Impact of Maternal Lifestyle and Dietary Habits during Pregnancy on Newborn Metabolic Profile
Abstract
1. Introduction
2. Materials and Methods
2.1. Routinely Newborn Screening Analysis
2.2. Questionnaire
2.3. Matrix Preparation
2.4. Statistical Analysis
3. Results
3.1. Correlation Analysis
3.1.1. Physical Activity
3.1.2. Use of Iodized Salt in the Diet
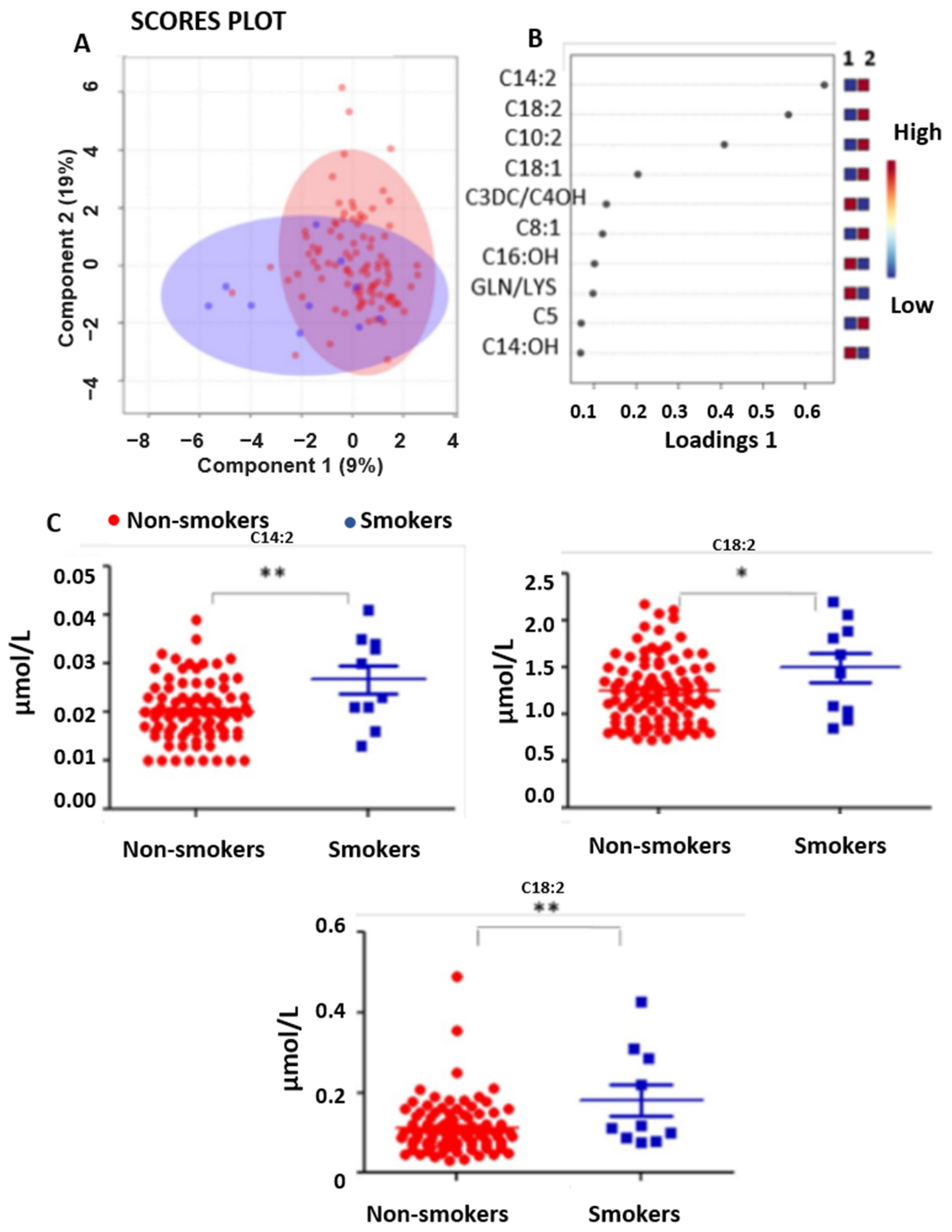
3.1.3. Smoking
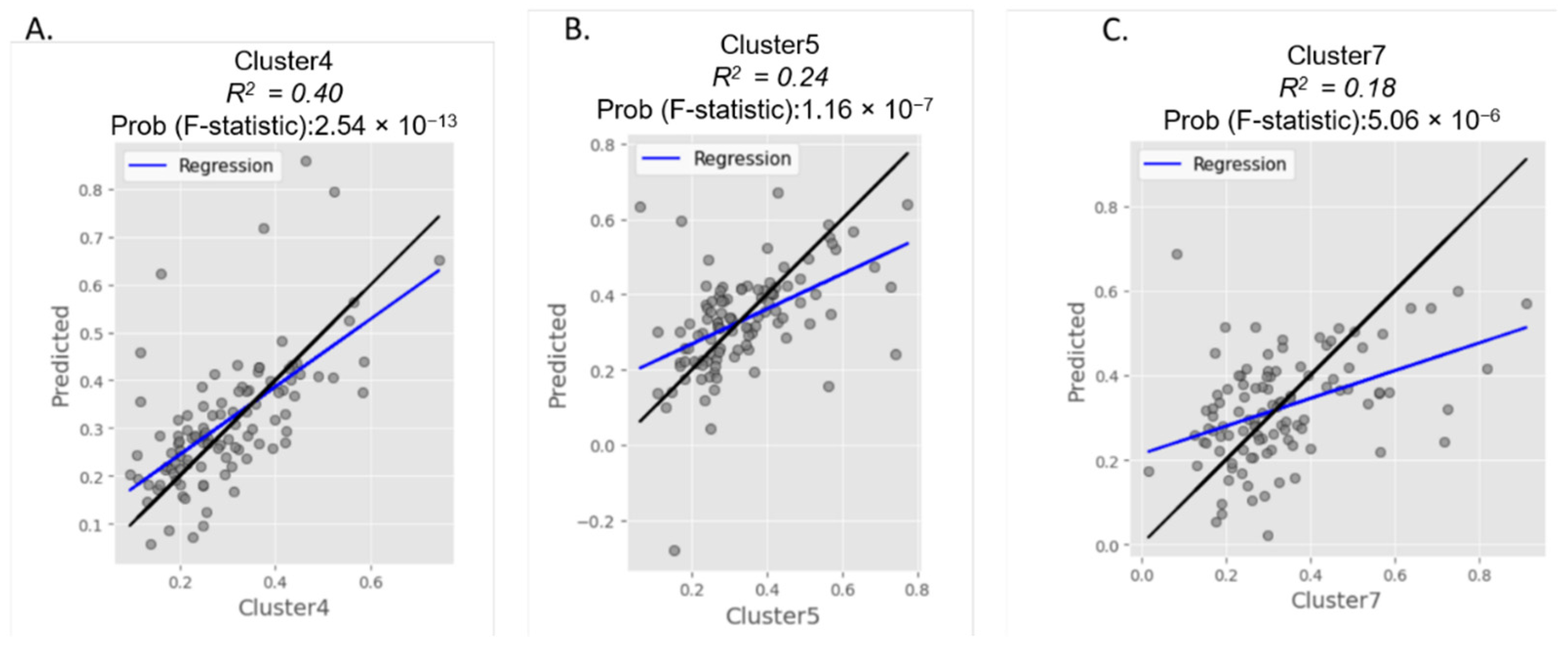
3.2. Cluster Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- la Marca, G. Mass spectrometry in clinical chemistry: The case of newborn screening. J. Pharm. Biomed. Anal. 2014, 101, 174–182. [Google Scholar] [CrossRef]
- Cicalini, I.; Valentinuzzi, S.; Pieragostino, D.; Consalvo, A.; Zucchelli, M.; Donzelli, S.; Ambrogi, D.; Brown, H.A.; Calton, L.J.; Stuppia, L.; et al. Analytical Evaluation of the Ideal Strategy for High-Throughput Flow Injection Analysis by Tandem Mass Spectrometry in Routine Newborn Screening. Metabolites 2021, 11, 473. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.-R. Screening newborns for metabolic disorders based on targeted metabolomics using tandem mass spectrometry. Ann. Pediatr. Endocrinol. Metab. 2015, 20, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Rossi, C.; Cicalini, I.; Rizzo, C.; Zucchelli, M.; Consalvo, A.; Valentinuzzi, S.; Semeraro, D.; Gasparroni, G.; Brindisino, P.; Gazzolo, D.; et al. A False-Positive Case of Methylmalonic Aciduria by Tandem Mass Spectrometry Newborn Screening Dependent on Maternal Malnutrition in Pregnancy. Int. J. Environ. Res. Public Health 2020, 17, 3601. [Google Scholar] [CrossRef] [PubMed]
- Ficicioglu, C. New tools and approaches to newborn screening: Ready to open Pandora’s box? Cold Spring Harb. Mol. Case Stud. 2017, 3, a001842. [Google Scholar] [CrossRef]
- Peng, G.; Tang, Y.; Gandotra, N.; Enns, G.M.; Cowan, T.M.; Zhao, H.; Scharfe, C. Ethnic variability in newborn metabolic screening markers associated with false-positive outcomes. J. Inherit. Metab. Dis. 2020, 43, 934–943. [Google Scholar] [CrossRef]
