Need for Multidimensional and Multidisciplinary Management of Depressed Preadolescents and Adolescents: A Review of Randomized Controlled Trials on Oral Supplementations (Omega-3, Fish Oil, Vitamin D3)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Literature Search
2.2. Inclusion and Exclusion Criteria
2.3. Quality Appraisal
2.4. Data Extraction
3. Results
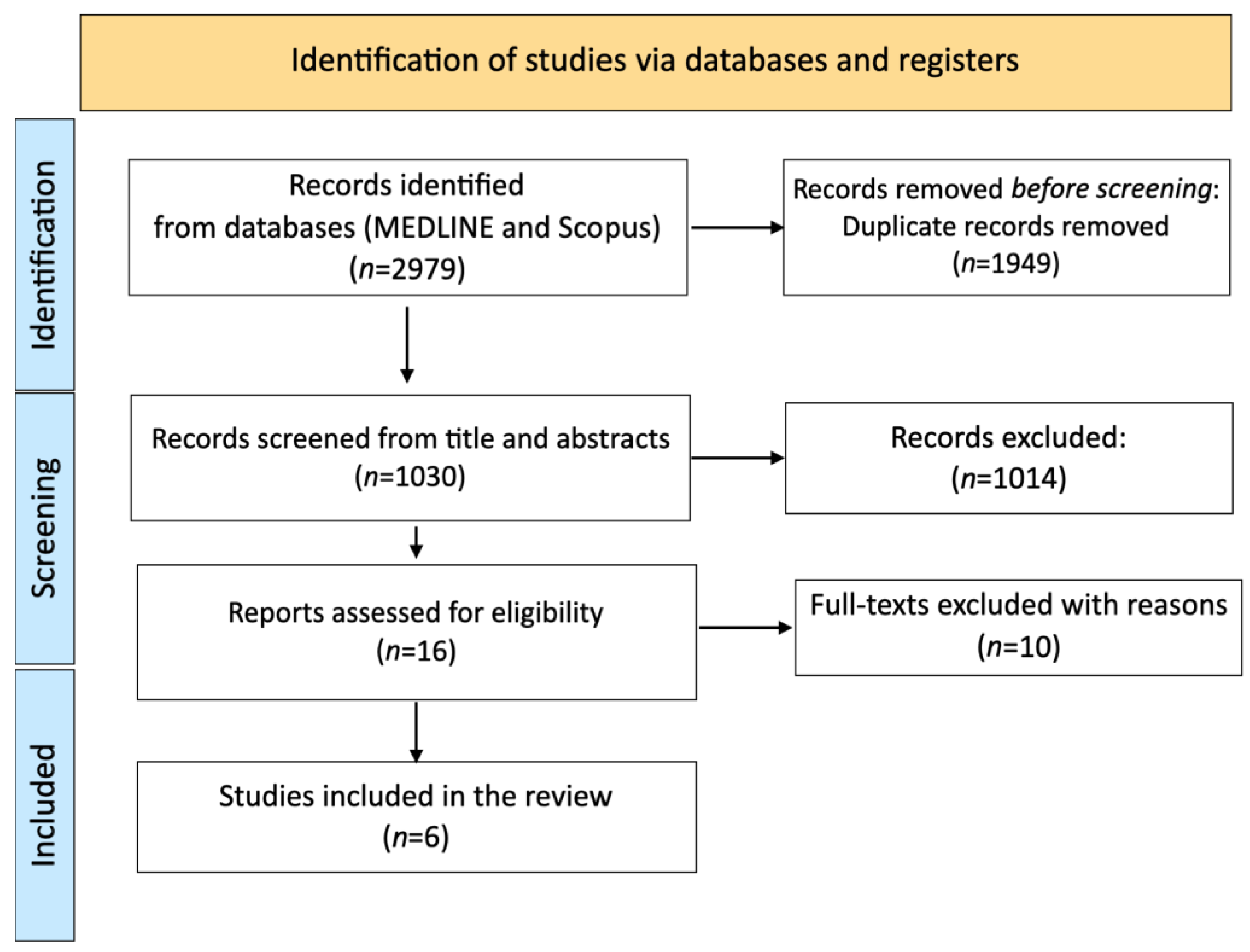
3.1. Study Selection
3.2. Study Characteristics
3.2.1. Population Characteristics
3.2.2. Intervention Style
3.2.3. Quality Assessment of Included Studies
3.3. Results for Depression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- The World Health Organization (WHO). Global Health Estimates. Available online: https://www.who.int/data/global-health-estimates (accessed on 1 August 2022).
- The National Institute of Mental Health (NIMH). Depression. Available online: https://www.nimh.nih.gov/health/topics/depression/index.shtml (accessed on 1 August 2022).
