An Isocaloric High-Fat Diet Regulates Partially Genetically Determined Fatty Acid and Carbohydrate Uptake and Metabolism in Subcutaneous Adipose Tissue of Lean Adult Twins
Abstract
:1. Introduction
2. Experimental Section
2.1. Study Participants
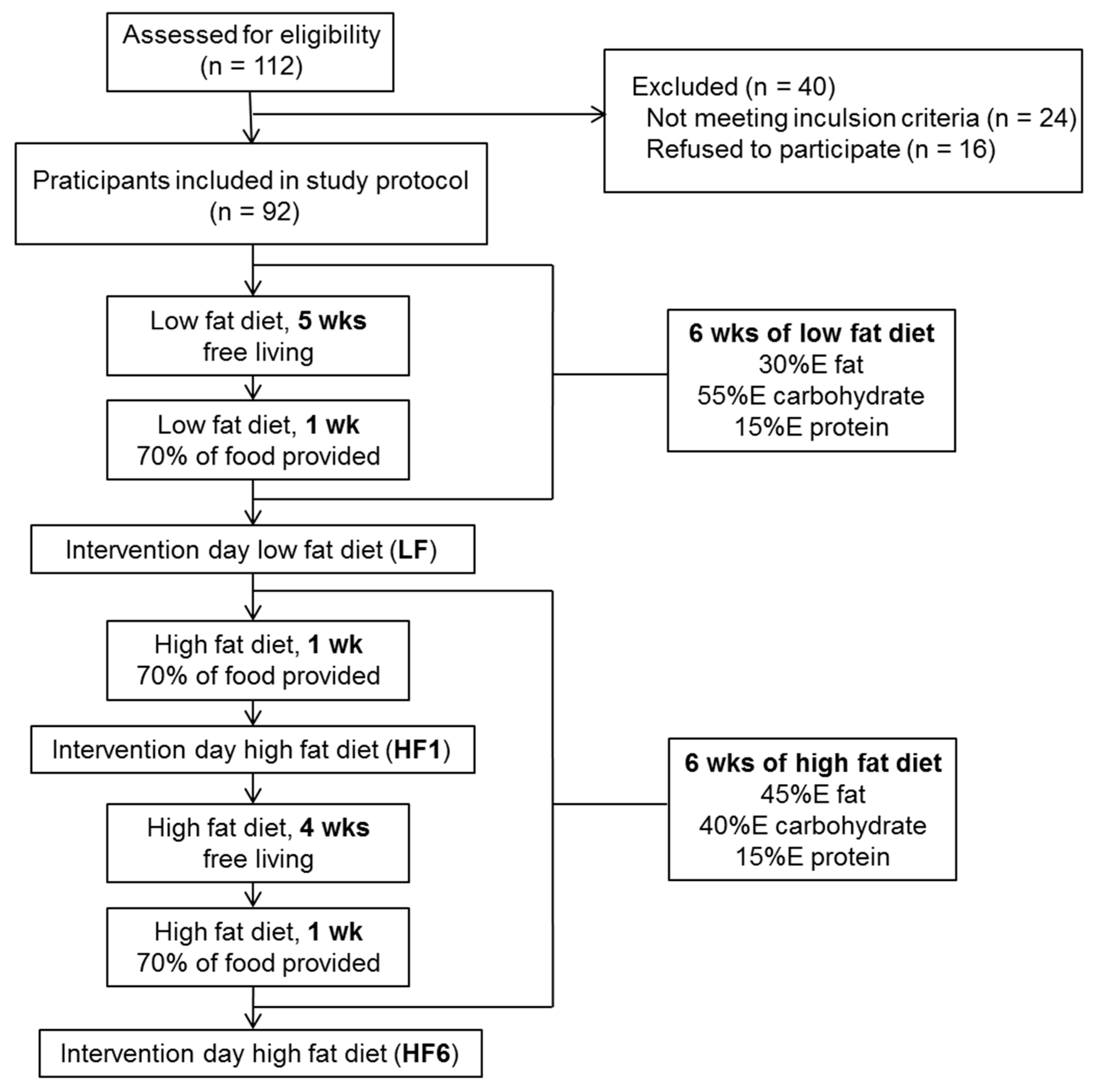
2.2. Study Design
2.3. Blood Analyses
2.4. Adipose Tissue Biopsy
2.5. Gene Expression Analyses
2.6. Statistical Analysis and Quantitative Genetics
3. Results
3.1. Body Weight, BMI, Serum Lipids, Glucose and Insulin
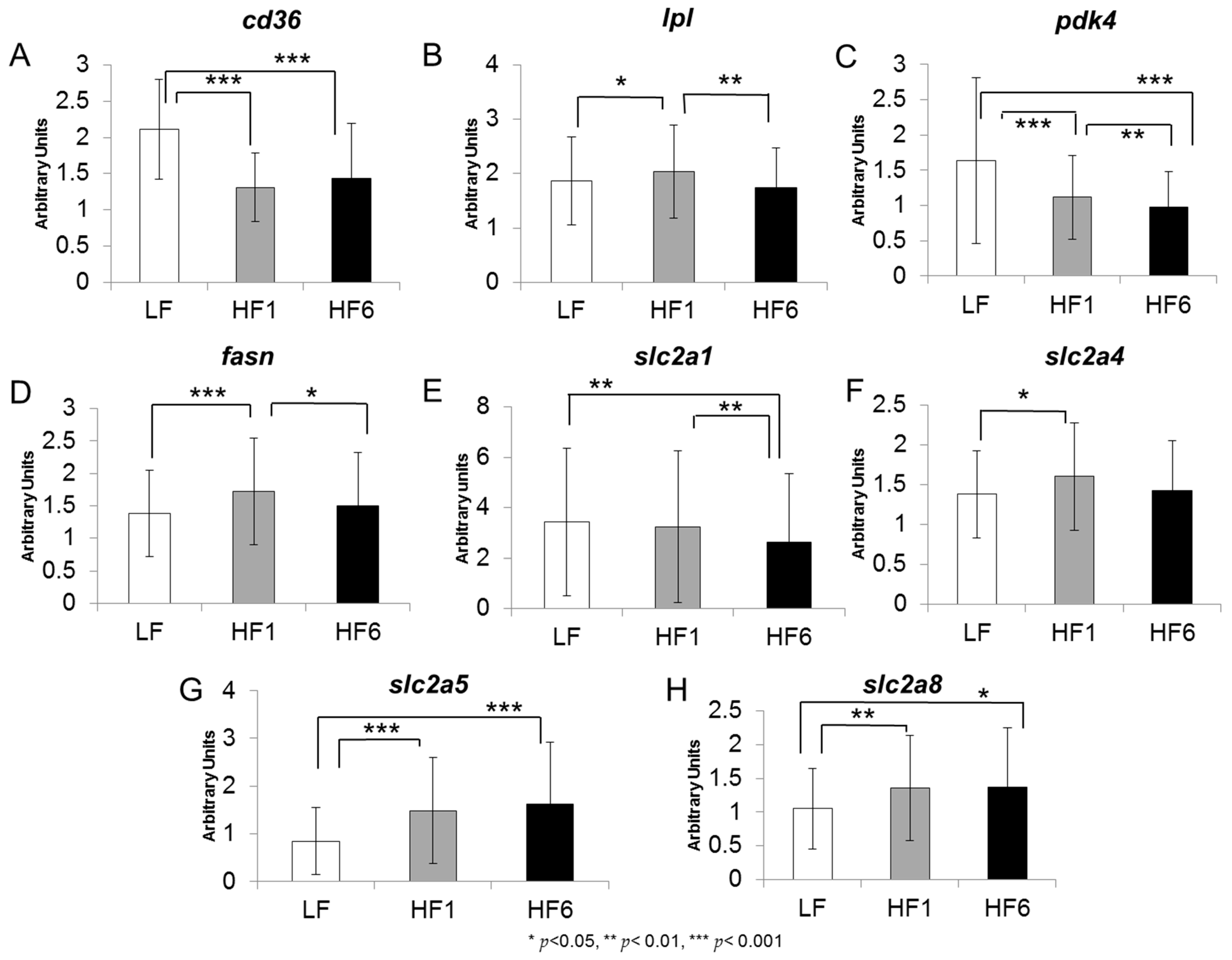
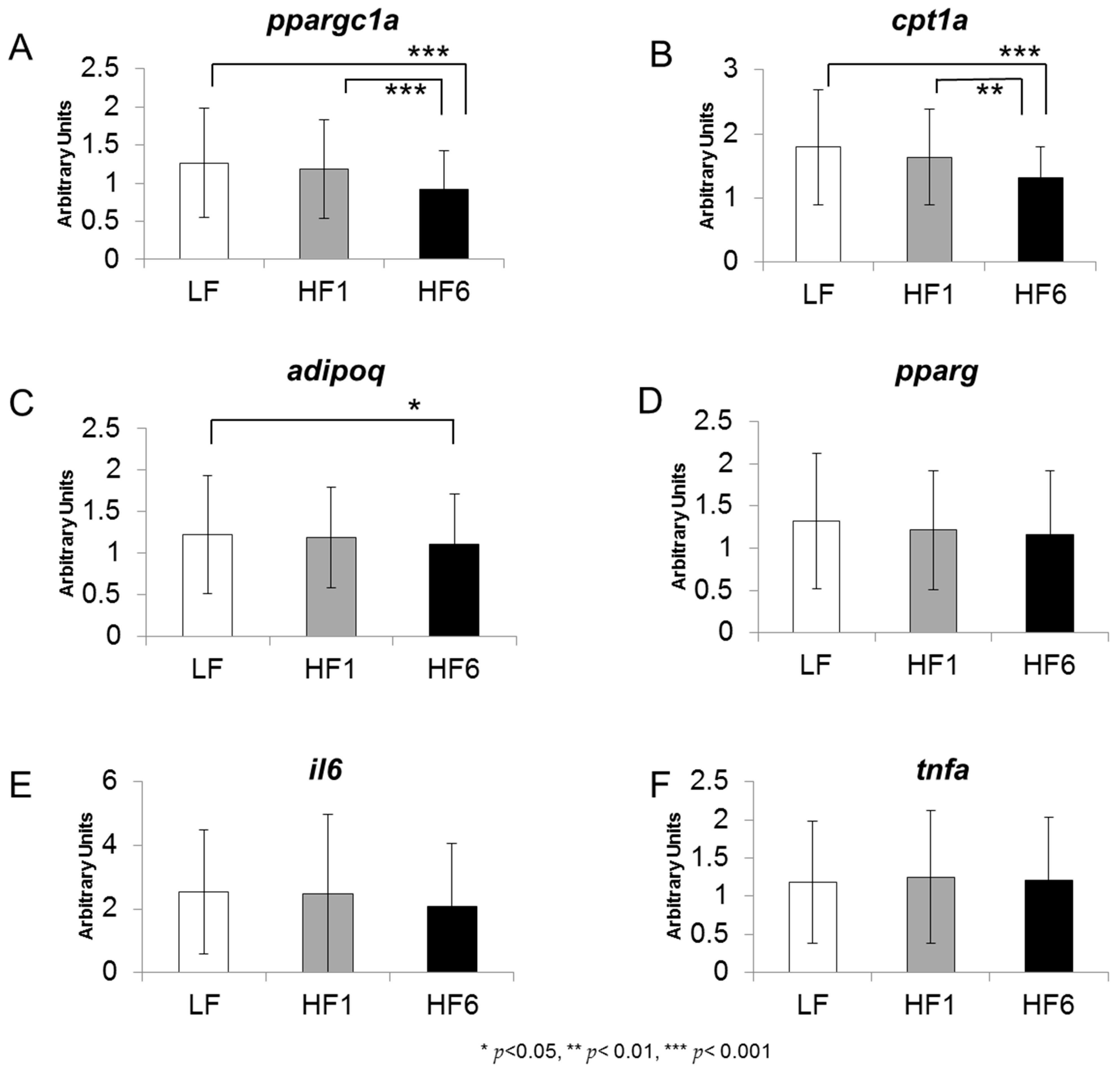
3.2. Gene Expression in Human s.c. Adipose tissue
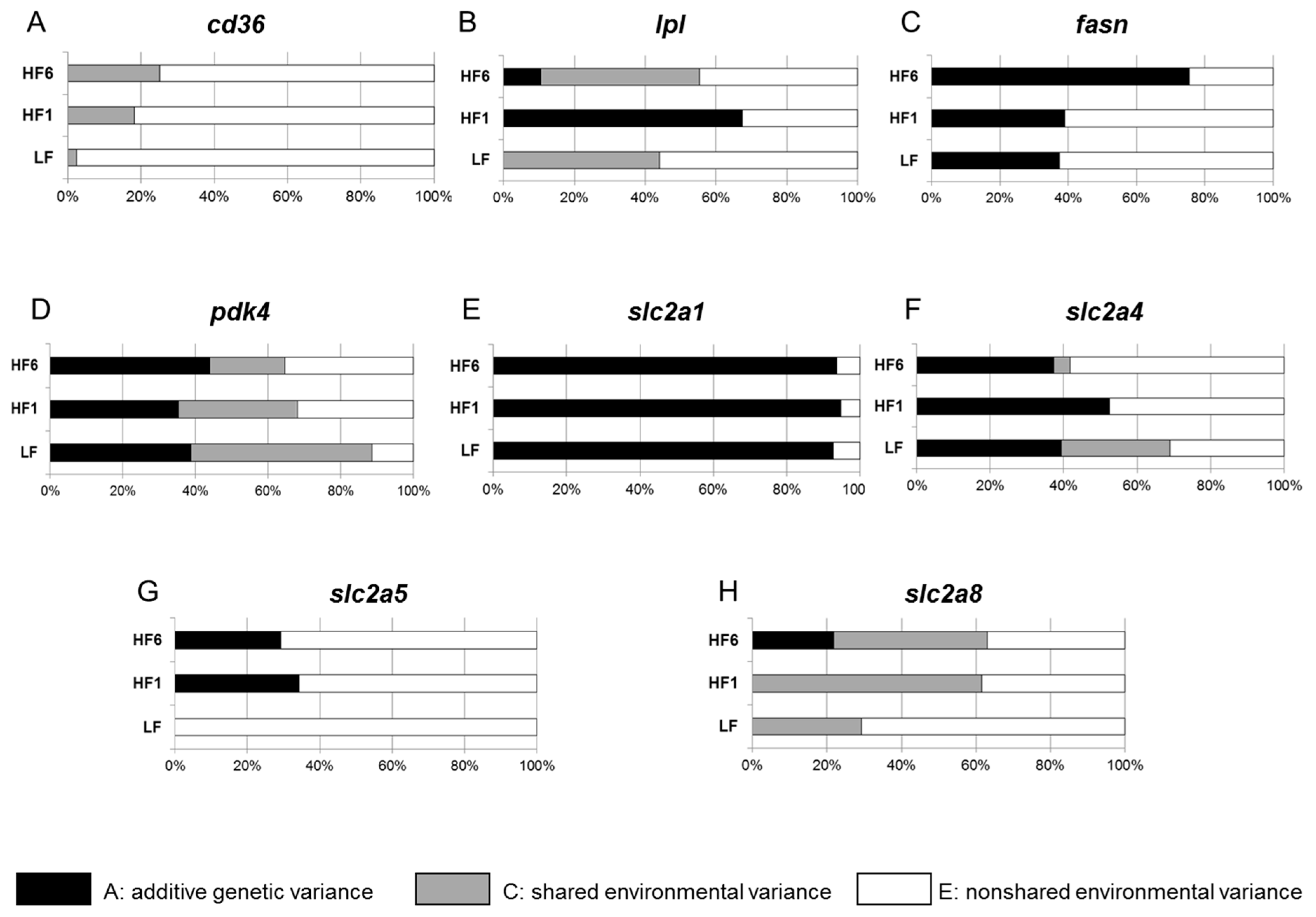
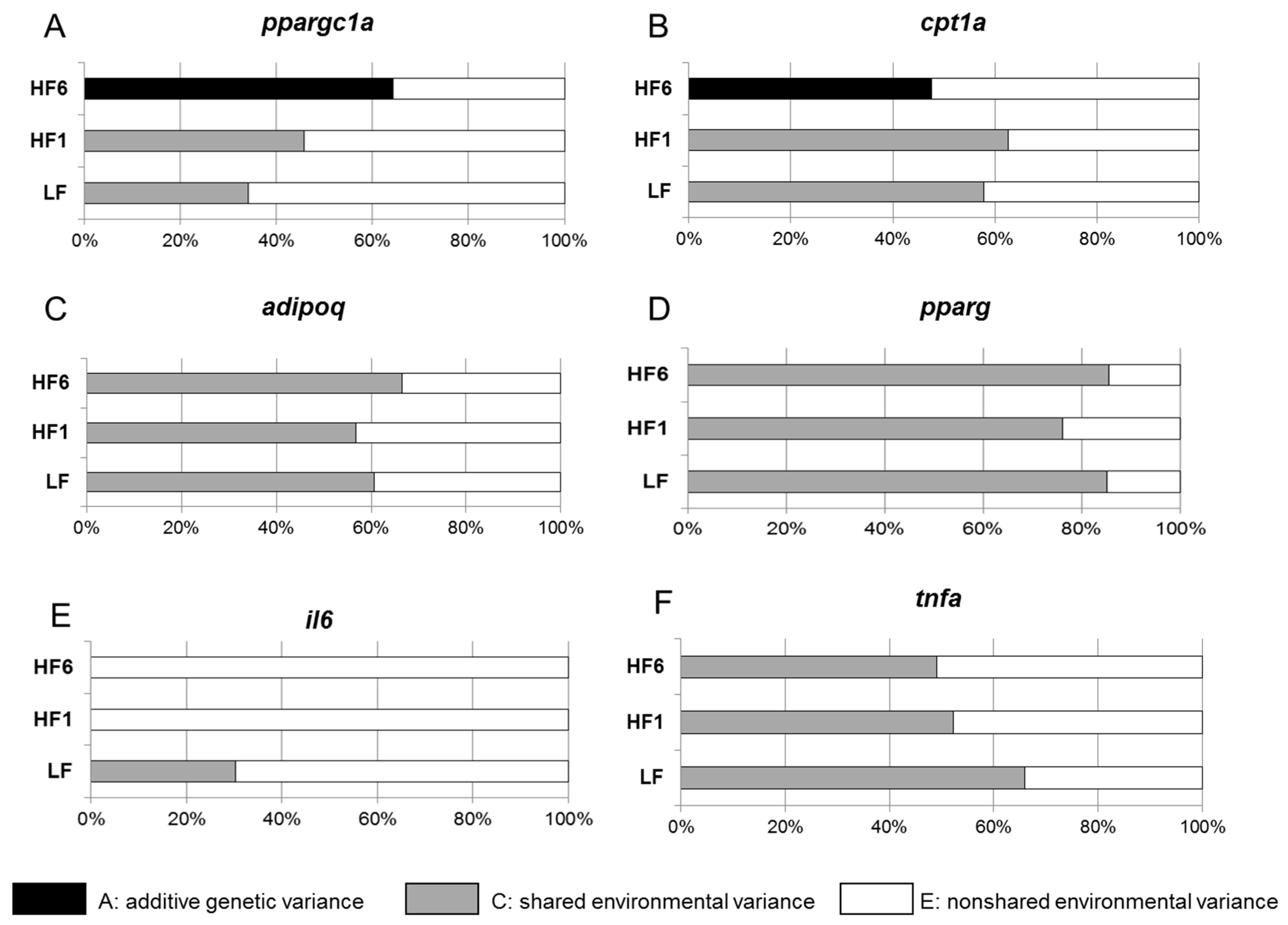
3.3. Determination of the Heritability of Gene Expression in s.c. Adipose Tissue
3.4. Correlation Analysis of the Gene Expression in s.c. Adipose Tissue
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Bremer, A.A.; Jialal, I. Adipose tissue dysfunction in nascent metabolic syndrome. J. Obes. 2013, 2013, 393192. [Google Scholar] [CrossRef]
- Collaboration NCDRF. Trends in adult body-mass index in 200 countries from 1975 to 2014: A pooled analysis of 1698 population-based measurement studies with 19.2 million participants. Lancet 2016, 387, 1377–1396. [Google Scholar] [CrossRef] [PubMed]
- Longo, M.; Zatterale, F.; Naderi, J.; Parrillo, L.; Formisano, P.; Raciti, G.A.; Beguinot, F.; Miele, C. Adipose Tissue Dysfunction as Determinant of Obesity-Associated Metabolic Complications. Int. J. Mol. Sci. 2019, 20, 2358. [Google Scholar] [CrossRef] [PubMed]
- Morigny, P.; Boucher, J.; Arner, P.; Langin, D. Lipid and glucose metabolism in white adipocytes: Pathways, dysfunction and therapeutics. Nat. Rev. Endocrinol. 2021, 17, 276–295. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Eckel, R.H. Lipoprotein lipase: From gene to obesity. Am. J. Physiol. Endocrinol. Metab. 2009, 297, E271–E288. [Google Scholar] [CrossRef]