- Fraser, J.L.; Venditti, C.P. Methylmalonic and propionic acidemias: Clinical management update. Curr. Opin. Pediatr. 2016, 28, 682–693. [Google Scholar] [CrossRef]
- Azzini, E.; Ruggeri, S.; Polito, A. Homocysteine: Its Possible Emerging Role in At-Risk Population Groups. Int. J. Mol. Sci. 2020, 21, 1421. [Google Scholar] [CrossRef]
- Silva, V.C.D.; Fernandes, L.; Haseyama, E.J.; Agamme, A.L.D.A.; Shinohara, E.M.G.; Muniz, M.T.C.; D’Almeida, V. Effect of vitamin B deprivation during pregnancy and lactation on homocysteine metabolism and related metabolites in brain and plasma of mice offspring. PLoS ONE 2014, 9, e92683. [Google Scholar] [CrossRef]
- Kominiarek, M.A.; Rajan, P. Nutrition Recommendations in Pregnancy and Lactation. Med. Clin. N. Am. 2016, 100, 1199–1215. [Google Scholar] [CrossRef]
- Pieragostino, D.; Cicalini, I.; Di Michele, S.; Fusilli, P.; Cotugno, G.; Ferrante, R.; Bucci, I.; Dionisi-Vici, C.; Stuppia, L.; De Laurenzi, V.; et al. A Case of Suspected Hyperphenylalaninemia at Newborn Screening by Tandem Mass Spectrometry during Total Parenteral Nutrition. Metabolites 2020, 10, 44. [Google Scholar] [CrossRef] [PubMed]
- Castell, E.C.; Sánchez-González, P.; Palazón-Bru, A.; Bosch-Giménez, V.; Manero-Soler, H.; Juste-Ruiz, M.; Rizo-Baeza, M.; Gil-Guillén, V.F. Highest Plasma Phenylalanine Levels in (Very) Premature Infants on Intravenous Feeding; A Need for Concern. PLoS ONE 2015, 10, e0138532. [Google Scholar]
- McCabe, E.R.B. Metabolite flux: A dynamic concept for inherited metabolic disorders as complex traits. Mol. Genet. Metab. 2019, 128, 14–18. [Google Scholar] [CrossRef]
- Lanpher, B.; Brunetti-Pierri, N.; Lee, B. Inborn errors of metabolism: The flux from Mendelian to complex diseases. Nat. Rev. Genet. 2006, 7, 449–460. [Google Scholar] [CrossRef]
- Ramezani Ahmadi, A.; Rayyani, E.; Bahreini, M.; Mansoori, A. The effect of glutamine supplementation on athletic performance, body composition, and immune function: A systematic review and a meta-analysis of clinical trials. Clin. Nutr. 2019, 38, 1076–1091. [Google Scholar] [CrossRef]