- Bharadwaj, P.; Pai, M.M.; Suziedelyte, A. Mental health stigma. Econ. Lett. 2017, 159, 57–60. [Google Scholar] [CrossRef]
- Chopra, C.; Mandalika, S.; Kinger, N. Does diet play a role in the prevention and management of depression among adolescents? A narrative review. Nutr. Health 2021, 27, 243–263. [Google Scholar] [CrossRef] [PubMed]
- Patel, V.; Flisher, A.J.; Hetrick, S.; McGorry, P. Mental health of young people: A global public-health challenge. Lancet 2007, 369, 1302–1313. [Google Scholar] [CrossRef] [PubMed]
- Khalid, S.; Williams, C.M.; Reynolds, S.A. Is there an association between diet and depression in children and adolescents? A systematic review. Br. J. Nutr. 2016, 116, 2097–2108. [Google Scholar] [CrossRef]
- Belfer, M.L. Child and adolescent mental disorders: The magnitude of the problem across the globe. J. Child Psychol. Psychiatry Allied Discip. 2008, 49, 226–236. [Google Scholar] [CrossRef]
- Scahill, L. Selective serotonin reuptake inhibitors in children and adolescents with major depression. Braz. J. Psychiatry 2005, 27, 91–92. [Google Scholar] [CrossRef]
- Zhou, X.; Teng, T.; Zhang, Y.; Del Giovane, C.; Furukawa, T.A.; Weisz, J.R.; Li, X.; Cuijpers, P.; Coghill, D.; Xiang, Y.; et al. Comparative efficacy and acceptability of antidepressants, psychotherapies, and their combination for acute treatment of children and adolescents with depressive disorder: A systematic review and network meta-analysis. Lancet Psychiatry 2020, 7, 581–601. [Google Scholar] [CrossRef]
- Luft, M.J.; Lamy, M.; DelBello, M.P.; McNamara, R.K.; Strawn, J.R. Antidepressant-Induced Activation in Children and Adolescents: Risk, Recognition and Management. Curr. Probl. Pediatr. Adolesc. Health Care 2018, 48, 50–62. [Google Scholar] [CrossRef]
- Lu, C.Y.; Zhang, F.; Lakoma, M.D.; Madden, J.M.; Rusinak, D.; Penfold, R.B.; Simon, G.; Ahmedani, B.K.; Clarke, G.; Hunkeler, E.M.; et al. Changes in antidepressant use by young people and suicidal behavior after FDA warnings and media coverage: Quasiexperimental study. BMJ 2014, 348, g3596. [Google Scholar] [CrossRef]
- US Food and Drug Administration. Suicidality in Children and Adolescents Being Treated with Antidepressant Medications. US Food and Drug Administration. 15 October 2004. Available online: https://www.fda.gov/drugs/postmarketdrugsafetyinformationpatientsandproviders/suicidalitychildrenandadolescentsbeingtreatedantidepressantmedications (accessed on 3 March 2020).
- Tulisiak, A.K.; Klein, J.A.; Harris, E.; Luft, M.J.; Schroeder, H.K.; Mossman, S.A.; Varney, S.T.; Keeshin, B.R.; Cotton, S.; Strawn, J.R. Antidepressant Prescribing by Pediatricians: A Mixed-Methods Analysis. Curr. Probl. Pediatr. Adolesc. Health Care 2017, 47, 15–24. [Google Scholar] [CrossRef]
- Riddle, M.A.; King, R.A.; Hardin, M.T.; Scahill, L.; Ort, S.I.; Chappell, P.; Rasmusson, A.; Leckman, J.F. Behavioral Side Effects of Fluoxetine in Children and Adolescents. J. Child Adolesc. Psychopharmacol. 1990, 1, 193–198. [Google Scholar] [CrossRef]
- Cipriani, A.; Zhou, X.; Del Giovane, C.; Hetrick, S.E.; Qin, B.; Whittington, C.; Coghill, D.; Zhang, Y.; Hazell, P.; Leucht, S.; et al. Comparative efficacy and tolerability of antidepressants for major depressive disorder in children and adolescents: A network metaanalysis. Lancet 2016, 388, 881–890. [Google Scholar] [CrossRef]
- Vitiello, B.; Davico, C. Twenty years of progress in paediatric psychopharmacology: Accomplishments and unmet needs. BMJ Ment. Health 2018, 21, e10. [Google Scholar] [CrossRef]