- Basu, D.; Goldberg, I.J. Regulation of lipoprotein lipase-mediated lipolysis of triglycerides. Curr. Opin. Lipidol. 2020, 31, 154–160. [Google Scholar] [CrossRef]
- Puchalowicz, K.; Rac, M.E. The Multifunctionality of CD36 in Diabetes Mellitus and Its Complications-Update in Pathogenesis, Treatment and Monitoring. Cells 2020, 9, 1877. [Google Scholar] [CrossRef]
- Goldberg, I.J.; Eckel, R.H.; Abumrad, N.A. Regulation of fatty acid uptake into tissues: Lipoprotein lipase- and CD36-mediated pathways. J. Lipid Res. 2009, 50, S86–S90. [Google Scholar] [CrossRef]
- Bonen, A.; Tandon, N.N.; Glatz, J.F.; Luiken, J.J.; Heigenhauser, G.J. The fatty acid transporter FAT/CD36 is upregulated in subcutaneous and visceral adipose tissues in human obesity and type 2 diabetes. Int. J. Obes. 2006, 30, 877–883. [Google Scholar] [CrossRef]
- Mueckler, M.; Thorens, B. The SLC2 (GLUT) family of membrane transporters. Mol. Aspects Med. 2013, 34, 121–138. [Google Scholar] [CrossRef]
- Charron, M.J.; Brosius, F.C., 3rd; Alper, S.L.; Lodish, H.F. A glucose transport protein expressed predominately in insulin-responsive tissues. Proc. Natl. Acad. Sci. USA 1989, 86, 2535–2539. [Google Scholar] [CrossRef] [PubMed]
- Holman, G.D. Structure, function and regulation of mammalian glucose transporters of the SLC2 family. Pflug. Arch. 2020, 472, 1155–1175. [Google Scholar] [CrossRef] [PubMed]
- Mobbs, C.V.; Makimura, H. Block the FAS, lose the fat. Nat. Med. 2002, 8, 335–336. [Google Scholar] [CrossRef] [PubMed]
- Berndt, J.; Kovacs, P.; Ruschke, K.; Kloting, N.; Fasshauer, M.; Schon, M.R.; Korner, A.; Stumvoll, M.; Bluher, M. Fatty acid synthase gene expression in human adipose tissue: Association with obesity and type 2 diabetes. Diabetologia 2007, 50, 1472–1480. [Google Scholar] [CrossRef] [PubMed]