- Herbst, E.A.F.; Holloway, G.P. Exercise increases mitochondrial glutamate oxidation in the mouse cerebral cortex. Appl. Physiol. Nutr. Metab. 2016, 41, 799–801. [Google Scholar] [CrossRef] [PubMed]
- Sonnewald, U.; Schousboe, A. Introduction to the Glutamate-Glutamine Cycle. Adv. Neurobiol. 2016, 13, 1–7. [Google Scholar] [PubMed]
- Chung, H.R. Iodine and thyroid function. Ann. Pediatr. Endocrinol. Metab. 2014, 19, 8–12. [Google Scholar] [CrossRef]
- Benvenga, S.; Lakshmanan, M.; Trimarchi, F. Carnitine is a naturally occurring inhibitor of thyroid hormone nuclear uptake. Thyroid 2000, 10, 1043–1050. [Google Scholar] [CrossRef] [PubMed]
- Benvenga, S.; Sindoni, A. L-carnitine supplementation for the management of fatigue in patients with hypothyroidism on levothyroxine treatment [Letter to the Editor]. Endocr. J. 2016, 63, 937–938. [Google Scholar] [CrossRef] [PubMed]
- Benvenga, S.; Feldt-Rasmussen, U.; Bonofiglio, D.; Asamoah, E. Nutraceutical Supplements in the Thyroid Setting: Health Benefits beyond Basic Nutrition. Nutrients 2019, 11, 2214. [Google Scholar] [CrossRef]
- An, J.H.; Kim, Y.J.; Kim, K.J.; Kim, S.H.; Kim, N.H.; Kim, H.Y.; Kim, N.H.; Choi, K.M.; Baik, S.H.; Choi, D.S.; et al. L-carnitine supplementation for the management of fatigue in patients with hypothyroidism on levothyroxine treatment: A randomized, double-blind, placebo-controlled trial. Endocr. J. 2016, 63, 885–895. [Google Scholar] [CrossRef]
- Maebashi, M.; Kawamura, N.; Sato, M.; Imamura, A.; Yoshinaga, K. Urinary excretion of carnitine in patients with hyperthyroidism and hypothyroidism: Augmentation by thyroid hormone. Metabolism 1977, 26, 351–356. [Google Scholar] [CrossRef]
- Yang, M.; Sun, M.; Jiang, C.; Wu, Q.; Jiang, Y.; Xu, J.; Luo, Q. Thyroid hormones and carnitine in the second trimester negatively affect neonate birth weight: A prospective cohort study. Front. Endocrinol. 2023, 14, 1080969. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, L.J.; MacDonald, S.M.; Shaw, M.D.; Kumar, R.; Saunders, R.W.; Parthipan, R.; Wilson, J.; Plane, J.M.C. Atmospheric iodine levels influenced by sea surface emissions of inorganic iodine. Nat. Geosci. 2013, 6, 108–111. [Google Scholar] [CrossRef]