- Le Noury, J.; Nardo, J.M.; Healy, D.; Jureidini, J.; Raven, M.; Tufanaru, C.; Abi-Jaoude, E. Restoring Study 329, Efficacy and harms of paroxetine and imipramine in treatment of major depression in adolescence. BMJ 2015, 351, h4320. [Google Scholar] [CrossRef]
- Jacka, F.N. Nutritional psychiatry: Where to next? EBioMedicine 2017, 17, 24–29. [Google Scholar] [CrossRef]
- Marx, W.; Moseley, G.; Berk, M.; Jacka, F.N. Nutritional psychiatry: The present state of the evidence. Proc. Nutr. Soc. 2017, 76, 427–436. [Google Scholar] [CrossRef]
- Khanna, P.; Chattu, V.K.; Aeri, B.T. Nutritional Aspects of Depression in Adolescents—A Systematic Review. Int. J. Prev. Med. 2019, 10, 42. [Google Scholar] [CrossRef]
- Liao, Y.; Xie, B.; Zhang, H.; He, Q.; Guo, L.; Subramanieapillai, M.; Fan, B.; Lu, C.; McIntyre, R.S. Efficacy of omega-3 PUFAs in depression: A meta-analysis. Transl. Psychiatry 2019, 9, 190. [Google Scholar] [CrossRef]
- Wang, J.; Um, P.; Dickerman, B.A.; Liu, J. Zinc, Magnesium, Selenium and Depression: A Review of the Evidence, Potential Mechanisms and Implications. Nutrients 2018, 10, 584. [Google Scholar] [CrossRef]
- van der Burg, K.P.; Cribb, L.; Firth, J.; Karmacoska, D.; Mischoulon, D.; Byrne, G.J.; Bousman, C.; Stough, C.; Murphy, J.; Oliver, G.; et al. EPA and DHA as markers of nutraceutical treatment response in major depressive disorder. Eur. J. Nutr. 2020, 59, 2439–2447. [Google Scholar] [CrossRef]
- Arab, A.; Mehrabani, S.; Moradi, S.; Amani, R. The association between diet and mood: A systematic review of current literature. Psychiatry Res. 2019, 271, 428–437. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Liu, H.; Suzuki, K.; Ma, S.; Liu, C. Linking What We Eat to Our Mood: A Review of Diet, Dietary Antioxidants, and Depression. Antioxidants 2019, 8, 376. [Google Scholar] [CrossRef] [PubMed]
- Kris-Etherton, P.M.; Petersen, K.S.; Hibbeln, J.R.; Hurley, D.; Kolick, V.; Peoples, S.; Rodriguez, N.; Woodward-Lopez, G. Nutrition and behavioral health disorders: Depression and anxiety. Nutr. Rev. 2021, 79, 247–260. [Google Scholar] [CrossRef] [PubMed]
- Murakami, K.; Sasaki, S. Dietary intake and depressive symptoms: A systematic review of observational studies. Mol. Nutr. Food Res. 2010, 54, 471–488. [Google Scholar] [CrossRef] [PubMed]
- Sarris, J.; Logan, A.C.; Akbaraly, T.N.; Amminger, G.P.; Balanzá-Martínez, V.; Freeman, M.P.; Hibbeln, J.; Matsuoka, Y.; Mischoulon, D.; Mizoue, T.; et al. Nutritional medicine as mainstream in psychiatry. Lancet Psychiatry 2015, 2, 271–274. [Google Scholar] [CrossRef]
- Ano, Y.; Ohya, R.; Kondo, K. Antidepressant-Like Effect of β-Lactolin, a Glycine-Threonine-Tryptophan-Tyrosine Peptide. J. Nutr. Sci. Vitaminol. 2019, 65, 430–434. [Google Scholar] [CrossRef]
- Holford, P. Depression: The nutrition connection. Prim. Care Ment. Health 2003, 1, 9–16. [Google Scholar]
- Poznanski, E.O.; Mokros, H. Children’s Depression Rating Scale-Revised (CDRS-R); WPS: Los Angeles, CA, USA, 1996. [Google Scholar]
- Beck, A.T.; Steer, R.A.; Brown, G.K. Manual for the Beck Depression Inventory-II; Psychological Corporation: San Antonio, TX, USA, 1996. [Google Scholar]
- Kovacs, M. Childrens Depression Inventory CDI; Psychodiagnostika a.s.: Bratislava, Slovakia, 1998. [Google Scholar]
- Tufanaru, C.; Munn, Z.; Aromataris, E.; Campbell, J.; Hopp, L. Chapter 3: Systematic Reviews of Effectiveness. Available online: https://jbi-global-wiki.refined.site/space/MANUAL/4688621/Chapter+3%3A+Systematic+reviews+of+effectiveness (accessed on 15 March 2023).