- Strable, M.S.; Ntambi, J.M. Genetic control of de novo lipogenesis: Role in diet-induced obesity. Crit. Rev. Biochem. Mol. Biol. 2010, 45, 199–214. [Google Scholar] [CrossRef]
- Du, L.; Heaney, A.P. Regulation of adipose differentiation by fructose and GluT5. Mol. Endocrinol. 2012, 26, 1773–1782. [Google Scholar] [CrossRef]
- Silva, J.C.P.; Marques, C.; Martins, F.O.; Viegas, I.; Tavares, L.; Macedo, M.P.; Jones, J.G. Determining contributions of exogenous glucose and fructose to de novo fatty acid and glycerol synthesis in liver and adipose tissue. Metab. Eng. 2019, 56, 69–76. [Google Scholar] [CrossRef]
- Elder, S.J.; Lichtenstein, A.H.; Pittas, A.G.; Roberts, S.B.; Fuss, P.J.; Greenberg, A.S.; McCrory, M.A.; Bouchard, T.J., Jr.; Saltzman, E.; Neale, M.C. Genetic and environmental influences on factors associated with cardiovascular disease and the metabolic syndrome. J. Lipid Res. 2009, 50, 1917–1926. [Google Scholar] [CrossRef]
- Bluher, M.; Bashan, N.; Shai, I.; Harman-Boehm, I.; Tarnovscki, T.; Avinaoch, E.; Stumvoll, M.; Dietrich, A.; Kloting, N.; Rudich, A. Activated Ask1-MKK4-p38MAPK/JNK stress signaling pathway in human omental fat tissue may link macrophage infiltration to whole-body Insulin sensitivity. J. Clin. Endocrinol. Metab. 2009, 94, 2507–2515. [Google Scholar] [CrossRef]
- Coburn, C.T.; Knapp, F.F., Jr.; Febbraio, M.; Beets, A.L.; Silverstein, R.L.; Abumrad, N.A. Defective uptake and utilization of long chain fatty acids in muscle and adipose tissues of CD36 knockout mice. J. Biol. Chem. 2000, 275, 32523–32529. [Google Scholar] [CrossRef]
- Hames, K.C.; Vella, A.; Kemp, B.J.; Jensen, M.D. Free fatty acid uptake in humans with CD36 deficiency. Diabetes 2014, 63, 3606–3614. [Google Scholar] [CrossRef] [PubMed]
