence of swallowing difficulty and tube feeding use as well as implementation of swallowing evaluation in long-term care settings in Japan. Geriatr. Gerontol. Int. 2014, 14, 577–581. [Google Scholar] [CrossRef]
- Kim, E.K.; Lee, S.K.; Choi, Y.H.; Tanaka, M.; Hirotsu, K.; Kim, H.C.; Lee, H.K.; Jung, Y.S.; Amano, A. Relationship between chewing ability and cognitive impairment in the rural elderly. Arch. Gerontol. Geriatr. 2017, 70, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Tsutsui, K.; Kaku, M.; Motokawa, M.; Tohma, Y.; Kawata, T.; Fujita, T.; Kohno, S.; Ohtani, J.; Tenjoh, K.; Nakano, M.; et al. Influences of reduced masticatory sensory input from soft-diet feeding upon spatial memory/learning ability in mice. Biomed. Res. 2007, 28, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Palmer, J.B.; Rudin, N.J.; Lara, G.; Crompton, A.W. Coordination of mastication and swallowing. Dysphagia 1992, 7, 187–200. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Hirayama, A. Effects of soft-diet feeding on synaptic density in the hippocampus and parietal cortex of senescence-accelerated mice. Brain Res. 2001, 902, 255–263. [Google Scholar] [CrossRef]


| Total | Diet Form | p Value | ||
|---|---|---|---|---|
| (n = 63) | Normal (n = 49) | Dysphagia (n = 14) | ||
| Age (years) * | 88.1 (6.1) | 87.6 (6.5) | 89.6 (4.1) | 0.278 † |
| Sex: male/female (n) | 15/48 | 11/38 | 4/10 | 0.635 § |
| GCDI (n) | 0.116 ‡ | |||
| 1/2/3/4/5 | 9/8/19/20/7 | 9/5/17/13/5 | 0/3/2/7/2 | |
| Cerebrovascular disease (n) | 20 | 16 | 4 | 0.722 § |
| BMI (kg/m2) * | 21.03 (3.05) | 21.69 (3.00) | 18.73 (2.24) | <0.001 † |
| MNA-SF | 0.444 ‡ | |||
| Normal (n) | 8 | 8 | 0 | |
| At risk (n) | 48 | 35 | 13 | |
| Malnutrition (n) | 7 | 6 | 1 | |
| ABC Dementia Scale * | 77.0 (19.8) | 79.7 (19.6) | 67.6 (18.2) | 0.043 † |
| Eichner Index (n) | 0.786 ‡ | |||
| A1/A2/A3/B1/B2/B3 | 47/0/1/4/9/2 | 37/0/1/2/8/1 | 10/0/0/2/1/1 | |
| Diet Form | p Value | d | 1-β | ||
|---|---|---|---|---|---|
| Normal (n = 49) | Dysphagia (n = 14) | ||||
| Masticatory time (s) | 28.90 (12.19) | 38.95 (17.7) | 0.027 | 0.731 | 0.660 |
| Number of cycles (n) | 37.15 (14.84) | 42.20 (20.78) | 0.241 | 0.306 | 0.168 |
| Cycle frequency (n/s) | 1.36 (0.37) | 1.13 (0.35) | 0.015 | 0.645 | 0.554 |
| Total change amount (mm) | 790.41 (300.89) | 1061.72 (446.15) | 0.024 | 0.805 | 0.530 |
| Speed (mm/s) | |||||
| Average | 29.32 (9.49) | 29.70 (13.43) | 0.922 | 0.036 | 0.052 |
| Maximum | 142.08 (54.88) | 140.26 (56.51) | 0.914 | 0.033 | 0.051 |
| Coefficient of variation | 0.79 (0.10) | 0.80 (0.11) | 0.799 | 0.077 | 0.057 |
| Cycle types | |||||
| Circular motion (n) | 24.93 (10.72) | 24.10 (14.77) | 0.787 | 0.070 | 0.056 |
| Linear motion (n) | 12.20 (9.98) | 18.10 (13.61) | 0.041 | 0.537 | 0.415 |
| Circular motion frequency (%) | 67.34 (16.10) | 56.75 (21.89) | 0.023 | 0.598 | 0.492 |
| Univariable Analysis | ||||
|---|---|---|---|---|
| Variables | Odds Ratio | 95% Confidence Interval | SE | p Value |
| Masticatory time | 0.347 | 0.004–0.021 | 0.004 | 0.005 |
| Cycle frequency | −0.243 | −0.604–0.006 | 0.153 | 0.055 |
| Total change amount | 0.256 | 0.000–0.001 | 0.000 | 0.043 |
| Circular motion frequency | −0.343 | −0.014–0.002 | 0.003 | 0.006 |
| Multivariable Analysis | ||||
|---|---|---|---|---|
| Variables | Odds Ratio | 95% Confidence Interval | SE | p Value |
| Masticatory time | 0.083 | −0.009–0.015 | 0.003 | 0.631 |
| Cycle frequency | −0.196 | −0.585–0.102 | 0.006 | 0.165 |
| Total change amount | 0.213 | 0.000–0.001 | 0.172 | 0.171 |
| Circular motion frequency | −0.307 | −0.013–−0.002 | 0.000 | 0.011 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakayama, E.; Tohara, H.; Sakai, M.; Iida, M.; Abe, K.; Ueda, K. Kinematic Features of Mandibular Movement during Mastication in Geriatric Individuals Who Are Provided with a Dysphagia Diet at Long-Term Care Facilities. Nutrients 2023, 15, 2273. https://doi.org/10.3390/nu15102273
Nakayama E, Tohara H, Sakai M, Iida M, Abe K, Ueda K. Kinematic Features of Mandibular Movement during Mastication in Geriatric Individuals Who Are Provided with a Dysphagia Diet at Long-Term Care Facilities. Nutrients. 2023; 15(10):2273. https://doi.org/10.3390/nu15102273
Chicago/Turabian StyleNakayama, Enri, Haruka Tohara, Mayu Sakai, Masato Iida, Kimiko Abe, and Koichiro Ueda. 2023. "Kinematic Features of Mandibular Movement during Mastication in Geriatric Individuals Who Are Provided with a Dysphagia Diet at Long-Term Care Facilities" Nutrients 15, no. 10: 2273. https://doi.org/10.3390/nu15102273
APA StyleNakayama, E., Tohara, H., Sakai, M., Iida, M., Abe, K., & Ueda, K. (2023). Kinematic Features of Mandibular Movement during Mastication in Geriatric Individuals Who Are Provided with a Dysphagia Diet at Long-Term Care Facilities. Nutrients, 15(10), 2273. https://doi.org/10.3390/nu15102273