- McNamara, R.K.; Strawn, J.R.; Tallman, M.J.; Welge, J.A.; Patino, L.R.; Blom, T.J.; DelBello, M.P. Effects of Fish Oil Monotherapy on Depression and Prefrontal Neurochemistry in Adolescents at High Risk for Bipolar I Disorder: A 12-Week Placebo-Controlled Proton Magnetic Resonance Spectroscopy Trial. J. Child Adolesc. Psychopharmacol. 2020, 30, 293–305. [Google Scholar] [CrossRef]
- Fristad, M.A.; Roley-Roberts, M.E.; Black, S.R.; Arnold, L.E. Moody kids years later: Long-term outcomes of youth from the Omega-3 and therapy (OATS) studies. J. Affect. Disord. 2021, 281, 24–32. [Google Scholar] [CrossRef]
- Trebatická, J.; Hradečná, Z.; Surovcová, A.; Katrenčíková, B.; Gushina, I.; Waczulíková, I.; Sušienková, K.; Garaiova, I.; Šuba, J.; Ďuračková, Z. Omega-3 fatty-acids modulate symptoms of depressive disorder, serum levels of omega-3 fatty acids and omega-6/omega-3 ratio in children. A randomized, double-blind and controlled trial. Psychiatry Res. 2020, 287, 112911. [Google Scholar] [CrossRef]
- Gabbay, V.; Freed, R.D.; Alonso, C.M.; Senger, S.; Stadterman, J.; Davison, B.A.; Klein, R.G. A Double-Blind Placebo-Controlled Trial of Omega-3 Fatty Acids as a Monotherapy for Adolescent Depression. J. Clin. Psychiatry 2018, 79, 17m11596. [Google Scholar] [CrossRef]
- Fristad, M.A.; Vesco, A.T.; Young, A.S.; Healy, K.Z.; Nader, E.S.; Gardner, W.; Seidenfeld, A.M.; Wolfson, H.L.; Arnold, L.E. Pilot Randomized Controlled Trial of Omega-3 and Individual-Family Psychoeducational Psychotherapy for Children and Adolescents With Depression. J. Clin. Child Adolesc. Psychol. 2019, 48 (Suppl. S1), S105–S118. [Google Scholar] [CrossRef]
- Libuda, L.; Timmesfeld, N.; Antel, J.; Hirtz, R.; Bauer, J.; Führer, D.; Zwanziger, D.; Öztürk, D.; Langenbach, G.; Hahn, D.; et al. Effect of vitamin D deficiency on depressive symptoms in child and adolescent psychiatric patients: Results of a randomized controlled trial. Eur. J. Nutr. 2020, 59, 3415–3424. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th ed.; American psychiatric Association: Washington, DC, USA, 2000. [Google Scholar]
- Poznanski, E.O.; Cook, S.C.; Carroll, B.J. A depression rating scale for children. Pediatrics 1979, 64, 442–450. [Google Scholar] [CrossRef]
- Poznanski, E.O.; Cook, S.C.; Carroll, B.J.; Corzo, H. Use of the Children’s Depression Rating Scale in an inpatient psychiatric population. J. Clin. Psychiatry 1983, 44, 200–203. [Google Scholar]
- Young, R.C.; Biggs, J.T.; Ziegler, V.E.; Meyer, D.A. A rating scale for mania: Reliability, validity and sensitivity. Br. J. Psychiatry 1978, 133, 429–435. [Google Scholar] [CrossRef]
- Shaffer, D.; Gould, M.S.; Brasic, J.; Ambrosini, P.; Fisher, P.; Bird, H.; Aluwahlia, S. A Children’s Global Assessment Scale (CGAS). Arch. Gen. Psychiatry 1983, 40, 1228–1231. [Google Scholar] [CrossRef]
- Bird, H.R.; Canino, G.; Rubio-Stipec, M.; Ribera, J.C. Further measures of the psychometric properties of the children’s global assessment scale. Arch. Gen. Psychiatry 1987, 44, 821–824. [Google Scholar] [CrossRef]
- Achenbach, T.M. Manual for the Child Behavior Checklist; University of Vermont Department of Psychiatry: Burlington, VT, USA, 1991. [Google Scholar]
- Bang, Y.R.; Park, J.H.; Kim, S.H. Cut-off scores of the children’s depression inventory for screening and rating severity in Korean adolescents. Psychiatry Investig. 2015, 12, 23–28. [Google Scholar] [CrossRef]
- Gioia, G.A.; Isquith, P.K.; Guy, S.C.; Kenworthy, L. Behavior rating inventory of executive function. Child Neuropsychol. 2000, 6, 235–238. [Google Scholar] [CrossRef]
- Döpfner, M.; Görtz-Dorten, A.; Lehmkuhl, G.; Breuer, D.; Goletz, H. DISYPS-II, Diagnostik-System für Psychische Störungen Nach ICD-10 und DSM-IV für Kinder und Jugendliche-II; Verlag Hans-Huber: Bern, Switzerland, 2008. [Google Scholar]
- Sonawalla, S.B.; Rosenbaum, J.F. Placebo response in depression. Dialogues Clin. Neurosci. 2002, 4, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Pruneti, C.; Cosentino, C.; Sgromo, M.; Innocenti, A. Skin Conductance Response as a decisive variable in individuals with a DSM-IV TR Axis I diagnosis. JMED Res. 2014, 2014, 565009. [Google Scholar] [CrossRef]