- Viguerie, N.; Vidal, H.; Arner, P.; Holst, C.; Verdich, C.; Avizou, S.; Astrup, A.; Saris, W.H.; Macdonald, I.A.; Klimcakova, E.; et al. Adipose tissue gene expression in obese subjects during low-fat and high-fat hypocaloric diets. Diabetologia 2005, 48, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Yost, T.J.; Jensen, D.R.; Haugen, B.R.; Eckel, R.H. Effect of dietary macronutrient composition on tissue-specific lipoprotein lipase activity and insulin action in normal-weight subjects. Am. J. Clin. Nutr. 1998, 68, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Savard, R.; Bouchard, C. Genetic effects in the response of adipose tissue lipoprotein lipase activity to prolonged exercise. A twin study. Int. J. Obes. 1990, 14, 771–777. [Google Scholar] [PubMed]
- Wang, T.; Zang, Y.; Ling, W.; Corkey, B.E.; Guo, W. Metabolic partitioning of endogenous fatty acid in adipocytes. Obes. Res. 2003, 11, 880–887. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, G.N.; Rossi-Valentim, R.; Buzelle, S.L.; Paula-Gomes, S.; Zanon, N.M.; Garofalo, M.A.R.; Frasson, D.; Navegantes, L.C.C.; Chaves, V.E.; Kettelhut, I.D.C. Differential regulation of glyceroneogenesis by glucocorticoids in epididymal and retroperitoneal white adipose tissue from rats. Endocrine 2017, 57, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Cadoudal, T.; Distel, E.; Durant, S.; Fouque, F.; Blouin, J.M.; Collinet, M.; Bortoli, S.; Forest, C.; Benelli, C. Pyruvate dehydrogenase kinase 4: Regulation by thiazolidinediones and implication in glyceroneogenesis in adipose tissue. Diabetes 2008, 57, 2272–2279. [Google Scholar] [CrossRef]
- Ruschke, K.; Fishbein, L.; Dietrich, A.; Kloting, N.; Tonjes, A.; Oberbach, A.; Fasshauer, M.; Jenkner, J.; Schon, M.R.; Stumvoll, M.; et al. Gene expression of PPARgamma and PGC-1alpha in human omental and subcutaneous adipose tissues is related to insulin resistance markers and mediates beneficial effects of physical training. Eur. J. Endocrinol. 2010, 162, 515–523. [Google Scholar] [CrossRef]