- Rebouche, C.J. Ascorbic acid and carnitine biosynthesis. Am. J. Clin. Nutr. 1991, 54, 1147S–1152S. [Google Scholar] [CrossRef] [PubMed]
- Lykkesfeldt, J.; Christen, S.; Wallock, L.M.; Chang, H.H.; A Jacob, R.; Ames, B.N. Ascorbate is depleted by smoking and repleted by moderate supplementation: A study in male smokers and nonsmokers with matched dietary antioxidant intakes. Am. J. Clin. Nutr. 2000, 71, 530–536. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cicalini, I.; Moffa, S.; Tommolini, M.L.; Valentinuzzi, S.; Zucchelli, M.; Bucci, I.; Chiacchiaretta, P.; Fontana, A.; Federici, L.; De Laurenzi, V.; et al. Impact of Maternal Lifestyle and Dietary Habits during Pregnancy on Newborn Metabolic Profile. Nutrients 2023, 15, 2297. https://doi.org/10.3390/nu15102297
Cicalini I, Moffa S, Tommolini ML, Valentinuzzi S, Zucchelli M, Bucci I, Chiacchiaretta P, Fontana A, Federici L, De Laurenzi V, et al. Impact of Maternal Lifestyle and Dietary Habits during Pregnancy on Newborn Metabolic Profile. Nutrients. 2023; 15(10):2297. https://doi.org/10.3390/nu15102297
Chicago/Turabian StyleCicalini, Ilaria, Samanta Moffa, Maria Lucia Tommolini, Silvia Valentinuzzi, Mirco Zucchelli, Ines Bucci, Piero Chiacchiaretta, Antonella Fontana, Luca Federici, Vincenzo De Laurenzi, and et al. 2023. "Impact of Maternal Lifestyle and Dietary Habits during Pregnancy on Newborn Metabolic Profile" Nutrients 15, no. 10: 2297. https://doi.org/10.3390/nu15102297
APA StyleCicalini, I., Moffa, S., Tommolini, M. L., Valentinuzzi, S., Zucchelli, M., Bucci, I., Chiacchiaretta, P., Fontana, A., Federici, L., De Laurenzi, V., Del Boccio, P., Rossi, C., & Pieragostino, D. (2023). Impact of Maternal Lifestyle and Dietary Habits during Pregnancy on Newborn Metabolic Profile. Nutrients, 15(10), 2297. https://doi.org/10.3390/nu15102297