- Pruneti, C.; Saccò, M.; Cosentino, C.; Sgromo, D. Relevance of Autonomic Arousal in the Stress Response in Psychopathology. J. Basic Appl. Sci. 2016, 12, 176–184. [Google Scholar] [CrossRef]
- Pruneti, C.; Guidotti, S.; Lento, R.M.; Renda, N. Dissociation between cognitive-behavioral and emotional-psycho- physiological aspects in Eating Disorders and its pre-post treatment stability. J. Psychopathol. 2022, 28, 30–38. [Google Scholar] [CrossRef]
- Eckshtain, D.; Kuppens, S.; Ugueto, A.; Ng, M.Y.; Vaughn-Coaxum, R.; Corteselli, K.; Weisz, J.R. Metaanalysis: 13 year follow up of psychotherapy effects on youth depression. J. Am. Acad. Child Adolesc. Psychiatry 2020, 59, 45–63. [Google Scholar] [CrossRef]
- Weisz, J.R.; Kuppens, S.; Ng, M.Y.; Eckshtain, D.; Ugueto, A.M.; Vaughn-Coaxum, R.; Jensen-Doss, A.; Hawley, K.M.; Krumholz Marchette, L.S.; Chu, B.C.; et al. What five decades of research tells us about the effects of youth psychological therapy: A multilevel metaanalysis and implications for science and practice. Am. Psychol. 2017, 72, 79–117. [Google Scholar] [CrossRef]
- Keller, M.B.; Ryan, N.D.; Strober, M.; Klein, R.G.; Kutcher, S.P.; Birmaher, B.; Hagino, O.R.; Koplewicz, H.; Carlson, G.A.; Clarke, G.N.; et al. Efficacy of paroxetine in the treatment of adolescent major depression: A randomized, controlled trial. J. Am. Acad. Child Adolesc. Psychiatry 2001, 40, 762–772. [Google Scholar] [CrossRef]
- Janicak, P.G.; Lipinski, J.; Davis, J.M.; Altman, E.; Sharma, R.P. Parenteral S-adenosyl-methionine (SAMe) in depression: Literature review and preliminary data. Psychopharmacol. Bull. 1989, 25, 238–242. [Google Scholar]
- Hibbeln, J.R. Fish consumption and major depression. Lancet 1998, 351, 1213. [Google Scholar] [CrossRef]
- Nemets, B.; Stahl, Z.; Belmaker, R.H. Addition of omega-3 fatty acid to maintenance medication treatment for recurrent unipolar depressive disorder. Am. J. Psychiatry 2002, 159, 477–479. [Google Scholar] [CrossRef]
- Fu, Y.; Wang, Y.; Gao, H.; Li, D.; Jiang, R.; Ge, L.; Tong, C.; Xu, K. Associations among Dietary Omega-3 Polyunsaturated Fatty Acids, the Gut Microbiota, and Intestinal Immunity. Mediat. Inflamm. 2021, 2021, 8879227. [Google Scholar] [CrossRef]
- Costantini, L.; Molinari, R.; Farinon, B.; Merendino, N. Impact of Omega-3 Fatty Acids on the Gut Microbiota. Int. J. Mol. Sci. 2017, 18, 2645. [Google Scholar] [CrossRef]
- Liang, S.; Wu, X.; Hu, X.; Wang, T.; Jin, F. Recognizing Depression from the Microbiota-Gut-Brain Axis. Int. J. Mol. Sci. 2018, 19, 1592. [Google Scholar] [CrossRef]
- Hakkarainen, R.; Partonen, T.; Haukka, J.; Virtamo, J.; Albanes, D.; Lönnqvist, J. Food and nutrient intake in relation to mental wellbeing. Nutr. J. 2004, 3, 14. [Google Scholar] [CrossRef]
- Morris, G.; Berk, M. The many roads to mitochondrial dysfunction in neuroimmune and neuropsychiatric disorders. BMC Med. 2015, 13, 68. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.; Reiche, E.M.V.; Murru, A.; Carvalho, A.F.; Maes, M.; Berk, M.; Puri, B.K. Multiple Immune-Inflammatory and Oxidative and Nitrosative Stress Pathways Explain the Frequent Presence of Depression in Multiple Sclerosis. Mol. Neurobiol. 2018, 55, 6282–6306. [Google Scholar] [CrossRef]
- Pruneti, C.; Guidotti, S. Cognition, Behavior, Sexuality, and Autonomic Responses of Women with Hypothalamic Amenorrhea. Brain Sci. 2022, 12, 1448. [Google Scholar] [CrossRef]
- Franzoni, F.; Scarfò, G.; Guidotti, S.; Fusi, J.; Asomov, M.; Pruneti, C. Oxidative Stress and Cognitive Decline: The Neuroprotective Role of Natural Antioxidants. Front. Neurosci. 2021, 15, 729757. [Google Scholar] [CrossRef]
- Black, C.N.; Bot, M.; Scheffer, P.G.; Cuijpers, P.; Penninx, B.W. Is depression associated with increased oxidative stress? A systematic review and meta-analysis. Psychoneuroendocrinology 2015, 51, 164–175. [Google Scholar] [CrossRef]