- Briant, L.J.B.; Dodd, M.S.; Chibalina, M.V.; Rorsman, N.J.G.; Johnson, P.R.V.; Carmeliet, P.; Rorsman, P.; Knudsen, J.G. CPT1a-Dependent Long-Chain Fatty Acid Oxidation Contributes to Maintaining Glucagon Secretion from Pancreatic Islets. Cell Rep. 2018, 23, 3300–3311. [Google Scholar] [CrossRef]
- Ohashi, K.; Munetsuna, E.; Yamada, H.; Ando, Y.; Yamazaki, M.; Taromaru, N.; Nagura, A.; Ishikawa, H.; Suzuki, K.; Teradaira, R.; et al. High fructose consumption induces DNA methylation at PPARalpha and CPT1A promoter regions in the rat liver. Biochem. Biophys. Res. Commun. 2015, 468, 185–189. [Google Scholar] [CrossRef]
- Douard, V.; Ferraris, R.P. Regulation of the fructose transporter GLUT5 in health and disease. Am. J. Physiol. Endocrinol. Metab. 2008, 295, E227–E237. [Google Scholar] [CrossRef] [PubMed]
- Legeza, B.; Balázs, Z.; Odermatt, A. Fructose promotes the differentiation of 3T3-L1 adipocytes and accelerates lipid metabolism. FEBS Lett. 2014, 588, 490–496. [Google Scholar] [CrossRef] [PubMed]
- Hajduch, E.; Litherland, G.J.; Turban, S.; Brot-Laroche, E.; Hundal, H.S. Insulin regulates the expression of the GLUT5 transporter in L6 skeletal muscle cells. FEBS Lett. 2003, 549, 77–82. [Google Scholar] [CrossRef]
- Varma, V.; Boros, L.G.; Nolen, G.T.; Chang, C.W.; Wabitsch, M.; Beger, R.D.; Kaput, J. Metabolic fate of fructose in human adipocytes: A targeted (13)C tracer fate association study. Metabolomics 2015, 11, 529–544. [Google Scholar] [CrossRef] [PubMed]
- Collins, J.M.; Neville, M.J.; Pinnick, K.E.; Hodson, L.; Ruyter, B.; van Dijk, T.H.; Reijngoud, D.-J.; Fielding, M.D.; Frayn, K.N. De novo lipogenesis in the differentiating human adipocyte can provide all fatty acids necessary for maturation. J. Lipid Res. 2011, 52, 1683–1692. [Google Scholar] [CrossRef] [PubMed]
- Wakil, S.J. Fatty acid synthase, a proficient multifunctional enzyme. Biochemistry 1989, 28, 4523–4530. [Google Scholar] [CrossRef]