- Lesch, K.P.; Bengel, D.; Heils, A.; Sabol, S.Z.; Greenberg, B.D.; Petri, S.; Benjamin, J.; Müller, C.R.; Hamer, D.H.; Murphy, D.L. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science 1996, 274, 1527–1531. [Google Scholar] [CrossRef]
- Arias, J.A.; Williams, C.; Raghvani, R.; Aghajani, M.; Baez, S.; Belzung, C.; Booij, L.; Busatto, G.; Chiarella, J.; Fu, C.H.; et al. The neuroscience of sadness: A multidisciplinary synthesis and collaborative review. Neurosci. Biobehav. Rev. 2020, 111, 199–228. [Google Scholar] [CrossRef] [PubMed]
- Caspi, A.; Sugden, K.; Moffitt, T.E.; Taylor, A.; Craig, I.W.; Harrington, H.; McClay, J.; Mill, J.; Martin, J.; Braithwaite, A.; et al. Influence of life stress on depression: Moderation by a polymorphism in the 5-HTT gene. Science 2003, 301, 386–389. [Google Scholar] [CrossRef] [PubMed]
- Caspi, A.; Hariri, A.R.; Holmes, A.; Uher, R.; Moffitt, T.E. Genetic sensitivity to the environment: The case of the serotonin transporter gene and its implications for studying complex diseases and traits. Am. J. Psychiatry 2010, 167, 509–527. [Google Scholar] [CrossRef] [PubMed]
- Pruneti, C.; Guidotti, S. Depression States, Behavioral, and Cognitive Components in Developmental age: Factorial analysis of a short assessment tool. Mediterr. J. Clin. Psychol. 2021, 9, 1–26. [Google Scholar] [CrossRef]
- Birmaher, B.; Ryan, N.D.; Williamson, D.E.; Brent, D.A.; Kaufman, J.; Dahl, R.E.; Perel, J.; Nelson, B. Childhood and adolescent depression: A review of the past 10 years. Part I. J. Am. Acad. Child Adolesc. Psychiatry 1996, 35, 1427–1439. [Google Scholar] [CrossRef]
- Dahl, R.E.; Birmaher, B.; Williamson, D.E.; Dorn, L.; Perel, J.; Kaufman, J.; Brent, D.A.; Axelson, D.A.; Ryan, N.D. Low growth hormone response to growth hormone-releasing hormone in child depression. Biol. Psychiatry 2000, 48, 981–988. [Google Scholar] [CrossRef]
- Herane-Vives, A.; Fischer, S.; de Angel, V.; Wise, T.; Cheung, E.; Chua, K.C.; Arnone, D.; Young, A.H.; Cleare, A.J. Elevated fingernail cortisol levels in major depressive episodes. Psychoneuroendocrinology 2018, 88, 17–23. [Google Scholar] [CrossRef]
- Cloninger, C.R.; Svrakic, D.M.; Przybeck, T.R. A psychobiological model of temperament and character. Arch. Gen. Psychiatry 1993, 50, 975–990. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 3rd ed.; Masson: Milano, Italy, 1980. [Google Scholar]
- Puig-Antich, J.; Blau, S.; Marx, N.; Greenhill, L.L.; Chambers, W. Prepubertal major depressive disorder: A pilot study. J. Am. Acad. Child Adolesc. Psychiatry 1978, 17, 695–707. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar]
- Kovacs, M.; Akistal, H.S.; Gatsonis, C. Childhood onset dysthymic disorder: Clinical feature and prospective naturalistic outcome. Arch. Gen. Psychiatry 1994, 51, 365–374. [Google Scholar] [CrossRef]
- Kovacs, M.; Obrosky, S.; George, C. The course of major depressive disorder from childhood to young adulthood: Recovery and recurrence in a longitudinal observational study. J. Affect. Disord. 2016, 203, 374–381. [Google Scholar] [CrossRef]
- Oh, Y.; Greenberg, M.T.; Willoughby, M.T. Family Life Project Key Investigators. Examining Longitudinal Associations between Externalizing and Internalizing Behavior Problems at Within- and Between-Child Levels. J. Abnorm. Child Psychol. 2020, 48, 467–480. [Google Scholar] [CrossRef]
- Quach, J.L.; Nguyen, C.D.; Williams, K.E.; Sciberras, E. Bidirectional Associations Between Child Sleep Problems and Internalizing and Externalizing Difficulties From Preschool to Early Adolescence. JAMA Pediatr. 2018, 172, e174363. [Google Scholar] [CrossRef]
- Pedersen, M.L.; Holen, S.; Lydersen, S.; Martinsen, K.; Neumer, S.P.; Adolfsen, F.; Sund, A.M. School functioning and internalizing problems in young schoolchildren. BMC Psychol. 2019, 7, 88. [Google Scholar] [CrossRef]
- Wada, K.; Sasaki, T.; Jitsuiki, H.; Takaishi, Y. One-year outcomes of unipolar depression patients with manic or hypomanic switch during acute antidepressant treatment. Int. J. Psychiatry Clin. Pract. 2013, 17, 219–222. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Kimoto, S.; Nagahama, T.; Kishimoto, T. Dosage-related nature of escitalopram treatment-emergent mania/hypomania: A case series. Neuropsychiatr. Dis. Treat. 2018, 14, 2099–2104. [Google Scholar] [CrossRef]
- Barbuti, M.; Pacchiarotti, I.; Vieta, E.; Azorin, J.M.; Angst, J.; Bowden, C.L.; Mosolov, S.; Young, A.H.; Perugi, G.; BRIDGE-II-Mix Study Group. Antidepressant-induced hypomania/mania in patients with major depression: Evidence from the BRIDGE-II-MIX study. J. Affect. Disord. 2017, 219, 187–192. [Google Scholar] [CrossRef]
- Hopkins, K.; Crosland, P.; Elliott, N.; Bewley, S. Diagnosis and management of depression in children and young people: Summary of updated NICE guidance. BMJ 2015, 350, h824. [Google Scholar] [CrossRef]
- Bachmann, C.J.; Aagaard, L.; Burcu, M.; Glaeske, G.; Kalverdijk, L.J.; Petersen, I.; Schuiling-Veninga, C.C.; Wijlaars, L.; Zito, J.M.; Hoffmann, F. Trends and patterns of antidepressant use in children and adolescents from five western countries, 2005–2012. Eur. Neuropsychopharmacol. 2016, 26, 411–419. [Google Scholar] [CrossRef]
- Zhou, X.; Hetrick, S.E.; Cuijpers, P.; Qin, B.; Barth, J.; Whittington, C.J.; Cohen, D.; Del Giovane, C.; Liu, Y.; Michael, K.D.; et al. Comparative efficacy and acceptability of psychotherapies for depression in children and adolescents: A systematic review and network metaanalysis. World Psychiatry 2015, 14, 207–222. [Google Scholar] [CrossRef]
- Doshi, P.; Dickersin, K.; Healy, D.; Vedula, S.S.; Jefferson, T. Restoring invisible and abandoned trials: A call for people to publish the findings. BMJ 2013, 346, f2865. [Google Scholar] [CrossRef] [PubMed]
- Bridge, J.A.; Iyengar, S.; Salary, C.B.; Barbe, R.P.; Birmaher, B.; Pincus, H.A.; Ren, L.; Brent, D.A. Clinical response and risk for reported suicidal ideation and suicide attempts in pediatric antidepressant treatment: A meta-analysis of randomized controlled trials. JAMA 2007, 297, 1683–1696. [Google Scholar] [CrossRef] [PubMed]
- Strawn, J.R.; Welge, J.A.; Wehry, A.M.; Keeshin, B.; Rynn, M.A. Efficacy and tolerability of antidepressants in pediatric anxiety disorders: A systematic review and meta-analysis. Depress. Anxiety 2015, 32, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Locher, C.; Koechlin, H.; Zion, S.R.; Werner, C.; Pine, D.S.; Kirsch, I.; Kessler, R.C.; Kossowsky, J. Efficacy and safety of selective serotonin reuptake inhibitors, serotonin norepinephrine reuptake inhibitors, and placebo for common psychiatric disorders among children and adolescents: A systematic review and metaanalysis. JAMA Psychiatry 2017, 74, 1011–1020. [Google Scholar] [CrossRef]
- Guidotti, S.; Coscioni, G.; Pruneti, C. Impact of COVID-19 on mental health and the role of personality: Preliminary data from a sample of Italian university students. Mediterr. J. Clin. Psychol. 2022, 10, 1–33. [Google Scholar] [CrossRef]

| Neurotransmitter | Precursor | Effects of Deficiency | Foods to Consume |
|---|---|---|---|
| Acetylcholine | Choline | Deterioration of memory and imagination; Fewer dreams; Increased confusion, forgetfulness, and disorganisation | Organic/free-range eggs; Organic or wild fish—especially salmon, mackerel, sardines, and fresh tuna |
| Serotonin | Tryptophan | Low mood, depression; Disrupted sleep cycle; Anxiety | Fish; Lean, organic poultry; Fruits, Avocado; Eggs; Low-fat cheese; Wheatgerm |
| Dopamine | Phenylalanine | Lack of focus and motivation; Poor attention and memory | Regular, balanced meals; Fruits and vegetables high in Vitamin C; Foods rich in B6 and Zinc; Wheatgerm; Fermented products |
| Norepinephrine and Epinephrine | Tyrosine | Low mood; Anxiety; Poor focus | Foods rich in Folic Acid; Magnesium, Manganese, Iron, Copper, Zinc, Vitamin C |
| Gamma-Amino Butyric Acid (GABA) | Glutamine | Self-criticism; Anxiety; Unable to relax | Dark green vegetables; Seeds and nuts; Potatoes; Bananas; Eggs |
| Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9 | Q10 | |
|---|---|---|---|---|---|---|---|---|---|---|
| McNamara et al. [35] | Y | Y | Y | Y | Y | Y | U | U | Y | Y |