- Schüler, R.; Seebeck, N.; Osterhoff, M.A.; Witte, V.; Flöel, A.; Busjahn, A.; Jais, A.; Brüning, J.C.; Frahnow, T.; Kabisch, S.; et al. VEGF and GLUT1 are highly heritable, inversely correlated and affected by dietary fat intake: Consequences for cognitive function in humans. Mol. Metab. 2018, 11, 129–136. [Google Scholar] [CrossRef]
- Schmidt, S.; Joost, H.G.; Schurmann, A. GLUT8, the enigmatic intracellular hexose transporter. Am. J. Physiol. Endocrinol. Metab. 2009, 296, E614–E618. [Google Scholar] [CrossRef]
- Herman, M.A.; Birnbaum, M.J. Molecular aspects of fructose metabolism and metabolic disease. Cell Metab. 2021, 33, 2329–2354. [Google Scholar] [CrossRef]
- Alexander, C.M.; Martin, J.A.; Oxman, E.; Kasza, I.; Senn, K.A.; Dvinge, H. Alternative Splicing and Cleavage of GLUT8. Mol. Cell. Biol. 2020, 41, e00480-20. [Google Scholar] [CrossRef]
- Novelle, M.G.; Bravo, S.B.; Deshons, M.; Iglesias, C.; Garcia-Vence, M.; Annells, R.; da Silva Lima, N.; Nogueiras, R.; Fernandez-Rojo, M.A.; Dieguez, C.; et al. Impact of liver-specific GLUT8 silencing on fructose-induced inflammation and omega oxidation. iScience 2021, 24, 102071. [Google Scholar] [CrossRef] [PubMed]
- Debosch, B.J.; Chen, Z.; Saben, J.L.; Finck, B.N.; Moley, K.H. Glucose transporter 8 (GLUT8) mediates fructose-induced de novo lipogenesis and macrosteatosis. J. Biol. Chem. 2014, 289, 10989–10998. [Google Scholar] [CrossRef] [PubMed]

| Monozygotic Twins | Dizygotic Twins | |
|---|---|---|
| (n = 68) | (n = 24) | |
| Gender (F/M) | 44/24 | 12/12 |
| Age (years) | 33.9 ± 14.9 | 24.5 ± 7.6 |
| Body height (m) | 1.71 ± 8.7 | 1.74 ± 11.6 |
| Body weight (kg) | 67.4 ± 11.9 | 68.0 ± 11.7 |
| BMI (kg/m2) | 23.0 ± 2.8 | 22.3 ± 2.2 |
| Waist/hip ratio | 0.82 ± 0.1 | 0.80 ± 0.1 |
| Serum total cholesterol (mmol/L) | 4.74 ± 0.9 | 4.15 ± 0.9 |
| Serum LDL cholesterol (mmol/L) | 2.84 ± 0.8 | 2.44 ± 0.6 |
| Serum HDL cholesterol (mmol/L) | 1.43 ± 0.3 | 1.24 ± 0.4 |
| Serum triacylglycerol (mmol/L) | 0.98 ± 0.4 | 1.01 ± 0.6 |