| Trebaticka et al. [37] | Y | Y | Y | Y | Y | Y | U | N | Y | Y |
| Gabbay et al. [38] | Y | Y | Y | Y | Y | Y | Y | N | Y | Y |
| Fristad et al. [36,39] | Y | Y | Y | U | U | U | Y | Y | Y | Y |
| Libuda et al. [40] | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Authors | Sample | Intervention | Measures | Outcomes |
|---|---|---|---|---|
| McNamara et al. [35] | n = 56 (9–21 years old). Inclusion criteria: (1) diagnosis of MDD or DD-NOS; (2) a CDRS-R score ≥ 40; (3) at least one biological parent with Bipolar I disorder. | Group 1: fish oil (2100 mg/day); Group 2: placebo; Duration: 12 weeks. | Primary outcome measure: CDRS-R; Secondary outcomes measures: YMRS, CGI-S, CGI-I, CGAS, CBCL | A significant decrease in CGI-S (p = 0.0042) and CGI-I (p = 0.036) scores was greater for the experimental group. |
| Trebaticka et al. [37] | n = 60 (7–18 years old). Inclusion criteria: diagnosis of MDD or MADD. | Group 1: Omega-3 FA-rich fish oil emulsion; Group 2: active comparator of Omega-6 FA-rich sunflower oil emulsion; Duration: 12 weeks. | CDI | The time-dependent treatment effect in the Om3 group was significant (F = 6.284, df = 6, p < 0.0001) with a significant decrease in CDI scores from baseline (p = 0.001 in week 2 to p < 0.0001 in week 12). |
| Gabbay et al. [38] | n = 51 (12–19 years old). Inclusion criteria: diagnosis of MDD. | Group 1: Omega-3 FA (max. dosages of 3.6 g/day); Group 2: placebo; Duration: 10 weeks. | CDRS-R, BDI-II | No superior efficacy of the experimental treatment to placebo in reducing the severity of symptoms of depression was observed. |
| Fristad et al. [36,39] | n = 72 (7–14 years old). Inclusion criteria: diagnosis of MDD, DD, or DD-NOS | Group 1: Omega-3; Group 2: PEP + placebo; Group 3: Omega-3 + PEP; Group 4: placebo; Duration: 12 weeks. | Primary outcome measure: CDRS-R; Secondary outcomes measures: BRIEF | Pre-post: The percentages of depressive symptom remission were: 77% for Omega-3 + PEP group; 61% for PEP + placebo group; 44% for the Omega-3 group; 56% for the placebo group. Follow up: Mood severity (CDRS-R: t = 2.13, p = 0.04, d = −0.035), executive functioning (BRIEF: t = −4.07, p = 0.000, d = 0.60), and the global one (CGAS: t = 3.89, p = 0.000, d = 0.63) continued to be significantly improved even if depressive symptoms (CDRS-R: t = 7.69, p = 0.002, d = −0.71) increased significantly from the end of the study to the follow-up. |
| Libuda et al. [40] | n = 113 (11.0–18.9 years old). Inclusion criteria: (1) diagnosis of hypovitaminosis D [25(OH)D ≤ 30 nmol/l]; (2) BDI-II score > 13 | Group 1: TAU + oral Vitamin D3 (2640 IU/day); Group 2: TAU + placebo; Duration: 12 weeks. | Primary outcome measure: BDI-II; Secondary outcome measures: DISYPS-II, Serum total 25(OH)D | A greater increase in 25(OH)D levels in the experimental group emerged (treatment difference: +14 ng/mL; 95% CI 4.86–23.77; p = 0.003) while BDI-II scores did not differ significantly (+1.3; 95% CI −2.22 to 4.81; p = 0.466). A significant difference on the DISYPS scale by the patients’ parents was observed (−0.68; 95% CI −1.23 to −0.13; p = 0.016). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pruneti, C.; Guidotti, S. Need for Multidimensional and Multidisciplinary Management of Depressed Preadolescents and Adolescents: A Review of Randomized Controlled Trials on Oral Supplementations (Omega-3, Fish Oil, Vitamin D3). Nutrients 2023, 15, 2306. https://doi.org/10.3390/nu15102306
Pruneti C, Guidotti S. Need for Multidimensional and Multidisciplinary Management of Depressed Preadolescents and Adolescents: A Review of Randomized Controlled Trials on Oral Supplementations (Omega-3, Fish Oil, Vitamin D3). Nutrients. 2023; 15(10):2306. https://doi.org/10.3390/nu15102306
Chicago/Turabian StylePruneti, Carlo, and Sara Guidotti. 2023. "Need for Multidimensional and Multidisciplinary Management of Depressed Preadolescents and Adolescents: A Review of Randomized Controlled Trials on Oral Supplementations (Omega-3, Fish Oil, Vitamin D3)" Nutrients 15, no. 10: 2306. https://doi.org/10.3390/nu15102306