| Serum-free fatty acids (mmol/L) | 0.52 ± 0.2 | 0.52 ± 0.4 |
| Fasted serum glucose (mg/dL) | 86.2 ± 9.5 | 86.2 ± 6.4 |
| Fasted serum insulin (mU/L) | 5.30 ± 4.1 | 4.96 ± 2.2 |
| HOMA IR index | 1.16 ± 01.0 | 1.05 ± 0.5 |
| HbA1c (%) | 5.02 ± 0.4 | 0.52 ± 0.4 |
| Monozygotic Twins | Dizygotic Twins | |||||
|---|---|---|---|---|---|---|
| LF | HF1 | HF6 | LF | HF1 | HF6 | |
| Body weight (kg) | 66.4 ± 11.9 | 66.3 ± 11.9 | 66.8 ± 12.1 | 67.2 ± 11.3 | 67.1 ± 11.0 | 67.6 ± 11.5 |
| BMI (kg/m2) | 22.7 ± 2.9 | 22.6 ± 2.9 | 22.8 ± 2.9 | 22.1 ± 2.0 | 22.0 ± 1.9 | 22.2 ± 2.0 |
| Total cholesterol (mmol/L) | 4.42 ± 0.8 | 4.57 ± 0.8 | 4.83 ± 0.9 * | 3.94 ± 0.8 | 4.18 ± 0.9 | 4.33 ± 0.8 |
| LDL (mmol/L) | 2.67 ± 0.7 | 2.76 ± 0.7 | 2.93 ± 0.8 | 2.38 ± 0.7 | 2.58 ± 0.7 | 2.65 ± 0.7 |
| HDL (mmol/L) | 1.30 ± 0.3 | 1.36 ± 0.4 | 1.46 ± 0.4 | 1.14 ± 0.3 | 1.21 ± 0.3 | 1.27 ± 0.3 |
| Triglycerides (mmol/L) | 0.97 ± 0.4 | 0.91 ± 0.3 | 0.90 ± 0.4 | 0.91 ± 0.5 | 0.86 ± 0.4 | 0.92 ± 0.4 |
| Free fatty acids (mmol/L) | 0.62 ± 0.2 | 0.55 ± 0.2 | 0.51 ± 0.2 § | 0.56 ± 0.2 | 0.63 ± 0.2 | 0.47 ± 0.2 # |
| Glucose (mg/dL) | 94.0 ± 13.2 | 93.6 ± 10.8 | 95.1 ± 10.7 | 93.9 ± 18.1 | 90.9 ± 12.4 | 90.9 ± 11.2 |
| Insulin (mU/L) | 4.78 ± 3.3 | 5.65 ± 4.0 | 5.30 ± 3.9 | 4.52 ± 2.7 | 5.29 ± 2.7 | 4.55 ± 2.8 |
| HOMA-IR | 1.06 ± 0.8 | 1.30 ± 1.0 $ | 1.26 ± 0.3 § | 1.02 ± 0.5 | 1.17 ± 0.5 ¶ | 1.15 ± 0.3 |
| Gene Name and Function | Dietary Regulation | ||
|---|---|---|---|
| LF vs. HF1 | LF vs. HF6 | HF1 vs. HF6 | |
| cd36 | ↓↓↓ | ↓↓↓ | ↔ |
| (hydrolysis of free fatty acids) | |||
| lpl | ↑ | ↔ | ↓ |
| (lipid transport) | |||
| pdk4 | ↓↓↓ | ↓↓↓ | ↓↓ |
| (glycerol-3-phosphate generation) | |||
| fasn | ↑ | ↔ | ↓ |
| (de novo fatty acid synthesis) | |||
| slc2a1 | ↔ | ↓ | ↓ |
| (glucose transport) | |||
| slc2a4 | ↑ | ↔ | ↔ |
| (glucose transport) | |||
| slc2a5 | ↑↑↑ | ↑↑↑ | ↔ |
| (fructose transport) | |||
| slc2a8 | ↑↑ | ↑ | ↔ |
| (glucose and fructose transport) | |||
| ppargc1a | ↔ | ↓ | ↓ |
| (fatty acid oxidation) | |||
| cpt1a | ↔ | ↓↓ | ↓↓ |
| (fatty acid oxidation) | |||
| adipoq | ↔ | ↓ | ↔ |
| (adipogenesis) | |||
| pparg | ↔ | ↔ | ↔ |
| (adipogenesis) | |||
| il6 | ↔ | ↔ | ↔ |
| (inflammation) | |||
| tnfa | ↔ | ↔ | ↔ |
| (inflammation) | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kruse, M.; Hornemann, S.; Ost, A.-C.; Frahnow, T.; Hoffmann, D.; Busjahn, A.; Osterhoff, M.A.; Schuppelius, B.; Pfeiffer, A.F.H. An Isocaloric High-Fat Diet Regulates Partially Genetically Determined Fatty Acid and Carbohydrate Uptake and Metabolism in Subcutaneous Adipose Tissue of Lean Adult Twins. Nutrients 2023, 15, 2338. https://doi.org/10.3390/nu15102338
Kruse M, Hornemann S, Ost A-C, Frahnow T, Hoffmann D, Busjahn A, Osterhoff MA, Schuppelius B, Pfeiffer AFH. An Isocaloric High-Fat Diet Regulates Partially Genetically Determined Fatty Acid and Carbohydrate Uptake and Metabolism in Subcutaneous Adipose Tissue of Lean Adult Twins. Nutrients. 2023; 15(10):2338. https://doi.org/10.3390/nu15102338
Chicago/Turabian StyleKruse, Michael, Silke Hornemann, Anne-Cathrin Ost, Turid Frahnow, Daniela Hoffmann, Andreas Busjahn, Martin A. Osterhoff, Bettina Schuppelius, and Andreas F. H. Pfeiffer. 2023. "An Isocaloric High-Fat Diet Regulates Partially Genetically Determined Fatty Acid and Carbohydrate Uptake and Metabolism in Subcutaneous Adipose Tissue of Lean Adult Twins" Nutrients 15, no. 10: 2338. https://doi.org/10.3390/nu15102338
APA StyleKruse, M., Hornemann, S., Ost, A.-C., Frahnow, T., Hoffmann, D., Busjahn, A., Osterhoff, M. A., Schuppelius, B., & Pfeiffer, A. F. H. (2023). An Isocaloric High-Fat Diet Regulates Partially Genetically Determined Fatty Acid and Carbohydrate Uptake and Metabolism in Subcutaneous Adipose Tissue of Lean Adult Twins. Nutrients, 15(10), 2338. https://doi.org/10.3390/nu15102338






